Abstract
Aims/Introduction
The aim of the present study was to use the sudomotor function test, Sudoscan, as a screening method for the evaluation of asymptomatic diabetic distal symmetric polyneuropathy in Chinese type 2 diabetes patients. As a result, more attention could be paid to those asymptomatic patients who could be easily neglected and underdiagnosed in everyday clinic.
Materials and Methods
A total of 394 Chinese type 2 diabetes patients were enrolled and tested for symptoms and clinical signs of neuropathy using the Neurological Symptom Score, Neuropathy Disability Score, and vibration perception threshold. Sudoscan was carried out, and the results were collected as the measurement of the electrochemical skin conductance of both hands and feet.
Results
In the present study, we found that the abnormal rate of Sudoscan results in patients with asymptomatic neuropathy was higher than those without neuropathy and those with symptomatic neuropathy. This study also showed that lower electrochemical skin conductance at the feet was significantly associated with increasing symptoms, Neurological Symptom Score (r = −0.124, P < 0.05), Neuropathy Disability Score (r = −0.3, P < 0.01) and vibration perception threshold value (r = −0.18, P < 0.05). Logistic analysis showed that age (odds ratio 1.042, 95% confidence interval 1.014−1.071, P < 0.05) and feet electrochemical skin conductance levels (odds ratio 0.98, 95% confidence interval 0.962–0.993, P < 0.01) were independently associated with diabetic distal symmetric polyneuropathy.
Conclusions
Sudoscan might be a promising tool to screen asymptomatic diabetic distal symmetric polyneuropathy in Chinese patients with type 2 diabetes mellitus.
Keywords: Distal symmetric polyneuropathy, Screening, Sudoscan
Introduction
Diabetic peripheral neuropathy (DPN) can remain undetected and dormant, leading to serious complications1, 2. The most common variety of diabetic peripheral neuropathy is a chronic, symmetrical, length‐dependent, sensorimotor polyneuropathy defined as distal symmetric polyneuropathy (DSPN). However, symptoms and presentations of DSPN can vary greatly3. It often involves distal small nerve fibers and presents as painful neuropathy, but up to 50% of patients with neuropathy might be asymptomatic – easily resulting in delayed diagnosis, reduced quality of life and increased mortality4, 5.
Clinical diabetic neuropathy is usually diagnosed according to symptoms including anesthesia, burning or prickling sensation, as well as by testing tools including vibration sensation (tuning fork) or touch (Semmes–Weinstein monofilament examination)6, 7, 8, 9. Conventional screening methods for DSPN consist of different questionnaires including the Michigan Neuropathy Screening Instrument, Neurological Symptom Score (NSS), Neuropathy Disability Score (NDS), Neuropathy Impairment Score–Lower Limb, Utah Early Neuropathy Scale and so on10, 11, 12, 13, 14. Each of these questionnaires emphasizes different clinical manifestations, and proves to be quite time‐consuming in everyday clinical use. However, it still remains to be a huge challenge for screening asymptomatic diabetic neuropathy in Chinese type 2 diabetes mellitus patients concerning the low detecting rate in everyday clinic. Although a definite neuropathy diagnosis is based on confirmatory tests, exemplified by nerve conduction studies (NCS), NCS such as electromyography aim to reflect the impairment of large myelinated fibers, and are difficult to carry out in everyday clinic because of the obvious disadvantage of being invasive and inconvenient. Therefore, selecting a effective, non‐invasive screening method to detect asymptomatic DSPN in the early stage still remains a problem.
Sudoscan (Impeto Medical, Paris, France) is a non‐invasive device for the assessment of sudomotor function through evaluation of sweat gland secretory function as an early reflection of sympathetic nerve impairment15. Sweat glands innervated by thin unmyelinated sympathetic C fibers could be easily affected in the early stage of diabetes, as well as prediabetes16. The electrical current applied to the patients automatically by the device (usually <4 V) could attract sodium chloride from sweat in the palms of their hands and soles of their feet. Results of the electrochemical skin conductance (ESC) values for hands and feet are generated from the device. As an easy and objective screening method for DSPN, SUDOSCAN has proved to be a sensitive and quantitative screening test in clinical use in Caucasian and Indian patients17, 18, 19. However, there was no relevant report regarding the screening of Sudoscan in Chinese asymptomatic patients. The present study was carried out to evaluate Sudoscan as a tool for assessing asymptomatic DSPN in Chinese patients with type 2 diabetes mellitus.
Materials and Methods
Study population
The study was carried out in Huashan Hospital, Shanghai, China, from September 2014 to September 2015. The ethics committee of Hua Shan Hospital approved the study. Voluntary outpatients diagnosed with type 2 diabetes aged between 18 and 80 years, with or without symptoms of neuropathy, were continually enrolled in the study. Exclusion criteria included undiagnosed hyperglycemia, type 1 diabetes mellitus patients, those under treatment with drugs that could have an effect on the sympathetic system (such as beta‐blockers and antineoplastic drugs), implantation of electrical implantable devices, history of seizures or epilepsy, lumbar sciatic nerve lesion, severe varices of the lower limbs, other metabolic diseases including thyroid disease or vitamin B12 deficiency, and any other advanced systemic condition including severe hepatic and renal dysfunction.
Sample size estimation
In the pilot experiment, we carried out the Sudoscan test in 62 patients (34 with non‐DPN and 28 with DPN). We assumed a two‐sided α error of 5%, and a statistical power of 80% to determine that a total of 165 patients were required per group. Finally, 394 patients were consecutively enrolled in the present study.
Physical examination
One trained nurse examined all the patients and recorded the results. Basic physical characteristics were recorded including height, weight, waist circumference and hip circumference measured by using standard methods. Body mass index and waist‐to‐hip ratio were calculated. Blood pressure was recorded in the supine position after 5 min of rest. The complete medical history (diabetes, hypertension, dyslipidemia, cardiovascular disease and other) was recorded for each patient.
Laboratory examination
Glycosylated hemoglobin A1c of all included patients was determined by high‐pressure liquid chromatography.
Peripheral neuropathy examination
Symptoms and signs of lower limbs were recorded, respectively. A composite score was calculated separately for neuropathic symptoms using the NSS score questionnaire, and for clinical examination using the NDS score.
Vibration perception value was detected with a biothesiometer carried out by a trained nurse.
Sudoscan test procedure
The Sudoscan device is composed of two sets of electrodes for the feet and hands, both of which are connected to a computer for recording and data analysis. The whole process of the test is non‐invasive, and no special preparation is required. Patients only need to place the palms of their hands and the soles of their feet on the electrodes for 2–3 min, and a low‐voltage (<4 V) electrical current stimulus is automatically applied by the device. The device can measure ESC values expressed in micro‐siemens (μS) for the hands and the feet (both right and left sides). We used the mean of the left and right ESC values for statistical analysis.
Diagnostic criterion for DSPN
According to previous standards, an NSS score of 3–4 points was considered as a mild neuropathy symptom, 5–6 points as a medium neuropathy symptom and 7–9 points as a severe neuropathy symptom. The results of the NDS score were divided into three classes as well. An NDS of 3–5 points was considered as mild neuropathy signs, 6–8 points as medium neuropathy signs and 9–10 points as severe neuropathy signs. The golden diagnosis standard of DSPN is dependent on both NSS and NDS scores (with an NDS score of ≥6, or an NDS score of 3–5 associated with an NSS score of ≥5)20.
We further divided all the patients into three categories based on their symptoms and clinical signs. The ‘no diabetic neuropathy’ group (group 1) referred to the patients without DSPN according to the NSS/NDS standard. The ‘asymptomatic neuropathy’ group (group 2) referred to the patients with NSS score <4 and NDS score >6. The ‘symptomatic neuropathy’ group (group 3) was defined by an NSS score >5, as well as an NDS score >3.
As for the Sudoscan test, we used 60 μS of mean feet ESC as the cut‐off for diagnosis of DSPN according to previous studies18, 19.
Statistical analysis
Continuous data are presented as mean ± standard deviation, and categorical variables as percentages, respectively. Analysis of variance was used to compare mean differences of ESC values between the groups. Categorical variables were compared by the χ2‐test. Correlation was determined using Spearman's rho rank tests. Logistic regression analysis was used to determine the association between biological determinants (age, sex, body mass index, waist‐to‐hip ratio, duration of type 2 diabetes mellitus etc.), feet ESC, hands ESC and DSPN. Spss 16.0 software (Spss, Chicago, IL, USA) was used for all statistical analyses. Significance was accepted at the P < 0.05 level.
Results
A total of 394 type 2 diabetic patients (244 men and 150 women) were enrolled in the study. We excluded the 37 patients who had been diagnosed as DPN and treated with neurotrophic drugs, and further divided patients into three groups according to their clinical symptoms and signs (as described in the diagnostic criterion).
The clinical and biochemical characteristics of the three groups of the remaining 357 participants are described in Table 1. Type 2 diabetes mellitus patients with symptomatic diabetic neuropathy had higher age, longer duration of diabetes and higher glycosylated hemoglobin A1c levels among the three groups. However, among the three groups, the asymptomatic neuropathy group had the highest rate of Sudoscan detecting abnormal results at up to 69%, compared with 34.6% in the no diabetic neuropathy group and 61.7% in symptomatic neuropathy group (Figure 1).
Table 1.
Basic clinical and biochemical characteristics of 357 type 2 diabetes mellitus study participants
| Clinical features | Group 1 (n = 258) | Group2 (n = 29) | Group 3 (n = 60) |
|---|---|---|---|
| Sex (male/female) | 162/96 | 23/6 | 36/24 |
| Age (years) | 57.3 ± 12.3* | 64.0 ± 13.4* | 65.4 ± 9.3* |
| Duration of diabetes (years) | 6 (1–10)* | 9 (1–13)* | 11 (6–18)* |
| Family history of T2DM (%) | 38.4 | 34.5 | 35.0 |
| Smoking (%) | 28.3 | 24.1 | 16.7 |
| Alcohol (%) | 15.1 | 20.7 | 11.7 |
| Dyslipidemia (%) | 28.7 | 13.8 | 20.0 |
| Height (cm) | 166.5 ± 8.4 | 161.8 ± 12.3 | 166.3 ± 7.8 |
| Weight (kg) | 69.5 ± 13.1 | 68.2 ± 7.6 | 66.5 ± 10.1 |
| BMI (kg/m2) | 24.5 ± 3.6 | 24.6 ± 2.36 | 23.5 ± 2.9 |
| Waist (cm) | 89.0 ± 10.1 | 89.0 ± 7.3 | 90.0 ± 8.7 |
| Hip (cm) | 96.4 ± 7.2 | 96.6 ± 5.0 | 96.1 ± 6.3 |
| WHR | 0.88 (0.8–0.9) | 0.9 (0.85–0.95) | 0.93 (0.89–0.97) |
| Systolic BP (mmHg) | 125.9 ± 11.6 | 125.0 ± 14.5 | 129.3 ± 13 |
| HbA1c (%) | 8.0 ± 2.0* | 7.5 ± 1.0* | 9.0 ± 2.2* |
| NSS score | 1 (0–4)* | 1 (0–2.5)* | 6 (5–7)* |
| NDS score | 1 (0–2)* | 7 (6–8)* | 5 (4–6)* |
| VPT value | 10.1 ± 5.8* | 15.7 ± 9.6* | 17.5 ± 8.5* |
| DSPN detected by Sudoscan (%) | 34.6* | 69.0* | 61.7* |
| Feet ESC value (μS) | 67.1 ± 16.6* | 57.8 ± 20.8* | 56.2 ± 20.3* |
| Hands ESC value (μS) | 66.4 ± 16.0* | 55.6 ± 20.0* | 61.1 ± 16.9* |
Continuous data are presented as mean ± standard deviation values and quartiles. *Significant difference between three groups (P < 0.05). Group 1, no diabetic neuropathy group; Group 2, asymptomatic neuropathy group; Group 3, symptomatic neuropathy group. BMI, body mass index; BP, blood pressure; DSPN, distal symmetric polyneuropathy; HbA1c, glycosylated hemoglobin A1c; NDS, Neuropathy Disability Score; NSS, Neuropathy Symptom Score; T2DM, type 2 diabetes mellitus; VPT, vibration threshold test; WHR, waist‐to‐hip ratio.
Figure 1.
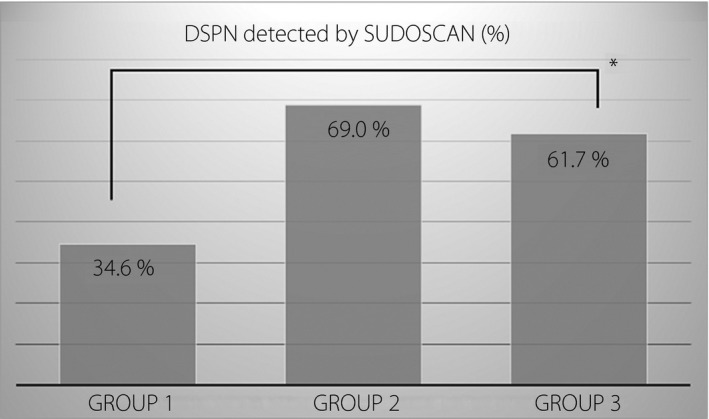
Distal symmetric polyneuropathy (DSPN) detected by Sudoscan in three groups divided according to symptoms and signs of lower limbs as described previously. Group 1, no diabetic neuropathy group; Group 2, asymptomatic neuropathy group; Group 3, symptomatic neuropathy group. *P < 0.01
An association was detected between NSS and NDS with both hands and feet ESC values, respectively. Increasing scores among the different evaluation scales were associated with decreasing ESC. Lower ESC was significantly associated with increasing symptoms by using the NSS score (r = −0.124, P < 0.05), increasing score on physical examination by using the NDS score (r = −0.3, P < 0.05) and increasing vibration threshold test value (r = −0.18, P < 0.05; Table 2). The association between traditional tests with feet ESC was higher in the subgroup of newly diagnosed DSPN patients than those who had already been diagnosed and treated with neurotrophic drugs. Lower ESC was associated with NSS score (r = −0.181, P < 0.01) and NDS score (r = −0.324, P < 0.01).
Table 2.
Spearman's correlations of hands and feet electrochemical skin conductance values with Neuropathy Symptom Score, Neuropathy Disability Score and vibration threshold test value in 357 Chinese type 2 diabetes mellitus patients
| Traditional Tests for DSPN | ESC value | Spearman's rho | P‐value |
|---|---|---|---|
| NSS score | Feet | −0.12a | 0.015 |
| Hands | −0.11 | 0.290 | |
| NDS score | Feet | −0.30a | <0.001 |
| Hands | −0.29a | <0.001 | |
| VPT value | Feet | −0.18a | <0.001 |
| Hands | −0.23a | <0.001 |
Significant difference of Spearman's rho between Neuropathy Symptom Score (NSS), Neuropathy Disability Score (NDS), vibration threshold test (VPT) value and electrochemical skin conductance (ESC) value (P < 0.05). DSPN, distal symmetric polyneuropathy.
On logistic regression analysis, we found that feet ESC remained independently associated with DSPN diagnosed by NSS/NDS score (odds ratio 0.98, 95% confidence interval 0.962–0.993), as well as age (odds ratio 1.042, 95% confidence interval 1.014–1.071). However, duration of diabetes, glycosylated hemoglobin A1c level, body mass index, waist‐to‐hip ratio and other factors were not independently associated with DSPN (Table 3).
Table 3.
Logistic analysis of clinical factors with the diagnosis of distal symmetric polyneuropathy by Neuropathy Symptom Score/Neuropathy Disability Score
| B‐value | OR ratio | CI | P‐value | |
|---|---|---|---|---|
| Age (years) | 0.04 | 1.04 | 1.01–1.07 | 0.003a |
| Duration of diabetes (years) | 0.02 | 1.02 | 0.98–1.07 | 0.173 |
| BMI (kg/m2) | −0.07 | 0.93 | 0.83–1.05 | 0.254 |
| Systolic BP (mmHg) | 0.00 | 0.99 | 0.97–1.03 | 0.960 |
| HbA1c (%) | 0.13 | 1.14 | 0.98–1.01 | 0.091 |
| Feet ESC (μS) | −0.023 | 0.98 | 0.962–0.993 | 0.004a |
| Hands ESC (μS) | −0.011 | 0.99 | 0.97–1.01 | 0.35 |
Significant difference of logistic analysis of clinical factors (P < 0.05). BMI, body mass index; BP, blood pressure; DSPN, distal symmetric polyneuropathy; HbA1c, glycosylated hemoglobin A1c; WHR, waist‐to‐hip ratio.
All the participants accepted Sudoscan without any complaint of discomfort, and no safety event was ever reported.
Discussion
The early detection of DSPN in clinical work has been a difficult problem, especially for those patients without obvious clinical symptoms21. Traditional screening tools for DSPN, such as the tuning fork and the 10‐g monofilament, are subjective and require the patient's full attention and cooperation, but are not quantitative to measure22, 23. Although a definite neuropathy diagnosis is based on confirmatory tests exemplified by the NCS, NCS such as electromyography aim to reflect the impairment of large myelinated fibers. The results are often normal in patients with early or small fiber predominant neuropathy21, 24. As we know, clinical tests of diabetic neuropathy are mostly based on testing of different peripheral nerves, usually involving the large type A alpha and beta myelinated nerve fibers. However, the unmyelinated, thin type C fibers of the sympathetic nervous system are usually neglected because of the limited evaluation methods. Sweat gland function controlled by sympathetic C fibers might be affected in the early pathological process of diabetes or prediabetes, as has been shown in patients with early diabetes by the use of different methods, including skin biopsies4, 25.
Sudoscan, reflecting the function of sympathetic C fibers, does not require patient preparation or full attention, and can be easily carried out by any non‐medical person in the outpatient clinic, and thus predicts early detection of DSPN. It has proven in several previous studies during the past 2–3 years to be much easier and more rapid to carry out, and is non‐invasive and well tolerated. However, there were no related studies of Sudoscan in screening Chinese type 2 diabetes mellitus patients without typical symptoms of DSPN15, 25, so the present study focused on those asymptomatic type 2 diabetes mellitus patients, possibly resulting in a delay in treatment due to their negligible manifestations.
In the present study, we investigated the results of the Sudoscan test in type 2 diabetes mellitus patients without any clinical manifestation of the lower limbs, and discovered a relatively higher detection rate by SUDOSCAN in asymptomatic DSPN patients, indicating that SUDOSCAN might be more sensitive in screening DSPN in asymptomatic DSPN patients. We found in the present study that lower ESC was associated with symptoms of peripheral neuropathy and conventional clinical tests. We further divided patients into subgroups according to their previous use of neurotrophic drugs, and showed a lower association between traditional tests with feet ESC in those who had previously been diagnosed with DPN and treated with neurotrophic drugs, such as Methycobal® and methylcobalamin, compared with type 2 diabetes mellitus patients who have never been diagnosed with DPN before. This finding might as a result of the recovery of small fibers after the use of neurotrophic medicine. Freedman17 studied the relationship between ESC and kidney disease in African and European American type 2 diabetes patients, and revealed different results due to racial difference, showing that ethnic difference might be one of the impact factors of ESC values. Previous studies mainly focused on the comparison of Sudoscan and other detecting methods, and showed a significant correlation between ESC value and other detecting methods including Michigan Neuropathy Screening Instrument score, vibration threshold test and electromyography results in Caucasian and African populations18, 19. Yet previous studies did not provide a perspective into the screening of Sudoscan in asymptomatic diabetic neuropathy patients, which proved to be equally important and easily neglected. Therefore, the present study is considered to be a pilot study in Chinese type 2 diabetes mellitus patients.
A number of limitations of the present study need to be clarified. Unlike previous published studies, which included a certain population of diabetic patients with established DSPN by diagnostic methods, such as skin biopsy, the present study did not provide a confirmed diagnosis of these type 2 diabetes mellitus patients. We used NSS/NDS score as the gold standard of DSPN, and further divided the whole group into three categories based on the cut‐off point in NSS and NDS scores. Also, the sample size of the present study was not large enough to provide the clinical risk factors of DSPN as described in previous studies. To further elucidate the diagnostic value of Sudoscan in screening asymptomatic diabetic DSPN patients, we need to establish a more confirmatory method as the gold standard instead, as well as expand the sample size.
The results suggested that the assessment of sudomotor function using Sudoscan through detection of impaired sudomotor function might provide a screening method for the detection of asymptomatic diabetic DSPN in Chinese type 2 diabetes mellitus patients.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation of China (81370884, to B Lu), the Shanghai New Excellent Youth Program (XYQ2013120, to B Lu), Fudan Zhuoxue Project (to B Lu), Pudong program from Pudong Municipal Commission of Health and Family Planning (PW2014D‐2, to B Lu), and Shanghai Science and Technology Committee Program (14411962200, to YM Li).
J Diabetes Investig 2017; 8: 363–368
Contributor Information
Bin Lu, Email: binlu@fudan.edu.cn.
Yiming Li, Email: yimingli@fudan.edu.cn.
References
- 1. Bruce A, Perkins F. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001; 24: 250–256. [DOI] [PubMed] [Google Scholar]
- 2. Andrew JM, Boulton M, Frcp. Diabetic neuropathies a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. [DOI] [PubMed] [Google Scholar]
- 3. Tesfaye S, Boulton AJM, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rage M, Van Acker N, Knaapen MW, et al Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser‐evoked potentials. J Neurol 2011; 258: 1852–1864. [DOI] [PubMed] [Google Scholar]
- 5. Apelqvist J, Bakker K, Van Houtum WH, et al Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev 2008; 24(Suppl 1): S181–S187. [DOI] [PubMed] [Google Scholar]
- 6. Garrow AP, Boulton AJ. Vibration perception threshold–a valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab Res Rev 2006; 22: 411–419. [DOI] [PubMed] [Google Scholar]
- 7. Hou Y, Liu S, Zhu T, et al Vibration perception threshold in diagnosing diabetic peripheral neuropathy by receiver operating characteristic curve. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2012; 37: 951–956. [DOI] [PubMed] [Google Scholar]
- 8. Pedro Jimenez‐Cohl MD. Thermal threshold research study on small fiber dysfunction in distal diabetic polyneuropathy. J Diabetes Sci Technol 2012; 6: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009; 50: 675–682, 682 e1. [DOI] [PubMed] [Google Scholar]
- 10. Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg 2006; 108: 477–481. [DOI] [PubMed] [Google Scholar]
- 11. Weintrob N, Amitay I, Lilos P, et al Bedside neuropathy disability score compared to quantitative sensory testing for measurement of diabetic neuropathy in children, adolescents, and young adults with type 1 diabetes. J Diabetes Complications 2007; 21: 13–19. [DOI] [PubMed] [Google Scholar]
- 12. Singleton JR. The Utah Early Neuropathy Scale a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst 2008; 13: 218–227. [DOI] [PubMed] [Google Scholar]
- 13. Bril V, Tomioka S, Buchanan RA, et al Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 2009; 26: 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herman WH, Pop‐Busui R, Braffett BH, et al Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012; 29: 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinik AI, Nevoret ML, Casellini C. The new age of sudomotor function testing: a sensitive and specific biomarker for diagnosis, estimation of severity, monitoring progression, and regression in response to intervention. Front Endocrinol (Lausanne) 2015; 6: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Z, Xu B, Lu J, et al Autonomic test by EZSCAN in the screening for prediabetes and diabetes. PLoS One 2013; 8: e56480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freedman BI, Bowden DW, Smith SC, et al Relationships between electrochemical skin conductance and kidney disease in Type 2 diabetes. J Diabetes Complications 2014; 28: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith AG, Lessard M, Reyna S, et al The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications 2014; 28: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yajnik CS, Kantikar VV, Pande AJ, et al Quick and simple evaluation of sudomotor function for screening of diabetic neuropathy. ISRN Endocrinol 2012; 2012: 103714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiong Q, Lu B, Ye H, et al The Diagnostic Value of Neuropathy Symptom and Change Score, Neuropathy Impairment Score and Michigan Neuropathy Screening Instrument for Diabetic Peripheral Neuropathy. Eur Neurol 2015; 74: 323–327. [DOI] [PubMed] [Google Scholar]
- 21. Quattrini C, Jeziorska M, Malik RA. Small fiber neuropathy in diabetes: clinical consequence and assessment. Int J Low Extrem Wounds 2004; 3: 16–21. [DOI] [PubMed] [Google Scholar]
- 22. Perkins BA, Olaleye D, Zinman B, et al. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001; 24: 250–256. [DOI] [PubMed] [Google Scholar]
- 23. Papanas N, Ziegler D. New vistas in the diagnosis of diabetic polyneuropathy. Endocrine 2014; 47: 690–698. [DOI] [PubMed] [Google Scholar]
- 24. Gronseth JDEGS. Distal symmetric polyneuropathy a definition for clinical research report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabil. Neurology 2005; 64: 199–207. [DOI] [PubMed] [Google Scholar]
- 25. Calvet JH, Dupin J, Winiecki H, et al Assessment of small fiber neuropathy through a quick, simple and non invasive method in a German diabetes outpatient clinic. Exp Clin Endocrinol Diabetes 2013; 121: 80–83. [DOI] [PubMed] [Google Scholar]


