Abstract
Background
Locoregional failure is a major problem associated with chemoradiation treatment for squamous cell esophageal carcinoma. The aim of this study was to assess the feasibility, efficacy, and toxicity of preoperative radiation (dose > 50 Gy) with platinum‐based chemotherapy followed by esophagectomy in locally advanced squamous cell carcinoma.
Methods
Data of patients with cT2‐cT4 or node positive squamous cell carcinoma of the esophagus who received trimodality treatment between February 2006 and June 2015 were reviewed.
Results
Forty‐four patients were treated with intensity‐modulated radiation therapy, volumetric‐modulated arc therapy or three‐dimensional radiation therapy. The median radiation dose was 60 Gy. The average volume of the lungs receiving 10 Gy was 48.1%, 20 Gy was 24.5%, and the average mean lung dose was 14 Gy. After chemoradiation, R0 resection was achieved in 31 patients (71%). Patients who received >60 Gy had a higher pathologic complete remission rate than those in the lower dose group (59.1% vs. 36.4%). R0 resection and radiation dose >60 Gy were associated with better overall survival in Cox proportional hazards regression analysis. The median follow‐up duration was 22.4 months and median survival was 25.6 months. Two‐year overall, progression‐free survival and locoregional control rates were 55.9%, 28.6%, and 56%, respectively. The most common grade 3–4 toxicities were esophagitis (63.6%) and neutropenia (25%). Grade 3–4 postoperative morbidities included surgical wound infection (2.3%), acute renal failure (2.3%), and anastomosis stricture (2.3%).
Conclusion
Trimodality treatment with a high preoperative radiation dose and chemotherapy yielded a good pathologic complete response rate, and long‐term survival with low toxicities.
Keywords: Chemoradiation, esophageal cancer, high‐dose radiation therapy, intensity‐modulated radiation therapy, trimodality treatment
Introduction
At present, surgery continues to be the major component of curative treatment for patients with operable thoracic esophageal cancer.1 Patients who undergo surgery alone have a median survival rate of 13.6–15.2 months, a two‐year survival rate of 34–37%,2, 3 and a 25–42% rate of locoregional failure.4, 5 The use of definitive concurrent chemoradiation has a median survival time of 12.5–22 months6, 7, 8, 9 and five‐year overall survival (OS) of 26%, but 46% of the patients experienced failure within the radiation (RT) field or had persistent disease.10
A recent meta‐analysis showed that preoperative chemoradiation followed by surgery, that is, trimodality treatment, significantly improved the rates of pathological complete response (pCR), R0 resection, and OS in patients with esophageal squamous cell carcinoma over surgery alone.11 However, compared with definitive concurrent chemoradiation in randomized controlled trials, trimodality treatment did not improve OS.12, 13 Of note is that these two trials used higher than standard RT doses in the control arm. The current standard RT dose is 40–50 Gy given in 15–25 fractions over three to five weeks.10, 14, 15 These results changed the use of definitive concurrent chemoradiation, which is reserved for cervical esophageal cancer, patients who decline surgery, and inoperable or unresectable tumors.
Over the past 15 years, RT delivery technology methods have improved. A few radiation dosimetric studies have explored the use of the new technology, intensity modulated radiation therapy (IMRT), which can maintain the therapeutic ratio by giving a high RT dose to the tumor while minimizing the dose to the surrounding organs.16, 17, 18 However, these results need to be confirmed in a real clinical setting. Studies have reported fewer postoperative morbidities and higher OS benefit using IMRT.19, 20, 21, 22 Data regarding high dose chemoradiation using IMRT followed by surgery is sparse. This retrospective study aimed to determine the pathological response rate, OS, progression‐free survival (PFS), locoregional control (LRC), and toxicities of high dose preoperative RT concurrently with chemotherapy and followed by esophagectomy among patients with esophageal squamous cell carcinoma. Potential prognostic factors associated with OS, particularly doses of less than or more than 60 Gy, were also evaluated.
Methods
Patients
Before commencement, a multidisciplinary gastrointestinal tumor board discussed all treatment options. Patients with cT2–cT4 or node positive thoracic esophageal cancer who were initially deemed unresectable or marginally resectable and received a preoperative RT (dose > 50 Gy) with platinum‐based chemotherapy followed by esophagectomy, were retrospectively identified. Tumor staging was based on the American Joint Committee on Cancer 2010 seventh edition staging criteria. Contrast computed tomography (CT) of the chest and abdomen, esophagogastroduodenoscopy, bone scintigraphy, and pathological confirmation of squamous cell carcinoma were included in the pretreatment work‐up. Although endoscopic ultrasound and positron emission tomography/CT (PET/CT) are standard procedures recommended in the National Comprehensive Cancer Network guidelines, issues of reimbursement and complete esophageal obstruction in some patients made endoscopic ultrasound and PET/CT optional in our study. All patients underwent jejunostomy before commencing chemoradiation. The Chulalongkorn University Institutional Review Board approved the study (No 296/59). Informed patient consent was obtained.
Treatment
All patients received preoperative chemotherapy and RT for five to seven weeks. The majority of the patients received cisplatin 80 mg/m2 or carboplatin AUC 5 administered on days 1 and 28, and intravenous infusion of 5‐fluorouracil (5‐FU) 1000 mg/m2/day on days 1–4 and 28–31.
Radiation techniques included IMRT, volumetric modulated arc therapy (VMAT) or three‐dimensional radiation therapy (3DRT). Megavoltage photon energy of 6 MV or higher was used. All patients underwent a treatment planning intravenous contrast CT simulation. The CT images were then transferred to a commercial planning system for structural delineation. The gross tumor volumes (GTVs) included the primary tumor (GTV‐primary) and involved regional lymph node(s) greater than 1 cm in diameter or node(s) with a necrotic center (GTV‐LN). The clinical target volume of gross lymph node (CTV‐GLN) was equal to GTV‐LN. The 0.5 cm lateral margin and 4 cm longitudinal margin of the GTV‐primary along the esophagus were extended to create the CTV‐low risk (CTV LR‐primary). Superior and inferior longitudinal margins were decreased to 2 cm beyond the GTV‐primary to obtain the CTV‐high risk (CTV HR‐primary). Elective nodal CTVs (CTV LR‐elective LN) encompassed the paratracheal, para‐esophageal, and supraclavicular nodes for the upper esophageal lesion; para‐esophageal nodes for the mid esophageal lesion; and the para‐esophageal, gastrohepatic, and celiac nodes for the distal esophageal lesion. A uniform margin of 1 cm to these CTVs (CTV‐GLN, CTV LR‐primary, CTV HR‐primary, CTV LR‐elective LN) was used to create planning target volumes (PTVs). All PTVs were at least 0.7 cm away from the spinal cord and were initially treated with 50 Gy in 25 fractions. A shrinking field boost of 10–14 Gy in 5–7 fractions was then delivered to the PTV HR‐primary and the PTV‐GLN. Patients were treated five days per week and all fields were treated each day. Dose–volume constraints were used to plan RT: for the spinal cord, maximum dose <50 Gy was used; for the lungs, V20 (volume of the lungs received 20 Gy) < 25%, V10 < 40%, and the mean lung dose <18 Gy were used; for the liver V30 < 30%, and median heart dose <30 Gy were used. Every effort was made to reduce exposure to the lungs, heart, spinal cord, and liver. The aim of RT treatment planning was to deliver an RT dose of up to 64 Gy to the PTV HR‐primary and PTV‐GLN; however, if the dose to normal organs exceeded dose volume constraints, the total doses for both the PTV HR‐primary and PTV‐GLN were reduced until normal tissue dose volume constraints were met. The Eclipse treatment planning system (Eclipse version 7.2.34, Varian, Palo Alto, CA, USA) was used to design RT plans with lung homogeneity corrections. The treatment was delivered by Varian linear accelerator (Varian Medical Systems Inc., Palo Alto, CA, USA) with dynamic 80‐Leaf multileaf collimators. Electronic portal images and cone beam CT were obtained at the start of RT therapy and performed at least once a week thereafter.
Weekly complete blood count and blood chemistry work‐ups were performed during the course of the chemoradiation. Acute and long‐term toxicities were graded according to the Common Terminology Criteria for Adverse Events version 3.0.23
After completion of chemoradiation therapy, follow‐up visits for physical examinations were scheduled every four weeks during the first three months. A multidisciplinary tumor board evaluated tumor response and resectability using CT and esophagogastroduodenoscopy at three months after chemoradiation therapy. If a complete response was not observed and the primary tumor did not attach to vital structures, such as the trachea or major vessels, patients were then considered for salvage transthoracic esophagectomy. One pathologist reviewed all pathological specimens. Pathologic stage was defined according to the tumor node metastasis classification. Patients with surgical pathology of esophagectomy and lymph node specimens without any viable residual tumor cells were considered to have achieved a complete response. After surgery, patients were followed for five years, which included physical examination, complete blood count, and blood chemistry at three‐month intervals for three years, then every six months thereafter for up to five years. CT scans of the chest and abdomen were performed every six months or when clinically indicated.
Statistical analysis
The end points of this study were the pathological response rate, OS, PFS, LRC, and toxicities. OS, PFS, and LRC analyses were computed using the Kaplan–Meier method and compared using log–rank statistics. OS was defined as the time period between the initial RT and any cause of death. PFS was defined as the time period since the initial RT of esophageal cancer until disease recurrence, progression, distant metastasis, or death. LRC was defined as the interval between the initial RT and disease recurrence or progression in the tumor bed and/or regional lymph node. A univariate logistic regression model was used to examine the association between clinicopathological factors and OS, LRC, and PFS. Factors with a P value of less than 0.25 in the univariate analysis were entered into the Cox proportional hazards regression analysis. SPSS version 17.0 was used for statistical analysis. Because of the large number of factors tested in the univariate analyses and our small patient sample, we used an adjusted P value of 0.004 or less as the significant level to prevent the risk of false positive results. Otherwise, a P value of 0.05 or less was considered statistically significant.
Results
From February 2006 to June 2015, 44 patients with squamous cell carcinoma of the thoracic esophagus were treated with high‐dose chemoradiation followed by esophagectomy; 36 (81.8%) were men and the median age was 60 years. Before treatment, most patients had clinical stage III (70.5%) cancer. Baseline PET/CT was carried out in seven patients (16%). Detailed patient characteristics are shown in Table 1. Fifty percent of the patients were irradiated with more than 60 Gy. When dividing patients in to two groups (i.e. ≤60 Gy vs. >60 Gy), there were proportionally more N2, stage IIIB, and tumor length >8 cm in the ≤60 Gy group. This was explained by the method of RT planning in which the medical physicist had to decrease the total dose to this group of patients to maintain an optimal dose to the surrounding normal tissue. As demonstrated in Table 2, normal tissue doses were comparable between both groups. The average volume of bilateral lungs receiving more than 20 Gy (V20) was 24.5%, the mean lung dose was 14 Gy, and the average median heart dose was 29.2 Gy.
Table 1.
Patient characteristics at baseline
| Characteristics | Radiation dose ≤ 60 Gy | Radiation dose > 60Gy | All | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Gender | ||||||
| Male | 19 | 86.4 | 17 | 77.3 | 36 | 81.8 |
| Female | 3 | 13.6 | 5 | 22.7 | 8 | 18.2 |
| Clinical T stage | ||||||
| T2 | 1 | 4.5 | 1 | 4.5 | 2 | 4.5 |
| T3 | 18 | 81.8 | 18 | 81.8 | 36 | 81.8 |
| T4 | 3 | 13.6 | 3 | 13.6 | 6 | 13.6 |
| Clinical N stage | ||||||
| N0 | 8 | 36.4 | 5 | 22.7 | 13 | 29.5 |
| N1 | 6 | 27.3 | 13 | 59.1 | 19 | 43.2 |
| N2 | 7 | 31.8 | 2 | 9.1 | 9 | 20.5 |
| N3 | 1 | 4.5 | 2 | 9.1 | 3 | 6.8 |
| Clinical stage grouping | ||||||
| IIA | 2 | 9.1 | 1 | 4.5 | 3 | 6.8 |
| IIB | 7 | 31.8 | 4 | 18.2 | 10 | 22.7 |
| IIIA | 4 | 18.2 | 13 | 59.1 | 18 | 40.9 |
| IIIB | 7 | 31.8 | 1 | 4.5 | 8 | 18.2 |
| IIIC | 2 | 9.1 | 3 | 13.6 | 5 | 11.4 |
| ECOG performance status | 0.0 | |||||
| 0–1 | 21 | 95.5 | 22 | 100 | 43 | 97.7 |
| 2 | 1 | 4.5 | 0 | 0 | 1 | 4.5 |
| Grading | 0.0 | |||||
| Grade 1 | 6 | 27.3 | 7 | 31.8 | 13 | 29.5 |
| Grade 2 | 9 | 40.9 | 9 | 40.9 | 18 | 40.9 |
| Grade 3 | 1 | 4.5 | 3 | 13.6 | 4 | 9.1 |
| Unknown | 6 | 27.3 | 3 | 13.6 | 9 | 20.5 |
| Location | ||||||
| Upper thoracic esophagus | 1 | 4.5 | 1 | 4.5 | 2 | 4.5 |
| Mid thoracic esophagus | 10 | 45.5 | 11 | 50 | 21 | 47.7 |
| Lower thoracic esophagus | 11 | 50 | 10 | 45.5 | 21 | 47.7 |
| Length | ||||||
| ≤8 cm | 15 | 68.2 | 17 | 77.3 | 32 | 72.7 |
| >8 cm | 7 | 31.8 | 5 | 22.7 | 12 | 27.3 |
| Weight loss | ||||||
| <10% | 14 | 63.6 | 14 | 63.6 | 28 | 63.6 |
| ≥10% | 8 | 36.4 | 8 | 36.4 | 16 | 36.4 |
ECOG, Eastern Cooperative Oncology Group.
Table 2.
Treatment modalities administered to patients
| Treatment parameters | Radiation dose ≤ 60 Gy | Radiation dose > 60 Gy | All | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Chemotherapy regimens | ||||||
| Cisplatin/5FU | 11 | 50.0 | 16 | 72.7 | 27 | 61.4 |
| Carboplatin/5FU | 8 | 36.4 | 6 | 27.3 | 14 | 31.8 |
| Other | 3 | 13.6 | 0 | 0 | 3 | 6.8 |
| Radiation technique | ||||||
| 3D conformal | 1 | 4.5 | 1 | 4.5 | 2 | 4.5 |
| IMRT | 15 | 68.2 | 20 | 90.9 | 35 | 79.5 |
| VMAT | 6 | 27.3 | 1 | 4.5 | 7 | 15.9 |
| Average dose | ||||||
| Mean lung dose (Gy) | 13.8 | 14.2 | 14 | |||
| Lung V20 (%) | 24.4 | 24.6 | 24.5 | |||
| Lung V10 (%) | 49.9 | 46.3 | 48.1 | |||
| Median heart dose (Gy) | 28.1 | 30.2 | 29.2 | |||
| Resection margin | ||||||
| R0 resection | 15 | 68.2 | 16 | 72.7 | 31 | 70.5 |
| R1 resection | 3 | 13.6 | 3 | 13.6 | 6 | 13.6 |
| R2 resection | 4 | 18.2 | 3 | 13.6 | 7 | 15.9 |
| Pathologic staging | ||||||
| pCR | 8 | 36.4 | 13 | 59.1 | 21 | 47.7 |
| Non pCR | 14 | 63.6 | 9 | 40.9 | 23 | 52.3 |
5FU, 5‐fluorouracil; IMRT, intensity‐modulated radiotherapy; pCR, pathologic complete response; VMAT, volumetric arc therapy.
V20 volume of the lung receiving 20 Gy; V10 volume of the lung receiving 10 Gy.
Intensity modulated radiation therapy, VMAT, and 3DRT were performed in 35, seven, and two patients, respectively. All patients were scheduled for concomitant chemotherapy. Forty‐one of the 44 patients (93.2%) received concurrent cisplatin or carboplatin plus 5‐FU. Three patients received weekly carboplatin AUC 2 and paclitaxel (50 mg/m2). The median time from post‐RT to surgery was four months. Thirty‐one patients (70.5%) underwent R0 resection.
Pathological response, locoregional control, and survival
The median follow‐up duration for the entire cohort was 22.4 months (range 5–121). Twenty‐one patients (47.7%) achieved a pCR. Patients who received >60 Gy had higher pCR rates than those in the lower dose group (59.1% vs. 36.4%). Median OS was 25.6 months and two‐year OS was 55.9% (Fig 1). The two‐year OS for those who received RT >60 Gy and ≤60 Gy doses were 73% and 35%, respectively (P = 0.026) (Fig 2). Two‐year PFS was 28.6% (Fig 3). Median PFS was 16.2 months. Locoregional recurrence was observed in 19 patients (43.2%), which translated into an estimated two‐year LRC of 56%. Seventeen patients (38.6%) developed distant failure. The most common metastatic sites were the lungs 13.6%, non‐regional nodal metastases 13.6%, and the liver 4.5%. To date, 32 patients have died during the follow‐up period. Of these patients, 31 died as a result of cancer recurrence and one patient developed pneumonia and sepsis after esophagectomy.
Figure 1.
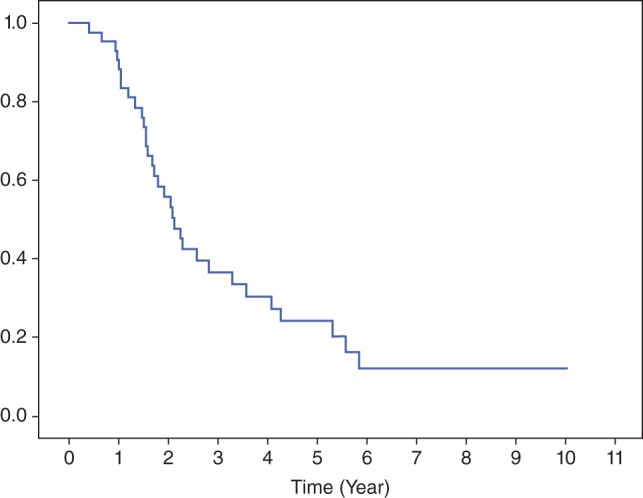
Overall survival curve.
Figure 2.
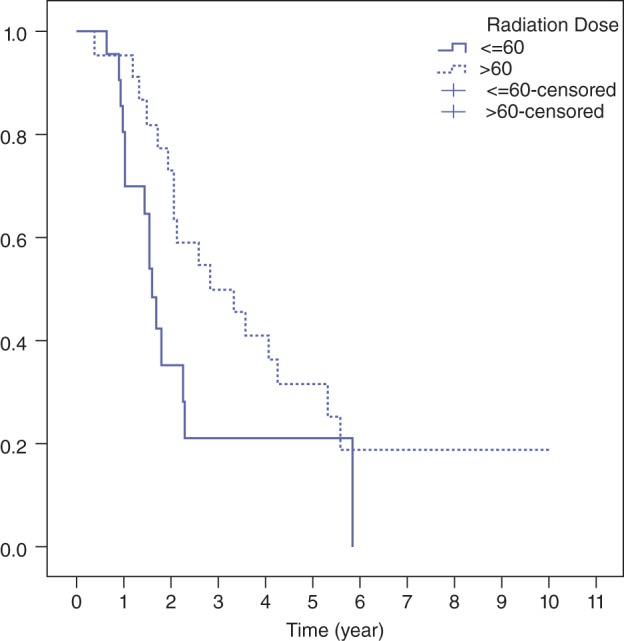
Overall survival curve stratified by radiation dose.
Figure 3.
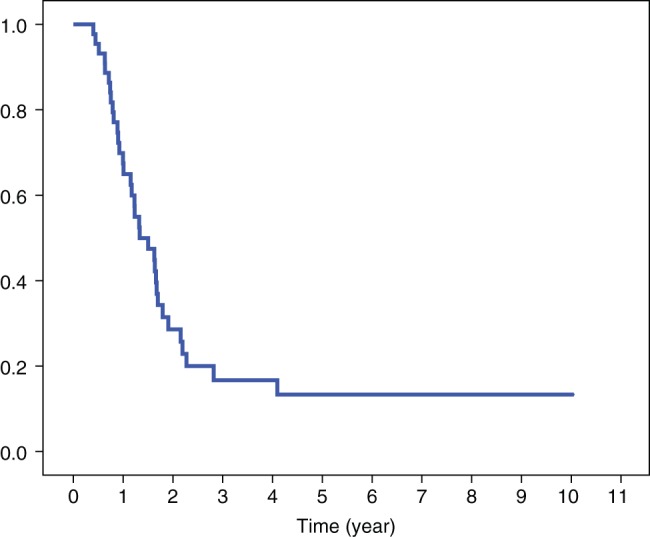
Progression‐free survival curve.
Univariate analysis
Multiple variables including age, gender, clinical stage, grading, location, length of tumor, weight loss, PTV dose, resection margin status, and pathological staging were selected for univariate analysis. Only resection margin status was significantly associated with two‐year OS. The two‐year OS in patients who underwent R0 resection was 71% compared with 21% who had R1–2 resection (P = 0.002). Patients who achieved pCR had a favorable two‐year PFS (50% vs. 13%; P = 0.002) compared with the non pCR group. Details of the univariate analysis are shown in Table 3.
Table 3.
Univariate analysis of OS, LRC, and PFS
| N | Two‐year OS | SD | P | Two‐year LRC | SD | P | Two‐year PFS | SD | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 36 | 56 | 9 | 0.123 | 52 | 9 | 0.387 | 28 | 8 | 0.635 |
| Female | 8 | 57 | 19 | 75 | 15 | 31 | 18 | |||
| Age | ||||||||||
| <60 | 24 | 67 | 10 | 0.085 | 58 | 11 | 0.896 | 34 | 11 | 0.402 |
| ≥60 | 20 | 43 | 11 | 54 | 11 | 23 | 10 | |||
| Clinical T stage | ||||||||||
| T2/T3 | 38 | 51 | 9 | 0.334 | 57 | 8 | 0.975 | 28 | 8 | 0.900 |
| T4 | 6 | 67 | 20 | 50 | 20 | 33 | 19 | |||
| Clinical N stage | ||||||||||
| N0 | 13 | 63 | 15 | 0.693 | 75 | 13 | 0.161 | 57 | 15 | 0.118 |
| N+ | 31 | 53 | 9 | 49 | 9 | 18 | 7 | |||
| Clinical stage grouping | ||||||||||
| II | 13 | 54 | 15 | 0.754 | 49 | 15 | 0.413 | 40 | 15 | 0.608 |
| III | 31 | 56 | 9 | 60 | 9 | 25 | 8 | |||
| Grading | ||||||||||
| Grade 1 | 13 | 59 | 14 | 0.093 | 73 | 14 | 0.292 | 48 | 16 | 0.176 |
| Grade 2 | 18 | 50 | 13 | 45 | 12 | 13 | 9 | |||
| Grade 3 | 4 | 75 | 22 | 50 | 25 | 50 | 25 | |||
| Unknown | 9 | 56 | 17 | 56 | 17 | 22 | 14 | |||
| Location | ||||||||||
| Upper/mid | 23 | 61 | 10 | 0.902 | 52 | 11 | 0.581 | 34 | 10 | 0.697 |
| Lower | 21 | 51 | 12 | 62 | 12 | 24 | 10 | |||
| Length | ||||||||||
| ≤8 cm | 32 | 56 | 9 | 0.316 | 53 | 9 | 0.657 | 18 | 7 | 0.068 |
| >8 cm | 12 | 56 | 15 | 67 | 14 | 57 | 15 | |||
| Weight loss | ||||||||||
| <10% | 28 | 50 | 10 | 0.268 | 51 | 10 | 0.543 | 16 | 7 | 0.065 |
| ≥10% | 16 | 67 | 12 | 66 | 13 | 53 | 13 | |||
| Dose | ||||||||||
| >5000–6000 | 22 | 35 | 12 | 0.026 | 41 | 12 | 0.104 | 18 | 10 | 0.143 |
| >6000 | 22 | 73 | 10 | 68 | 10 | 36 | 10 | |||
| Resection margin | ||||||||||
| R0 | 31 | 71 | 9 | 0.002* | 61 | 9 | 0.277 | 36 | 9 | 0.021 |
| R1–R2 | 13 | 21 | 12 | 45 | 14 | 10 | 9 | |||
| Pathologic staging | ||||||||||
| pCR | 21 | 71 | 11 | 0.055 | 78 | 10 | 0.031 | 50 | 12 | 0.002* |
| non pCR | 23 | 44 | 10 | 39 | 10 | 13 | 7 | |||
Factors with P < 0.004 (adjusted P value for multiple comparison).
LRC, locoregional control; OS, overall survival; pCR, pathologic complete response; PFS, progression‐free survival; SD, standard deviation.
Multivariate analysis
Resection margin status and RT dose were significantly related to OS with a hazard ratio (HR) of 0.290 (95% confidence interval [CI] 0.135–0.624; P = 0.002) and 0.389 (95% CI 0.181–0.839; P = 0.016), respectively, in favor of R0 resection (Fig 4) and >60 Gy group (Fig 2). Only pCR was associated with improved LRC (HR 0.342, 95% CI 0.123–0.950; P = 0.04) and PFS (HR 0.324, 95% CI 0.152–0.689; P = 0.003).
Figure 4.
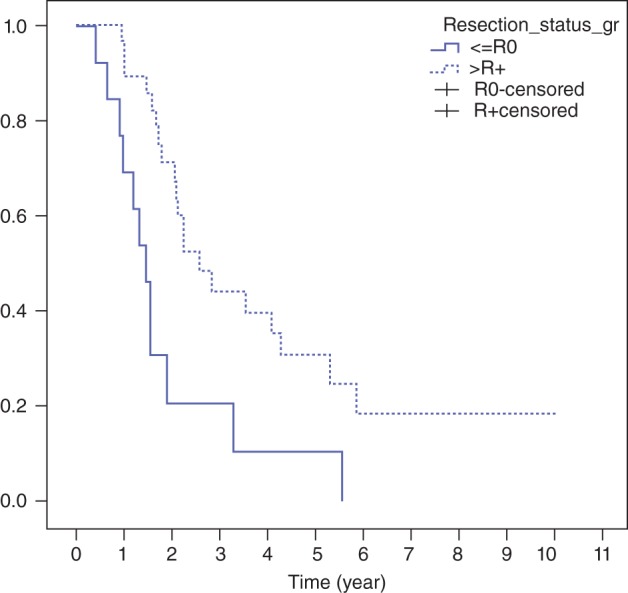
Overall survival stratified by resection margin.
Toxicities
Acute toxicities during chemoradiation are listed in Table 4. Grade 3–4 acute toxicities occurred in 29 patients (66%). The most common acute grade 3–4 toxicities were dysphagia (63.6%) and neutropenia (25%). Treatment‐related grade 1–2 weight loss was observed in 26 patients (59.1%).
Table 4.
Acute adverse events during chemoradiation
| Acute toxicities | Grade 1–2 | Grade 3–4 | ||
|---|---|---|---|---|
| N | % | N | % | |
| Anemia | 20 | 45.5 | 2 | 4.5 |
| Neutropenia | 12 | 27.3 | 11 | 25.0 |
| Thrombocytopenia | 17 | 38.6 | 3 | 6.8 |
| Dysphagia | 3 | 6.8 | 28 | 63.6 |
| Nausea | 5 | 11.4 | 1 | 2.3 |
| Vomiting | 8 | 18.2 | 0 | 0.0 |
| Cough | 4 | 9.1 | 0 | 0.0 |
| Creatinine rising | 0 | 0.0 | 0 | 0.0 |
| High fever | 0 | 0.0 | 1 | 2.3 |
| Diarrhea | 1 | 2.3 | 2 | 4.5 |
| Fatigue | 1 | 2.3 | 1 | 2.3 |
| Weight loss | 26 | 59.1 | 2 | 4.5 |
The incidence of surgical‐related toxicity was low and well tolerated. There was one postoperative death from pneumonia and sepsis. One patient had grade 4 acute renal failure. Two patients experienced wound infection or anastomotic stricture requiring surgical re‐intervention. Two patients experienced respiratory failure requiring ventilator support and subsequently recovered. Common grade 1–2 complications, such as cardiac arrhythmia (15.9%), high fever (15.9%), wound infection (11.4%), and anastomotic stricture (11.4%), were observed. Details of surgical‐related complications are shown in Table 5.
Table 5.
Post‐operative morbidity and mortality
| Surgically‐related complications | Grade 1–2 | Grade 3–5 | ||
|---|---|---|---|---|
| N | % | N | % | |
| Pneumonia | 4 | 9.1 | 1† | 2.3 |
| Wound infection | 5 | 11.4 | 1 | 2.3 |
| Anastomosis leakage | 3 | 6.8 | 0 | 0.0 |
| Anastomotic stricture | 5 | 11.4 | 1 | 2.3 |
| Cardiac arrhythmia | 7 | 15.9 | 0 | 0.0 |
| High fever | 7 | 15.9 | 1† | 2.3 |
| Pleural effusion | 3 | 6.8 | 0 | 0.0 |
| Acute renal failure | 3 | 6.8 | 1 | 2.3 |
| Chyle leak | 1 | 2.3 | 0 | 0.0 |
| Respiratory failure | 0 | 0 | 2 | 4.5 |
Same patient.
Discussion
Most patients with thoracic squamous cell carcinoma of the esophagus present in locally advanced stages, especially in Thailand. Patients are usually referred for either palliative RT or curative chemoradiation. For unresectable or potentially resectable cases, chemoradiation is the mainstay treatment. The major problem with chemoradiation treatment is the combination of persistent disease and locoregional failure (50%:50%).10 It has been postulated that locoregional recurrence can be overcome by using higher doses of RT. However, the INT 0123 trial showed that a high RT dose (64.8 Gy) plus concurrent 5‐FU and cisplatin showed no significant difference in median survival (13.0 vs. 18.1 months) or two‐year survival (31% vs. 40%) compared with the standard dose arm (50 Gy), in which more than half of the patients experienced locoregional failure and persistence of the disease post‐treatment.24
The second strategy, preoperative chemoradiation followed by esophagectomy versus chemoradiation alone, was examined in two randomized trials. The results revealed that although there was no significant difference in terms of OS, the trimodality treatment had less locoregional relapses in an FFCD 9102 study (34% vs. 43%)12 and improved two‐year local control in a German trial (64% vs. 41%), compared with chemoradiation alone.13 Of note, these two studies used doses higher than 50 Gy in the chemoradiation alone arm and used 3DRT.
On the other hand, the Stanford series demonstrated that preoperative standard dose IMRT (50.4 Gy) followed by surgery in 12 patients had a two‐year LRC of 83% and pCR of 18%.22 In another study, 232 esophageal cancer patients from a cohort were treated with IMRT or 3DRT concurrently with chemotherapy, and 47% of patients underwent esophagectomy. In multivariable analysis, surgical resection resulted in improved OS (HR 0.444; P < 0.0001), while IMRT was associated with a decrease in grade 3 or higher toxicity.25 On the contrary, 108 patients treated with preoperative (46%) or definitive IMRT concurrently with chemotherapy yielded a comparable two‐year OS using the trimodality technique (63.7%) versus chemoradiation (52.3%) alone (P = 0.2059).26 Because of these conflicting results, we wondered if combining the two postulates for overcoming locoregional failure and recurrence would work if a new RT methodology, such as IMRT or VMAT, was used. We carefully considered the risk versus benefit of integrating both postulates based on available data at the time of the study design. The aim was to achieve a good tumor response while lowering RT toxicities to nearby organs such as the lungs and heart enough so that, in cases of less than clinical complete remission, surgeons could perform salvage transthoracic esophagectomy and still achieve a lower surgical‐related morbidity and mortality rate.
Our results showed a two‐year OS of 55.9%, which was higher than in the FFCD 9102 (34%) and German trials’ (39.9%) trimodality arms. The median survival in this study (25.6 months) was also higher than the data reported by the FFCD 9102 (17.7 months) and the German trial (16.4 months). Our two‐year LRC was 56%, compared with 64–66% reported in the aforementioned trials, and the 46–56% locoregional failure rate in patients treated with definitive chemoradiation in the RTOG 8501 and INT0123 trials.10, 12, 13, 24 Moreover, the pCR rate in our study was 48%, which was far better than the 22–40% reported by other studies that used an RT dose of <50 Gy.13, 15, 26, 27, 28 Furthermore, our results revealed that patients who received >60 Gy achieved pCR as high as 59%. pCR had significantly better LRC and PFS in multivariate analysis. This may be because of the use of a higher RT dose, longer intervals (>3 months) between completion of neoadjuvant chemoradiation and surgery, and more favorable prognostic factors in the >60 Gy group. We also demonstrated in multivariate analysis that patients who underwent R0 resection and received an RT dose of >60 Gy had favorable OS outcomes.
Although there has been no randomized trial to support the dose‐response relationship in esophageal cancer, one systematic review showed that the probability of pCR improved with increasing doses of RT.29 The use of IMRT or VMAT lowered the lung dose and achieved the study's pre‐established dose volume constraints of the lungs (V20 = 24.5%, mean lung dose = 14 Gy) and the heart (median dose 29.2 Gy), without exceeding the tolerance levels. This result is comparable to other IMRT or VMAT studies which demonstrated that the lung V20 was between 21% and 25% and the mean heart dose was 21–22 Gy.18, 22, 30 We did not find any difference in terms of survival outcome for patients who received 3DRT versus IMRT/VMAT. However, we recommend that when giving higher than standard RT dose to the PTV while keeping the optimal dose to the surrounding tissue, it is easier to achieve an optimal RT plan using IMRT/VMAT than 3DRT.
Moderate grade toxicities were observed in this study. Grade 3–4 acute toxicities occurred in 66% of the patients, higher than 25–40% found in standard dose IMRT studies.22, 25 The most common acute grade 3–4 toxicities were dysphagia (63.6%) and neutropenia (25%). The grade 3–4 dysphagia experienced by our sample was higher than the 42% previously report ed.13 The higher grade 3–4 dysphagia may be a result of the higher RT dose used in our study. However, all patients in our study had prophylactic jejunostomy to prevent malnutrition. Only 4.5% had grade 3–4 weight loss. The rate of grade 3–4 neutropenia during chemoradiation in this study was comparable or lower than 36% when compared with other reports.14, 31, 32 For example, Roeder et al. treated 27 patients with 56 Gy of IMRT, and 26% of the patients experienced grade 3–4 leukopenia.32 This suggests that the use of high dose IMRT did not significantly alter hematotoxicity, even though IMRT had more monitor units compared with 3DRT. As for the other treatment‐related grade 3 weight loss, only 4.5% was observed in this study, which was lower than the 11% previously reported by Tepper et al. 14 This discrepancy may have been a result of the use of prophylactic jejunostomy in this study for every patient before commencing treatment.
Other complications, such as surgical complications, were tolerable. The in‐hospital postoperative mortality rate in our study corroborates data previously reported by other trimodality treatment studies to be 4–11%.13, 15, 19, 27, 33 One patient in our study died post‐operatively because of pneumonia and sepsis five months after commencing RT. This patient underwent R1 resection and the pathological report showed ypT2N0. He received 54 Gy RT preoperatively, lung V20 = 28%, lung V10 = 55%, and mean lung dose of 16 Gy, which did not differ from other patients. In the two patients who suffered from grade 4 respiratory failure, one received an RT dose of 64 Gy, lung V20 = 32%, lung V10 = 59%, and mean lung dose of 16 Gy, while the other received an RT dose of 60 Gy, lung V20 = 13%, lung V10 = 50%, and mean lung dose of 12 Gy. A possible explanation for these two respiratory failures may have been the preoperative forced expiratory volume in one second, which has been shown to be independently associated with postoperative pulmonary complications in patients receiving preoperative IMRT, 40–45 Gy, and concurrent chemotherapy followed by thoracic esophagectomy.19 However, pulmonary function data of these two patients were within normal limits.
Some of the limitations of this study are its retrospective nature, relatively small patient sample, short follow‐up, and selection bias toward more favorable patients in the >60 Gy group. Cross‐study comparison is, however, limited by the difference in patient characteristics and end point definitions. The risk versus benefit of conducting the study in locally advanced esophageal cancer patients was carefully considered. IMRT provided a higher tumor dose and lower lung dose, which resulted in a higher response rate, improved resection margins, and a low postoperative mortality rate.
In conclusion, our study indicated the efficacy, safety, and feasibility of high dose RT with chemotherapy followed by transthoracic esophagectomy in locally advanced esophageal cancer patients; however, further investigation in a larger prospective randomized controlled trial is required.
Disclosure
No authors report any conflict of interest.
Acknowledgment
The authors would like to thank Ms. Buntipa Netsawang, Ms. Sranya Phaisawang, and Ms. June Ohata for their statistical analysis, preparation, and editing of the manuscript.
References
- 1. Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol 2003; 4: 481–8. [DOI] [PubMed] [Google Scholar]
- 2. Fok M, Sham JS, Choy D, Cheng SW, Wong J. Postoperative radiotherapy for carcinoma of the esophagus: A prospective, randomized controlled study. Surgery 1993; 113: 138–47. [PubMed] [Google Scholar]
- 3. Medical Research Council Oesophageal Cancer Working Group . Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002; 359: 1727–33. [DOI] [PubMed] [Google Scholar]
- 4. Law SY, Fok M, Wong J. Pattern of recurrence after oesophageal resection for cancer: Clinical implications. Br J Surg 1996; 83: 107–11. [DOI] [PubMed] [Google Scholar]
- 5. Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001; 19: 305–13. [DOI] [PubMed] [Google Scholar]
- 6. Coia LR, Engstrom PF, Paul AR, Stafford PM, Hanks GE. Long‐term results of infusional 5‐FU, mitomycin‐C and radiation as primary management of esophageal carcinoma. Int J Radiat Oncol Biol Phys 1991; 20: 29–36. [DOI] [PubMed] [Google Scholar]
- 7. Leichman L, Herskovic A, Leichman CG et al. Nonoperative therapy for squamous‐cell cancer of the esophagus. J Clin Oncol 1987; 5: 365–70. [DOI] [PubMed] [Google Scholar]
- 8. Seitz JF, Giovannini M, Padaut‐Cesana J et al. Inoperable nonmetastatic squamous cell carcinoma of the esophagus managed by concomitant chemotherapy (5‐fluorouracil and cisplatin) and radiation therapy. Cancer 1990; 66: 214–9. [DOI] [PubMed] [Google Scholar]
- 9. Herskovic A, Martz K, Al‐Sarraf M et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992; 326: 1593–8. [DOI] [PubMed] [Google Scholar]
- 10. Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation Therapy Oncology Group. JAMA 1999; 281: 1623–7. [DOI] [PubMed] [Google Scholar]
- 11. Sjoquist KM, Burmeister BH, Smithers BM et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta‐analysis. Lancet Oncol 2011; 12: 681–92. [DOI] [PubMed] [Google Scholar]
- 12. Bedenne L, Michel P, Bouché O et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25: 1160–8. [DOI] [PubMed] [Google Scholar]
- 13. Stahl M, Stuschke M, Lehmann N et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005; 23: 2310–7. [DOI] [PubMed] [Google Scholar]
- 14. Tepper J, Krasna MJ, Niedzwiecki D et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26: 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 16. Nutting CM, Bedford JL, Cosgrove VP, Tait DM, Dearnaley DP, Webb S. A comparison of conformal and intensity‐modulated techniques for oesophageal radiotherapy. Radiother Oncol 2001; 61: 157–63. [DOI] [PubMed] [Google Scholar]
- 17. Chandra A, Guerrero TM, Liu HH et al. Feasibility of using intensity‐modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005; 77: 247–53. [DOI] [PubMed] [Google Scholar]
- 18. Wu VW, Sham JS, Kwong DL. Inverse planning in three‐dimensional conformal and intensity‐modulated radiotherapy of mid‐thoracic oesophageal cancer. Br J Radiol 2004; 77: 568–72. [DOI] [PubMed] [Google Scholar]
- 19. Hsu FM, Lee YC, Lee JM et al. Association of clinical and dosimetric factors with postoperative pulmonary complications in esophageal cancer patients receiving intensity‐modulated radiation therapy and concurrent chemotherapy followed by thoracic esophagectomy. Ann Surg Oncol 2009; 16: 1669–77. [DOI] [PubMed] [Google Scholar]
- 20. Lee HK, Vaporciyan AA, Cox JD et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: Correlation with pulmonary dose‐volume histogram parameters. Int J Radiat Oncol Biol Phys 2003; 57: 1317–22. [DOI] [PubMed] [Google Scholar]
- 21. Lin SH, Wang L, Myles B et al. Propensity score‐based comparison of long‐term outcomes with 3‐dimensional conformal radiotherapy vs intensity‐modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012; 84: 1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. La TH, Minn AY, Su Z et al. Multimodality treatment with intensity modulated radiation therapy for esophageal cancer. Dis Esophagus 2010; 23: 300–8. [DOI] [PubMed] [Google Scholar]
- 23. National Cancer Institute. Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events Version 3.0. 2003. [Cited 24 Feb 2017.] Available from URL: http://ctep.cancer.gov.
- 24. Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002; 20: 1167–74. [DOI] [PubMed] [Google Scholar]
- 25. Freilich J, Hoffe SE, Almhanna K et al. Comparative outcomes for three‐dimensional conformal versus intensity‐modulated radiation therapy for esophageal cancer. Dis Esophagus 2015; 28: 352–7. [DOI] [PubMed] [Google Scholar]
- 26. Shridhar R, Chuong M, Weber J et al. Outcomes of definitive or preoperative IMRT chemoradiation for esophageal cancer. J Radiat Oncol 2012; 1: 347–54. [Google Scholar]
- 27. Mariette C, Dahan L, Mornex F et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014; 32: 2416–22. [DOI] [PubMed] [Google Scholar]
- 28. Ajani JA, Correa AM, Hofstetter WL et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012; 23: 2638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geh JI, Bond SJ, Bentzen SM, Glynne‐Jones R. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: Evidence of a radiation and chemotherapy dose response. Radiother Oncol 2006; 78: 236–44. [DOI] [PubMed] [Google Scholar]
- 30. Yin L, Wu H, Gong J et al. Volumetric‐modulated arc therapy vs. c‐IMRT in esophageal cancer: A treatment planning comparison. World J Gastroenterol 2012; 18: 5266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anvari K, Aledavood SA, Toussi MS et al. A clinical trial of neoadjuvant concurrent chemoradiotherapy followed by resection for esophageal carcinoma. J Res Med Sci 2015; 20: 751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roeder F, Nicolay NH, Nguyen T et al. Intensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancer. Radiat Oncol 2014; 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tachimori Y, Kanamori N, Uemura N, Hokamura N, Igaki H, Kato H. Salvage esophagectomy after high‐dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009; 137: 49–54. [DOI] [PubMed] [Google Scholar]


