Abstract
Aims/Introduction
To assess the time to initiation of insulin therapy, and concurrently investigate both patient‐ and physician‐related factors associated with delaying insulin therapy in Korean patients with type 2 diabetes uncontrolled by oral hypoglycemic agents (OHAs).
Materials and Methods
This prospective, observational disease registry study was carried out across 69 centers in Korea. Type 2 diabetes patients who had received two or more OHAs within the past 5 years, had a glycated hemoglobin ≥8% in the past 6 months and had not received insulin were included. Data recorded on data collection forms during a 12‐month period were analyzed.
Results
Of 2168 patients enrolled, 1959 were evaluated and classified as the insulin‐initiated or insulin‐delayed group. Insulin was prescribed for just 20% of the patients during a 1‐year follow‐up period, and less than half (44.5%) of the patients who were taking two OHAs started insulin after 6 years. Patient‐related factors for delay in insulin initiation included older age, shorter duration of diabetes and lower glycated hemoglobin. Physician‐related factors included age (~50 to <60 years), sex (women) and number (<1000) of patients consulted per month. Patient refusal (33.6%) and physicians’ concerns of patient non‐compliance (26.5%) were the major physician‐reported reasons for delaying insulin therapy. Inconvenience of insulin therapy (51.6%) and fear of injection (48.2%) were the major reasons for patient refusal.
Conclusions
Insulin initiation is delayed in patients with type 2 diabetes uncontrolled by two or more OHAs in Korea. Patient‐ and physician‐related factors associated with this delay need to be addressed for better diabetes management.
Keywords: Insulin therapy, Physician and patient behaviors, Type 2 diabetes
Introduction
Globally, an estimated 382 million people are living with diabetes, and this number is expected to rise to 592 million by 20351. According to 2010 estimates, there are 3.3 million people with diabetes living in Korea, and this number is expected to rise to 4.3 million by 20302. The aging population, rapid economic development, increase in central obesity and a westernized lifestyle have led to the rise in diabetes prevalence2, 3, which in turn has resulted in a high burden in terms of morbidity, mortality and healthcare costs in Korea2, 4, 5. Diabetes is the fifth leading cause of death in Korea2, 4.
For the management of diabetes, numerous oral hypoglycemic agents (OHAs) are available, and recommendations for their use either as monotherapy or in combination are described in the diabetes management guidelines including the American Diabetes Association (ADA), and the joint ADA and European Association for the Study of Diabetes (EASD) guidelines6, and the Korean Diabetes Association guideline7. Although OHAs either as monotherapy or combination therapy are often initially successful in lowering the glycated hemoglobin (HbA1c) level, they are unable to restore normal HbA1c in the long term8, 9. Timely initiation of insulin therapy has been suggested in these patients, as it reduces diabetes‐related complications, improves the cardiovascular profile, lowers insulin resistance, reverses glucotoxicity and helps preserve β‐cell function for a longer duration than OHAs alone10, 11, 12.
Although evidence supports timely insulin initiation, significant concerns about weight gain, fear of injections, risk of hypoglycemia, patient adherence, pain associated with injections and self‐monitoring of blood glucose hinder its application in clinical practice13. Failure to initiate insulin therapy timely can result in poor glycemic control, reduced life expectancy and a compromised quality of life14, and is associated with a number of diabetes‐related complications including blindness, organ damage and loss of circulation to limbs resulting in amputation.
Despite known benefits of timely insulin initiation, insulin is not initiated at the appropriate time in a considerable proportion of patients who require insulin. The reasons for which such resistance to initiation of insulin therapy are predicated must be understood in order to explore the means of reversing the trend of delayed insulin initiation. Exploring patients’ concerns and beliefs regarding insulin therapy is crucial to formulating effective strategies for timely insulin initiation and assisting physicians in delivering patient‐centered care. Furthermore, there are barriers to initiating insulin originating from physicians. Thus, it is important to assess the magnitude of, and the reasons for, resistance to insulin initiation in real‐life healthcare settings in order to create the evidence base to formulate better diabetes care practices. The objectives of the current study were to investigate the time to onset of insulin therapy in Korean patients with type 2 diabetes, uncontrolled by two or more OHAs combination therapy (HbA1c ≥8.0%) and to elucidate specific factors that contribute to the delay of insulin initiation among Korean physicians and patients.
Materials and Methods
The present study was a multicenter, prospective, observational disease registry study carried out across 69 centers (53 general hospitals and 16 private hospitals) in Korea from September 2009 to October 2012. This study was carried out in accordance with the ethical principles laid down in the declaration of Helsinki and guidelines for Good Clinical Practice, and was approved by the ethics committees of each participating center. Written informed consent was obtained from each patient before initiation of the study.
Study patients and procedure
The present study included adults with type 2 diabetes aged >20 years who had started taking two or more OHAs within the past 5 years (the date of which could be validated through medical record review), had HbA1c ≥8% in the past 6 months and had not received insulin therapy on a regular basis. The eligibility criteria for the initiation of insulin therapy was based on the Korean Diabetes Association guideline for diabetes 2011 (www.diabetes.or.kr). Patients participating in another drug trial were excluded from the study.
The start of insulin therapy and change or discontinuation of existing therapy was based solely on the clinical judgment of the investigator. No study‐specific interventions were performed.
Data collection
Data were recorded on data collection forms for variables including patient demographics (i.e., age, sex and body mass index), mean HbA1c and fasting plasma glucose levels, duration of diabetes, duration of treatment with two or more OHAs, and history of diabetes‐related complications.
Data on each physician's age, sex, specialty, number of type 2 diabetes patients consulted per month, presence of nurses trained in diabetes education and the level of agreement with consensus guidelines were also collected on the data collection forms.
During the follow‐up period, dates of insulin prescription were collected through patients’ medical record review. The data were collected from the patients’ medical records at baseline, and at within 1 month of 3, 6, 9 and 12 months after baseline by the investigators in the case report form. For patients whose data at month 12 after baseline were not collected, the last recorded data were used as end‐of‐study data. At the final visit, both physicians and patients were asked why they did not start insulin through a questionnaire (Appendix S1).
Statistical analysis
Patients were classified into an insulin‐initiated group or an insulin‐delayed group. Statistical analysis was based on all patients who were included in the analysis set. All recorded data were descriptively analyzed. Continuous variable data are summarized as means ± standard deviations, median and range. Categorical variable data are presented as frequencies and percentages. Where necessary, 95% confidence intervals (CIs) are provided, and all statistical tests were carried out at a significance level of 5% using a two‐sided test. For primary analysis, survival time was defined as the time from start date of using two OHAs to the onset of insulin therapy in patients with type 2 diabetes uncontrolled by two or more agents. A survival curve was drawn using Kaplan–Meier estimates followed by median survival time and its 95% CI. The effects of patient and physician factors on whether to start insulin were evaluated using a multivariate logistic regression model. All statistical analyses were carried out using Sas version 9.1 (SAS Institute, Cary, North Carolina, USA).
Results
Patient classification
Of the 2168 enrolled patients, 209 patients were excluded from the study owing to incomplete data. Of the remaining 1959 patients, 386 (19.7%) initiated insulin during the 1‐year follow‐up period. Among the 1573 patients who did not start insulin during the 1‐year follow‐up period, 1205 (76.6%) were followed up until the study end (Figure S1).
Baseline characteristics of patients and physicians
Overall, the mean age of the patients was 57.0 ± 11.3 years, with no statistically significant (P = 0.0757) difference between groups. The mean duration of diabetes of the study population was 7.9 ± 6.4 years. HbA1c and fasting plasma glucose levels were significantly higher in the insulin‐initiated group than in the insulin‐delayed group (HbA1c: 9.7 ± 1.5 vs 8.8 ± 0.9%, P < 0.0001; fasting plasma glucose: 203.9 ± 75.8 vs 171.7 ± 49.0 mg/dL, P < 0.0001; Table 1).
Table 1.
Baseline and demographic characteristics
| Insulin‐initiated group (n = 386) | Insulin‐delayed group (n = 1573) | Total (n = 1959) | P‐value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (years) | 56.0 ± 12.0 | 57.3 ± 11.1 | 57.0 ± 11.3 | 0.0757 |
| BMI (kg/m2) | 25.3 ± 4.3 | 25.6 ± 3.5 | 25.7 ± 3.7 | 0.0154 |
| HbA1c (%) | 9.7 ± 1.5 | 8.8 ± 0.9 | 9.0 ± 1.1 | <0.0001 |
| FPG (mg/dL) | 203.9 ± 75.8 | 171.7 ± 49.0 | 178.1 ± 56.8 | <0.0001 |
| Duration of diabetes (years) | 8.5 ± 6.9 | 7.8 ± 6.2 | 7.9 ± 6.4 | 0.1191 |
| Microvascular complications, n (%) | 144 (37.3) | 513 (32.6) | 657 (33.5) | 0.0801 |
| Macrovascular complications, n (%) | 73 (18.9) | 259 (16.5) | 332 (17.0) | 0.2509 |
| Physician characteristics | ||||
| Age (years) | 43.5 ± 7.1 | 43.5 ± 7.4 | 43.5 ± 7.3 | 0.8489 |
| <40, n (%) | 123 (31.9) | 531 (33.8) | 654 (33.4) | <0.0001 |
| 40 to <50 | 204 (52.8) | 729 (46.3) | 933 (47.6) | |
| 50 to <60 | 45 (11.7) | 293 (18.6) | 338 (17.3) | |
| ≥60 | 14 (3.6) | 20 (1.3) | 34 (1.7) | |
| Male, n (%) | 296 (76.7) | 1022 (65.0) | 1318 (67.3) | <0.0001 |
| Specialty in medicine, n (%) | ||||
| Endocrinology | 346 (88.6) | 1468 (93.3) | 1814 (92.6) | 0.0131 |
| Internal medicine (others) | 40 (10.4) | 105 (6.7) | 145 (7.4) | |
| Patients consulted per month | 730.9 ± 336.3 | 757.6 ± 271.8 | 752.3 ± 285.8 | 0.1485 |
| <500, n (%) | 56 (14.5) | 152 (9.6) | 208 (10.6) | <0.0001 |
| 500 to <1000 | 258 (66.8) | 1286 (81.8) | 1544 (78.8) | |
| ≥1000 | 72 (18.7) | 135 (8.6) | 207 (10.6) | |
| No. beds in hospital | 780.2 ± 345.6 | 811.8 ± 410.0 | 806.1 ± 399.3 | 0.1480 |
| <500, n (%) | 30 (9.1) | 210 (14.1) | 240 (13.2) | 0.0072 |
| 500 to <1000 | 220 (66.9) | 1014 (67.8) | 1234 (67.6) | |
| ≥1000 | 79 (24.0) | 271 (18.1) | 350 (19.2) | |
| Work with diabetes education nurse, n (%) | ||||
| Yes | 277 (71.8) | 1381 (87.8) | 1658 (84.6) | <0.0001 |
| No | 109 (28.2) | 192 (12.2) | 301 (15.4) | |
| Support ADA/EASD guideline, n (%) | ||||
| Yes | 302 (78.2) | 1152 (73.2) | 1454 (74.2) | 0.0441 |
| No | 84 (21.8) | 421 (26.8) | 505 (25.8) | |
Unless specified otherwise, values are presented as means ± standard deviations.
ADA, American Diabetes Association; BMI, body mass index; EASD, European Association for the Study of Diabetes; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin.
The mean age of the physicians was 43.5 ± 7.3 years, with no statistically significant differences among the groups (P = 0.8489), and the majority of physicians were men (67.3%). There was a higher proportion of physicians in the 40–50 years age group in the insulin‐initiated group than in the insulin‐delayed group (52.8 vs 46.3%, P < 0.0001). Most physicians specialized in endocrinology (92.6%). Physicians working with diabetes education nurses were more common in the insulin‐delayed group than in the insulin‐initiated group (87.8 vs 71.8%, P < 0.0001). Physicians who supported ADA/EASD guidelines were more common in the insulin‐initiated group than in the insulin‐delayed group (78.2 vs 73.2%, P = 0.0441).
Time to onset of insulin therapy from the start of therapy with two OHAs
Figure 1 shows Kaplan–Meier estimates of time to onset of insulin therapy from the start date of two OHAs in patients with type 2 diabetes. Among the patients whose HbA1c was <9.0%, approximately 14 and 22% of patients initiated insulin at 45 and 60 months, respectively. Even among the patients whose HbA1c was >9.0%, approximately 34% initiated insulin therapy at 45 months, and 53% at 60 months. Overall, it took approximately 72 months for insulin therapy to be initiated in 44.5% of the patients.
Figure 1.
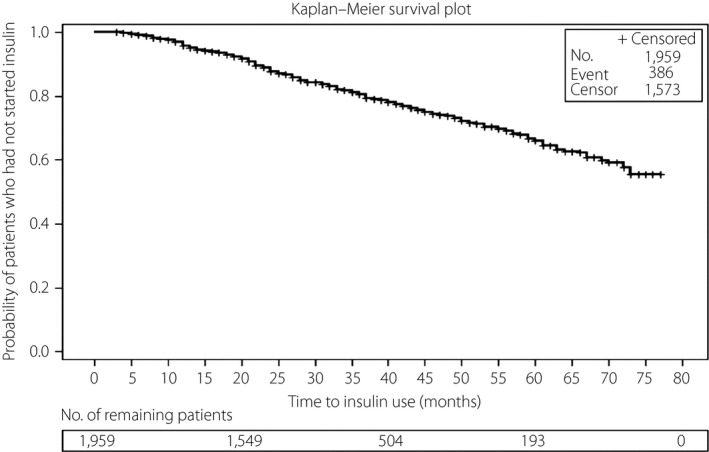
Time to onset of insulin therapy from the start date of combination therapy using two oral hypoglycemic agents.
Factors associated with initiation of insulin therapy
A multivariate logistic regression analysis was carried out to assess the factors associated with the initiation of insulin therapy. Patients aged younger than 40 years were almost 2.3‐fold more likely to have initiated insulin therapy than patients aged 60 years or older (odds ratio [OR] 2.274, 95% CI: 1.305–3.963). Initiation of insulin therapy was nearly 70.0% more likely in patients with HbA1c ≥9% than in patients with HbA1c <9% (OR 0.308, 95% CI: 0.235–0.404). A long duration of diabetes (≥10 years) was also associated with initiation of insulin therapy. A history of microvascular or macrovascular complications was not significantly associated with initiation of insulin therapy (Table 2).
Table 2.
Patient‐related factors associated with initiation of insulin therapy
| Patient characteristics | OR (95% CI)a |
|---|---|
| Age (years) | |
| <40 | 2.274 (1.305–3.963) |
| 40 to <50 | 1.511 (1.019–2.240) |
| 50 to <60 | 1.345 (0.971–1.864) |
| ≥60 | 1.00 |
| Sex, n (%) | |
| Male | 0.802 (0.610–1.055) |
| Female | 1.00 |
| BMI (kg/m2) | |
| <25 | 1.00 |
| ≥25 | 0.961 (0.731–1.263) |
| HbA1c (%) | |
| <9% | 0.308 (0.235–0.404) |
| ≥9% | 1.00 |
| Duration of diabetes (years) | |
| <5 | 0.777 (0.553–1.090) |
| 5 to <10 | 0.643 (0.456–0.907) |
| ≥10 | 1.00 |
| History of microvascular complication | |
| Yes | 1.00 |
| No | 1.124 (0.842–1.500) |
| History of macrovascular complication | |
| Yes | 1.00 |
| No | 0.744 (0.525–1.056) |
Multivariate logistic regression.
BMI, body mass index; CI, confidence interval; HbA1c, glycated hemoglobin; OR, odds ratio.
Physicians aged older than 60 years were 70% more likely to initiate insulin therapy than physicians aged 50–59 years (OR 0.290, 95% CI: 0.145–0.581), and younger physicians aged <50 years also were almost four‐ to sixfold more likely to initiate insulin therapy than physicians aged 50–59 years. Male physicians were 2.2‐fold more likely to initiate insulin therapy compared with their female counterparts (OR 2.232, 95% CI: 1.523–3.271). Furthermore, physicians consulting more than 1000 patients with diabetes per month were more likely to initiate insulin therapy (Table 3). However, physicians supporting the ADA/EASD guideline or not was not associated with the initiation of insulin therapy.
Table 3.
Physician‐related factors associated with initiation of insulin therapy
| Physician characteristics | OR (95% CI)a |
|---|---|
| Age (years) | |
| <40 | 1.854 (0.952–3.610) |
| 40 to <50 | 1.306 (0.722–2.363) |
| 50 to <60 | 0.290 (0.145–0.581) |
| ≥60 | 1.00 |
| Sex, n (%) | |
| Male | 2.232 (1.523–3.271) |
| Female | 1.00 |
| Specialty in medicine | |
| Endocrinology | 1.00 |
| Internal medicine, others | 1.594 (0.967–2.629) |
| No. diabetes patients per month | |
| <500 | 0.156 (0.082–0.295) |
| 500 to <1000 | 0.176 (0.110–0.282) |
| ≥1000 | 1.00 |
| Work with nurses trained in diabetes education, n (%) | |
| Yes | 1.00 |
| No | 1.403 (0.980–2.009) |
| Support ADA/EASD guideline, n (%) | |
| Yes | 1.00 |
| No | 0.996 (0.705–1.406) |
Multivariate logistic regression.
ADA, American Diabetes Association; CI, confidence interval; EASD, European Association for the Study of Diabetes; OR, odds ratio.
Reasons for not initiating insulin therapy
The major patient‐reported reasons for delaying insulin therapy included inconvenience (51.6%), fear of injection (48.2%) and concern about the pain of injections (14.0%). Nearly one in three patients reported that they considered the initiation of insulin therapy as the end of life (survey response: think ‘it is the end of life,’ 31.1%). Simultaneously, patient refusal (33.6%), concerns about patient compliance (26.5%) and concerns about hypoglycemia (14.2%) were the major physician‐reported reasons for delaying insulin therapy in patients with type 2 diabetes (Table 4).
Table 4.
Reasons associated with delaying insulin therapy
| Percentage of patients (%) | |
|---|---|
| Patient‐reported reasons (n = 415) | |
| Inconvenience | 51.6 |
| Fear of injection | 48.2 |
| Think ‘it is the end of life’ | 31.1 |
| Concern about injection pain | 14.0 |
| No confidence in glucose control | 7.2 |
| Concern about hypoglycemia | 2.9 |
| Concern about weight gain | 1.5 |
| Prefer oral drugs | 1.5 |
| Prefer diet and exercise | 1.2 |
| Inability to self‐inject | 1.0 |
| Physician‐reported reasons (n = 1220) | |
| Patient's refusal | 33.6 |
| Concern about patient's compliance | 26.5 |
| Concern about hypoglycemia | 14.2 |
| Controllable with OADs | 12.2 |
| Patient's lack of DM knowledge | 6.0 |
| Need to follow up before insulin use | 3.9 |
| Lost to follow up | 3.0 |
| Diet change is needed | 2.6 |
| Lack of time to explain | 2.3 |
| Concern about weight gain | 2.1 |
| Lack of efficacy | 1.6 |
| Comorbid condition | 1.2 |
| Physician's decision | 0.7 |
| Lack of patient's will | 0.6 |
Multiple choices were given. DM, diabetes mellitus; OAD, oral antidiabetic.
Discussion
To the best of our knowledge, this is the first study to assess the time to initiation of insulin therapy and concurrently investigate both patient‐ and physician‐related factors associated with delaying insulin therapy in patients who were uncontrolled by two OHAs in a prospective manner.
Current treatment guidelines by the ADA and the joint ADA/EASD advocate timely initiation of insulin in patients who do not achieve glycemic targets with two OHAs15. In the current study, approximately 25.0, 34.0 and 44.0% of patients initiated insulin after almost 3.75, 5 and 6 years from the start date of two OHAs, respectively, showing a wide gap between the recommended medical practice and the actual care that patients with diabetes receive. Patient‐related factors associated with delayed initiation of insulin included older age, relatively low HbA1c level (<9%) and shorter duration of diabetes. In contrast, physician‐related factors contributing to their resistance toward initiating therapy included age between 50 and 60 years, female sex, and consulting <1000 patients per month.
Despite the benefits of timely insulin initiation, the transition from OHAs to insulin therapy is often delayed in patients with diabetes14, 16, 17, 18. In the current study, approximately one in two patients initiated insulin after almost 6 years. According to data published in the USA, patients with inadequate glycemic control carry the burden of HbA1c ≥8.0% for approximately 5 years before subcutaneous insulin therapy is initiated19. In another longitudinal cohort of 3891 patients with type 2 diabetes receiving OHAs, just 41.9% of patients added insulin therapy despite failures to attain HbA1c <8.0%17. Thus, data from the current study show that the trend of delaying insulin initiation is in line with global trends; this highlights the need for concerted efforts to effect changes in modalities of diabetes care.
Timely initiation of insulin therapy is crucial for achieving glycemic control and preventing diabetes‐related complications19, 20, 21. In the MOdaliTy of Insulin treatment eValuation (MOTIV) registry, timely initiation of insulin therapy results in better achievement of glycemic goals with a lower insulin dosage and a low frequency of hypoglycemic events in patients with type 2 diabetes22. In the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial, basal insulin therapy for more than 6 years maintained near‐normal glycemic control and slowed progression of dysglycemia, with a neutral effect on cardiovascular outcomes, and a low rate of severe hypoglycemia and modest weight gain23.
The delay in initiation of insulin therapy is a major issue in managing people with diabetes in primary care24, and warrants a thorough understanding of the factors that modulate such a delay. The current study included an assessment of patient‐ and physician‐related factors affecting insulin initiation in patients with diabetes. Insulin therapy was more likely to be initiated in patients with HbA1c levels of at least 9% than in those with HbA1c levels below 9% (OR 1.00 vs 0.308, 95% CI: 0.235–0.404). HbA1c values are considered sufficiently high to warrant direct insulin initiation in patients treated with/without OHAs10, 15. Overall, 74% of physicians agreed with the ADA/EASD guidelines, and although the rate of support was over 70% in both groups, there was a gap between guideline support and actual insulin initiation. The importance of diabetes education and communication in addressing the underlying cause of delay in insulin initiation was addressed in a survey of primary care physicians25. Nurses trained in diabetes education often play key roles in helping patients make the decision to initiate insulin therapy, teach skills of injection and provide support in assessing diabetes‐related complications as they arise26. In contrast, in the current study, physicians working with nurses trained in diabetes education tended to delay initiation of insulin therapy, which might require further investigation.
Several studies examined factors that contribute to physician unwillingness to initiate insulin therapy. Riddle27 reported the underuse of insulin therapy in North America, and pointed out that some physicians worry that insulin therapy might promote insulin resistance or increase the risk of cardiovascular events, both of which are reported as major concerns for many physicians in the survey carried out by Hayes et al.24 Additionally, physician's perception of fear of hypoglycemia, weight gain and impaired quality of life in patients with diabetes might delay insulin initiation28, 29, 30.
In the current study, emotional or psychological issues of patients were commonly reported as reasons for delaying insulin therapy. Physicians delayed insulin therapy mostly because patients refused it (33.6%) or because of concern about the patient's compliance (26.5%) rather than concerns about hypoglycemia (14.2%) or weight gain (2.1%) – two well‐known adverse effects of insulin therapy. This can be understood from the trends on patient‐reported reasons for delaying insulin therapy in the current study; the major patient‐reported reasons for not starting insulin therapy in the current study were inconvenience (51.6%), fear of injection (48.2%) and thinking that ‘it is the end of life’ (31.1%). Concerns about hypoglycemia and weight gain were reported by just 2.9 and 1.5% of patients, respectively. Similarly, a qualitative study among patients with type 2 diabetes in a primary care clinic reported needle phobia, injection pain, inconvenience and negative beliefs (such as insulin could cause organ damage, insulin is required lifelong, insulin is for more severe disease only and their diabetes was not serious enough) as factors for influencing insulin acceptance among these patients31. The psychological and emotional response of patients might have further exacerbated physicians’ unwillingness to initiate insulin treatment in the current study, and adequate psychosocial counseling must precede and accompany actual insulin initiation to empower patients. Thus, identifying patient concerns about diabetes and insulin therapy is vital to support physicians in delivering patient‐centered care.
The main limitation of the present study relates first to its observational nature, as the data obtained cannot be used to discern the cause‐and‐effect relationships between the variables. Second, the study's patients and providers did not constitute a representative sample of the total population, which could result in bias with respect to hospital selection and potential confounders. Finally, insulin initiation could be influenced by the nation's culture and medical insurance system. Therefore, these findings have limitations with respect to generalization to other countries.
In summary, the current study shows that there is a delay in insulin initiation in people with type 2 diabetes. Less than half of the patients uncontrolled by two OHAs initiated insulin after almost 6 years. Numerous patient‐ and physician‐related reasons are associated with delays in insulin therapy initiation. Among them, major reasons delaying insulin therapy were associated with fear or misunderstanding of insulin therapy. Therefore, more education providing the exact details of insulin therapy will be required for clinicians as well as patients. Furthermore, exploring a patient's concerns about insulin is crucial to assist physicians in delivering patient‐centered care. By understanding this, physicians could address concerns and aim to modify their patients’ misconceptions toward insulin therapy and formulate appropriate health strategies to overcome these barriers.
Disclosure
All authors except NHK have reported receiving payments from Sanofi‐Aventis for speaking at investigator meetings, which does not alter the authors’ adherence to all the policies on sharing data and materials.
Supporting information
Figure S1 Patient disposition. Data are the number of study participants.
Appendix S1 Questionnaire format.
Acknowledgments
This study was sponsored by Sanofi‐Aventis. This study was also partly supported by a grant from the Korean Health Technology R&D Project (HI14C2750), Ministry of Health & Welfare, Korea. The authors thank Su Jin Sim of Sanofi (Korea) for providing statistical analysis, and Jeevan Scientific Technology Limited (Hyderabad, India) and Anahita Gouri of Sanofi (India) for providing writing assistance in the drafting of this manuscript. The authors also acknowledge all investigators of the DIPP‐FACTOR (Delay of Insulin initiation in patients with type 2 diabetes mellitus inadequately controlled with oral hypoglycemic agents [Analysis of Patients‐ and Physicians‐related FACTORs]) study: So Young Nah (Chungjoo St. Mary's Hospital), Jung Kook Kim (Kyungbook National University Hospital), Ji Hyun Ahn (Choong Ang University Hospital), Jong Hwa Kim (Se Jong Hospital), Hyung Jin Kim (Myung Ji Hospital), Kwang Eun Lee (Yonsei Green Internal Medicine Clinic), Kyoung Soon Chang (Chang Internal Medicine Clinic), Young Eun Cho (Dabos Hospital), Jong Kon Lee (Leejongkon Internal Medicine Clinic), Il Hoi Kim (Kimilhoi Internal Medicine Clinic), Soon Hee Park (Sahmyook Medical Center), Sung Geun Lee (Leesaem Internal Medicine Clinic), Sung Pyo Sohn (Bong Seng Memorial Hospital), Choon Hee Chung, Young Goo Shin (Wonju Severance Christian Hospital), Yu‐Bae Ahn (St Vincent's Hospital), Hyun Suk Son (Sonhyunsuk Internal Medicine Clinic), Hyung Joon Yu (Hallym University Medical Center), Hye‐Soon Kim (Keimyung University Dongsan Medical Center), Chung Gu Cho (Wonkwang University Hospital), Jeong‐Hyun Park (Inje University Busan Paik Hospital), Yoon Hee Choi, Kun Ho Yoon, Bong Yun Cha (Catholic University Seoul St. Mary's Hospital), Tae Keun Oh (Chungbuk National University Hospital), Chan Hee Chung (Soonchunhyang University. Bucheon Hospital), Kang Seo Park (Eulji University Daejun Hospital), Kwan Woo Lee (Ajou University Hospital), Hyuk Sang Kwon (Catholic University Yeouido St. Mary's Hospital), Sei Hyun Baik (Korea University Guro Hospital), Dong Lim Kim (Kon Kuk University Medical Center), Bo Kyung Koo (Seoul National University Boramae Medical Center), Suk Chon (Kyung Hee University Hospital), Jung Min Kim (St. Carollo Hospital), Kyung Ho Lim (Inje University Seoul Baik Hospital), Seok Man Son (Pusan National University Yangsan Hospital), Nan Hee Kim (Korean University Ansan Hospital), Bong Soo Cha (Yonsei University Severance Hospital), Chul‐Woo Ahn (Yonsei University Kangnam Severance Hospital), Kyung Ah Han (Eulji University Nowon Eulji Hospital), Hak Chul Jang (Seoul National University Bundang Hospital), Sang Jin Kim (Soon Chun Hyang University Cheonan Hospital), Yong Soo Park (Hanyang University Guri Hospital), Sung Rae Cho (Changwon Fatima Hospital), Yong Wook Cho (Cha Medical School Bundang Cha Hospital), Ie Byung Park (Gachon University Gil Hospital), Jong Ryeal Hahm (Gyeong Sang National University Hospital), Mi Kyung Kim (Maryknoll Hospital), Young Il Kim (Ulsan University Hospital), Se In Hong (Kwangjoo Bohoon Hospital), Seok O Park (Kwangmyung Sungae Hospital), Dong Jun Kim (Inje University Ilsan Paik Hospital), Kyoung Ah Kim (Dong Guk University Ilsan Hospital), Duk Kyu Kim (Dong‐A University Medical Center), Sang Joon Lee (Pureun Mirae Internal Medicine Clinic), Tae Wha Kim (Hanyang University Seoul Hospital), Kwang Jae Lee (Daedong Hospital), Sun Hwa Lee (Incheon Christian Hospital), Kyung Soo Ko (Inje University Sanggye Paik Hospital), Dong Hoon Shin (Hanil Hospital), In Won Kim (Sejin Internal Medicine Clinic) and Seok Woo Kang (Seoul Red Cross Hospital).
J Diabetes Investig 2017; 8: 346–353
References
- 1. IDF Diabetes Atlas Sixth Edition [Internet]. International Diabetes Federation; 2013. Available from: http://www.idf.org/worlddiabetesday/toolkit/gp/facts-figures Accessed August 22, 2014.
- 2. Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J 2011; 35: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeon JY, Kim DJ, Ko SH, et al Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Statistics Korea: statistical results about cause of death [Internet]; 2009. Available from: http://www.index.go.kr Accessed August 22, 2014 (Korean).
- 5. Lee KW. Costs of diabetes mellitus in Korea. Diabetes Metab J 2011; 35: 567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . Executive summary: standards of medical care in Diabetes‐2012. Diabetes Care 2012; 35(Suppl 1): S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook MN, Girman CJ, Stein PP, et al Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care 2005; 28: 995–1000. [DOI] [PubMed] [Google Scholar]
- 9. Riedel AA, Heien H, Wogen J, et al Secondary failure of glycemic control for patients adding thiazolidinedione or sulfonylurea therapy to a metformin regimen. Am J Manag Care 2007; 13: 457–463. [PubMed] [Google Scholar]
- 10. Korean Diabetes Association [Internet] . The Korean Diabetes Association guideline for diabetes; 2011. Available from: http://www.diabetes.or.kr Accessed September 26, 2011 (Korean).
- 11. Brunton S. Insulin regimens for type 2 diabetes mellitus. J Fam Pract 2006; 55: 10S–17S. [Google Scholar]
- 12. Nicasio J, McFarlane SI. Early insulin therapy and the risk of cardiovascular disease in type 2 diabetes. Therapy 2005; 2: 685–688. [Google Scholar]
- 13. Nakar S, Yitzhaki G, Rosenberg R, et al Transition to insulin in type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications 2007; 21: 220–226. [DOI] [PubMed] [Google Scholar]
- 14. Goodall G, Sarpong EM, Hayes C, et al The consequences of delaying insulin initiation in UK type 2 diabetes patients failing oral hyperglycaemic agents: a modelling study. BMC Endocr Disord 2009; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nathan DM, Buse JB, Davidson MB, et al Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubino A, McQuay LJ, Gough SC, et al Delayed initiation of subcutaneous insulin therapy after failure of oral glucose‐lowering agents in patients with type 2 diabetes: a population‐based analysis in the UK. Diabet Med 2007; 24: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 17. Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med 2007; 22: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care 2003; 9: 213–217. [PubMed] [Google Scholar]
- 19. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004; 27: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 20. Home PD, Boulton AJ, Jimenez J, et al Issues relating to the early or earlier use of insulin in type 2 diabetes. Pract Diabetes Int 2003; 20: 63–71. [Google Scholar]
- 21. 3rd Dailey GE. Early insulin: an important therapeutic strategy. Diabetes Care 2005; 28: 220–221. [DOI] [PubMed] [Google Scholar]
- 22. Kim SS, Kim IJ, Kim YK, et al Duration of diabetes and effectiveness of insulin in the management of insulin‐naïve Korean patients uncontrolled on oral antidiabetic drugs: a sub‐analysis of the MOdaliTy of Insulin treatment eValuation (MOTIV) registry results. Acta Diabetol 2014; 51: 655–661. [DOI] [PubMed] [Google Scholar]
- 23. ORIGIN Trial Investigators , Gerstein HC, Bosch J, et al Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 24. Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract 2008; 62: 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yap KK, Yiyi Y, Aruna C. Barriers to insulin initiation in type 2 diabetes mellitus‐ a single institution study among the physicians. AACE 2013; A209 (Abstract)
- 26. Levich BR. Diabetes management: optimizing roles for nurses in insulin initiation. J Multidiscip Healthc 2011; 4: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev 2002; 18(Suppl 3): S42–S49. [DOI] [PubMed] [Google Scholar]
- 28. Peyrot M, Rubin RR, Lauritzen T, et al Resistance to insulin therapy among patients and providers: results of the cross‐national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care 2005; 28: 2673–2679. [DOI] [PubMed] [Google Scholar]
- 29. Wallace TM, Matthews DR. Poor glycaemic control in type 2 diabetes: a conspiracy of disease, suboptimal therapy and attitude. QJM 2000; 93: 369–374. [DOI] [PubMed] [Google Scholar]
- 30. Elgrably F, Costagliola D, Chwalow AJ, et al Initiation of insulin treatment after 70 years of age: patient status 2 years later. Diabet Med 1991; 8: 773–777. [DOI] [PubMed] [Google Scholar]
- 31. Abu Hassan H, Tohid H, Mohd Amin R, et al Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract 2013; 14: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Patient disposition. Data are the number of study participants.
Appendix S1 Questionnaire format.


