Abstract
A 62‐year‐old Japanese female with primary lung adenocarcinoma received seven cycles of nivolumab as an eighth line of chemotherapy until she presented with hemoptysis. After transcatheter arterial embolization therapy, she received subsequent chemotherapy with paclitaxel and S−1. Four weeks later, a chest computed tomography examination revealed infiltrative shadows mainly in the right lung field, in addition to enlargement of the lung metastasis in the right middle lung lobe. Bronchofiberscopic examination revealed infiltration of lymphocytes without any malignant cells in the right segment 1 of the lung, which suggested interstitial lung disease. Corticosteroid therapy not only improved the infiltrative shadows but also reduced the lung metastasis. Even after the infiltrative shadows improved, the lung metastasis reduced further. This phenomenon resembles manifestation of pseudoprogression during treatments with immune checkpoint inhibitors, such as nivolumab.
Keywords: Interstitial lung disease, lung adenocarcinoma, nivolumab, pseudoprogression
Introduction
Immune checkpoint inhibitors (ICIs), such as nivolumab or pembrolizumab, which are antibodies against programmed cell death protein 1, have become key drugs against advanced non‐small cell lung cancers.1, 2, 3, 4, 5, 6 While pseudoprogression is occasionally observed during nivolumab therapy,7 interstitial lung disease (ILD) is also observed at a ratio of 3–5% as an immune‐related adverse event,3, 4 and ILDs caused during nivolumab therapy generally show a radiologic pattern of cryptogenic organizing pneumonia and exhibit a good response against corticosteroid therapy.8 In addition, nivolumab may enhance the risk of ILD when osimertinib is used after novolumab.9 Interestingly, some melanoma cases of ILD induced by nivolumab or ipilimumab show both improvement of ILD and reduction of tumor size by corticosteroid therapy, and antitumor immunity might partially contribute to these phenomena.10, 11
Here we report a case of lung adenocaricinoma with pseudoprogression and ILD during chemotherapy after nivolumab treatment.
Case report
A 62‐year‐old Japanese female with a 32 pack‐year smoking history was diagnosed with primary lung adenocarcinoma lacking epidermal growth factor receptor‐mutation in the right lower lobe, with a clinical stage of T2N2M0, stage IIIA. It was unknown whether her lung cancer harbored anaplastic lymphoma kinase‐rearrangement. She received chemoradiotherapy as first line therapy. A year later, her lung cancer relapsed with a single brain metastasis and multiple lung metastases. She subsequently received gamma knife radiotherapy and six regimens of chemotherapy for six years and three months, followed by nivolumab treatment. After receiving seven cycles of nivolumab (12 weeks later), she presented with hemoptysis. Chest computed tomography (CT) revealed that the lung metastasis in the middle lobe had slightly enlarged within the range of stable disease according to Response Evaluation Criteria in Solid Tumors, version 1.1 (Fig 1a,b).12 Bronchofiberscopic examination revealed that her right basal bronchus was responsible for hemoptysis (Fig 1c). She received transcatheter arterial embolization therapy against the right lower lobe bronchial artery. In addition to the endoscopic gross view, positron emission tomography‐CT revealed accumulation of 18F‐fluorodeoxyglucose in the lung metastasis, and her carcinoembryonic antigen serum level was increased (Fig 1b,d). Based on these factors, we considered her lung cancer to be clinically progressive. The patient subsequently received chemotherapy with paclitaxel and S−1.13 Four weeks later, when 20 weeks had passed since the initiation of nivolumab, she visited our hospital with a fever and cough. CT examination revealed infiltrative shadows in the bilateral lung fields mainly in the right, in addition to enlargement of the lung metastasis (Fig 2c), which suggested ILD with a radiologic pattern of cryptogenic organizing pneumonia and progressive disease by RECIST v1.1. Chemotherapy was discontinued. She was admitted to our hospital, and bronchofiberscopic examination revealed infiltration of the lymphocytes without any cancer cells or bacteria in the right lung segment 1. We diagnosed grade III ILD, and 1000 mg of methylprednisolone was administered daily for three days. She then received 60 mg of prednisolone daily, which was altered to dexamethasone because of hypokalemia induced by prednisolone. The corticosteroid therapy improved the infiltrative shadow (Fig 2d). Surprisingly, during the tapering of dexamethasone, the lung metastasis also decreased in size, and reduced even further after the infiltrative shadows improved (Fig 2a–e). The anti‐tumor effect was considered a partial response through pseudoprogression. Her carcinoembryonic antigen serum level also decreased (Fig 2e). Nineteen weeks after the initiation of paclitaxel and S−1, her lung cancer became progressive disease (Fig 2e).
Figure 1.
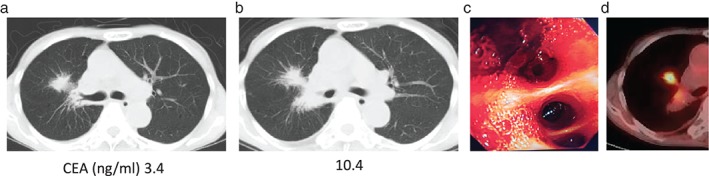
Chest computed tomography and carcinoembryonic antigen serum level (normal upper limit of 5 ng/ml). After (a) three and (b) seven cycles of nivolumab. (c) The right intermediate bronchus of the patient. (d) Positron emission tomography‐computed tomography after transcatheter arterial embolization therapy.
Figure 2.
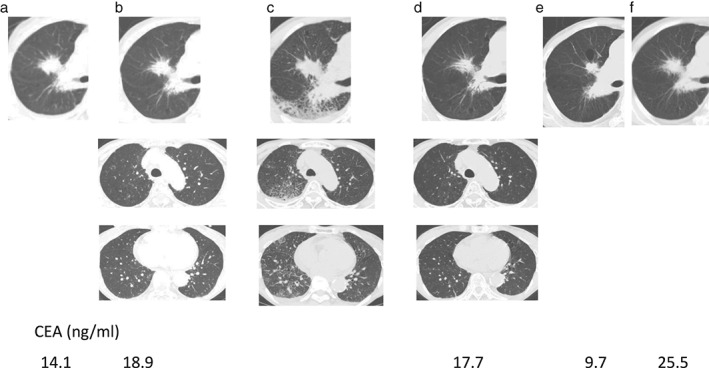
Chest computed tomography and carcinoembryonic antigen (CEA) serum level during a ninth chemotherapy regimen with paclitaxel and S−1. (a) Prior to the ninth chemotherapy and (b–f) two, four, six, 11 and 19 weeks later, respectively.
Discussion
It is well known that ICIs, such as nivolumab or pembrolizumab, occasionally induce pseudoprogression.7, 14 Although not completely clarified, one possible mechanism for pseudoprogression is an increase of tumor volume via lymphocytic infiltration.15 In our case, corticosteroid therapy might have induced early tumor reduction, probably through a rapid decrease of lymphocytic infiltration, which might also have occurred in the region of ILD. While almost all instances of pseudoprogression are reported to appear during treatment with an immune‐checkpoint‐inhibitor (ICI), our case indicates that pseudoprogression may also occur after cessation of ICI treatment. Therefore, paclitaxel + S‐1 appeared to have triggered both pseudoprogression and ILD. The improved immune responses from nivolumab and increased antigen presentation from destructed cancer cells after chemotherapy might have been responsible for these two phenomena. The reduction in tumor size may resemble an abscopal effect, in which radiation enhances ICIs responses, although we cannot exclude the possibility of a direct cytotoxic effect of paclitaxel and S−1.16, 17
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank the radiographic examination staff of Osaka Medical Center for Cancer and Cardiovascular Diseases for their assistance with diagnosis.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guibert N, Mazières J. Nivolumab for treating non‐small cell lung cancer. Expert Opin Biol Ther 2015; 15: 1789–97. [DOI] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 7. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015; 33: 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishino M, Ramaiya NH, Awad MM et al. PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin Cancer Res 2016; 22: 6051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kotake M, Murakami H, Kenmotsu H, Naito T, Takahashi T. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann Oncol 2017; 28: 669–70. [DOI] [PubMed] [Google Scholar]
- 10. Sano T, Uhara H, Mikoshiba Y, et al. Nivolumab‐induced organizing pneumonia in a melanoma patient. Jpn J Clin Oncol 2016; 46: 270–2. [DOI] [PubMed] [Google Scholar]
- 11. Berthod G, Lazor R, Letovanec I et al. Pulmonary sarcoid‐like granulomatosis induced by ipilimumab. J Clin Oncol 2012; 30: e156–9. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 13. Aono N, Ito Y, Nishino K et al. A retrospective study of the novel combination of paclitaxel and S1 for pretreated advanced non‐small cell lung cancer. Chemotherapy 2012; 58: 454–60. [DOI] [PubMed] [Google Scholar]
- 14. Hodi FS, Hwu WJ, Kefford R et al. Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016; 34: 1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res 2009; 15: 7116–8. [DOI] [PubMed] [Google Scholar]
- 16. Seyedin SN, Schoenhals JE, Lee DA et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy 2015; 7: 967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribeiro Gomes J, Schmerling RA, Haddad CK et al. Analysis of the abscopal effect with anti‐PD1 therapy in patients with metastatic solid tumors. J Immunother 2016; 39: 367–72. [DOI] [PubMed] [Google Scholar]


