Abstract
Background
Epithelial to mesenchymal transition (EMT) is a complex and dynamic molecular event in lung cancer metastasis that has not yet been thoroughly investigated. EMT transcriptional factors, such as Snail, play a central role in regulation of the EMT process. In this study, we sought to identify an association between p300 and Snail in lung cancer, as well as the engagement of p300 in Snail acetylation.
Methods
We transfected p300 small interfering RNA into lung cancer cells to detect Snail and E‐cadherin expression levels by real time‐PCR. Immunoprecipitation assay was conducted to determine Snail acetylation in vivo. Bacteria‐expressed Snail was purified to analyze Snail acetylation in vitro. We further mutated lysine 187 for identifying acetylated residue in Snail.
Results
Snail transcription in lung cancer cells was repressed by p300 knockdown. E‐cadherin expression was increased by transfection of p300 small interfering RNA in a dose‐dependent manner. Immunoprecipitation and Western blot assay with anti‐acetylated lysine antibody were used to confirm that Snail was acetylated by p300. A sequence coding snail gene was cloned into glutathione S‐transferase‐tagged vector and the fusion protein was purified using glutathione. We observed Snail acetylation in vitro by incubation of recombinant Snail and p300 histone acetyltransferase domain with acetyl coenzyme A. The reduced Snail acetylation level was related to lysine mutation at position 187 of Snail.
Conclusion
There was a correlation between Snail and p300 expressions in lung cancer. Moreover, p300 acetylates Snail both in vivo and in vitro, and K187 may be involved in this modification.
Keywords: Acetylation, lung cancer, p300, Snail
Introduction
It is broadly accepted that lung cancer is the leading cause of cancer death and represents one of the most common malignancies worldwide. Non‐small‐cell lung cancer (NSCLC) is the most common type of lung cancer and the five‐year survival rate is extremely low. The majority of advanced lung cancer patients die of cancer metastasis rather than as a result of the primary tumor. This occurs as some cells leave the primary tumor and migrate separately to distant sites and then acquire mesenchymal characteristics. Numerous investigations have confirmed the importance of epithelial‐mesenchymal transition (EMT) as a key step during tumor metastasis. Several signaling pathways are involved in the EMT process to promote cancer metastasis, including transforming growth factor‐β, Wnt, and Notch.1 Moreover, evidence has shown that EMT is regulated by a group of transcriptional factors, which increase the metastatic potential of tumor cells by repressing E‐cadherin, an EMT marker. These factors belong to the Snail superfamily, the ZEB family, or basic helix‐loop‐helix proteins. EMT transcriptional factors could induce the mesenchymal phenotype, thus resulting in the malignancy of different types of tumors, such as lung cancer.
Snail is a zinc‐finger transcriptional factor, which is able to bind to the E‐BOX motif on the E‐cadherin promoter, as well as recruit histone deacetylases (HDACs) to inhibit E‐cadherin expression. Snail is overexpressed in several types of human cancers and is assumed to be a crucial mediator in triggering the EMT process and cell invasiveness. In addition, it has been reported that the EMT‐induction activity of Snail may be regulated at a transcriptional or translational level by a variety of mechanisms. A recent observation demonstrated that Snail functions were regulated by phosphorylation. A number of kinases phosphorylate Snail at different residues to manipulate its function in cell migration and invasion. Snail is also phosphorylated by glycogen synthase kinase (GST)3‐β at two domains. Interestingly, phosphorylation of the first domain destabilized Snail, while modification of the other promoted nuclear export.2 Conversely, Snail function is positively regulated by ataxia telangiectasia mutated, Lats2, and p21‐activated kinase 1‐dependent phosphorylation to enhance tumor metastasis.3, 4, 5, 6 Recent studies have shown that the histone acetyltransferase (HAT), p300, is associated with the metastatic ability of cancer cells, and Snail is upregulated by p300 to suppress E‐cadherin expression.7, 8, 9, 10 Snail is acetylated by VPA (a histone deacetyltransferase inhibitor) treatment in colorectal cancer, thus contributing to Snail stabilization and nuclear localization.11 CREB‐binding protein (CBP) was discovered to interact with Snail though its HAT domain. Moreover, lysines at position 146 and 187 are substrates for CBP‐mediated acetylation. Although DNA‐binding ability or nuclear import are not influenced by acetylation, the half‐life of Snail is positively modulated. This modification is indispensable for Snail function in promoting tumor progression, which leads to poor prognosis in head and neck cancers.12 It is possible that Snail triggers tumor cell migration and invasion and exerts its metastasis‐inducing role via post‐translational modifications (PTMs). However, the involvement of PTMs in affecting the Snail‐regulated EMT process still requires in‐depth investigation, particularly the acetylation of Snail in human cancer metastasis.
In the present study, we sought to identify an association between p300 and Snail in lung cancer, as well as the engagement of p300 in Snail acetylation. Firstly, we verified that p300 silence leads to Snail transcription repression and that E‐cadherin is upregulated by p300 inhibition in a dose‐dependent manner in A549 lung cancer cells. Furthermore, p300 acetylated Snail in vivo. We then explored Snail acetylation in vitro and confirmed this phenomenon using an in vitro acetylation assay. Lysine 187 mutation affected Snail acetylation status. Therefore, we consider that elucidation of p300‐mediated acetylation of Snail might provide a better understanding of the mechanisms of EMT and lung cancer progression.
Methods
Cell culture and transfection
Human lung cancer cell lines A549, YTMLC‐9, NL9980, and L9981 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (GIBCO, Thermo Fisher Scientific, Waltham, MA, USA), 50 U/mL penicillin, and 50 μg/mL streptomycin sulfate at 37°C with a 5% CO2 incubator. NL9980 and L9981 were isolated and established from a human lung large cell carcinoma cell line (WCQH‐9801). The L9981 cells displayed low nm23‐H1 expression and higher invasion and metastasis potential.13 Cell transfection with plasmids or small interfering (si)RNA was carried out using Lipo2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions. The p300 siRNA was synthesized by RiboBio (Guangzhou, China), and cells were transfected with 50, 100, or 200 nM siRNA, then lysed for real time (RT)‐PCR assay.
Quantitative real‐time‐PCR
The total RNA was extracted using an RNAsimple Total RNA Kit (Tiangen Biotech, Beijing, China). The RT‐PCR reaction was performed with a FastQuant RT Kit and SuperReal PreMix Plus (SYBR Green) (Tiangen Biotech) using an ABI Prism 7900HT fast RT‐PCR system (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Amplification was carried out at 95°C for five minutes, followed by 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds. In each experiment, all samples were tested in triplicate. The relative amount of messenger (m)RNA was calculated using the 2−ΔΔCt method after normalization to endogenous glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) mRNA levels. Primer sequences to detect Snail, p300, E‐cadherin (CDH1), and GAPDH expression are shown in Table 1.
Table 1.
Primers to detect Snail, p300, CDH1 and GAPDH expression by RT‐PCR
| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| p300 | Forward | GGGACTAACCAATGGTGGTG |
| Reverse | GTCATTGGGCTTTTGACCAT | |
| Snail | Forward | TCTAGGCCCTGGCTGCTAC |
| Reverse | GCCTGGCACTGGTACTTCTT | |
| CDH1 | Forward | TGCCCAGAAAATGAAAAAGG |
| Reverse | GTGTATGTGGCAATGCGTTC | |
| GAPDH | Forward | GACCCCTTCATTGACCTCAAC |
| Reverse | CTTCTCCATGGTGGTGAAGA |
CDH1, E‐cadherin; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; RT, real time.
Plasmids and recombinant proteins
The sequence coding for Snail was generated from pCMV‐Tag2B‐Snail by PCR with the forward primer 5′‐TCGGAATTCATGCCGCGCTCTTTCCTCG‐3′ and reverse primer 5′‐TTTCTCGAGTCAGCGGGGACATCCTG‐3′. The products were double‐digested by EcoR I and Xho I and then cloned in a pGEX‐6p‐1 vector (Amersham Biosciences, Buckinghamshire, UK) to construct GST‐tagged Snail (pGEX‐Snail). The Snail‐K187R mutation was generated by PCR‐based site directed mutagenesis (QuickChange Kit, Stratagene, La Jolla, CA, USA) using pCMV‐Tag2B‐Snail as a template with the forward primer 5′‐GAACCTGCGGGAGGGCCTTCTCTAGG‐3′ and reverse primer 5′‐CCTAGAGAAGGCCCTCCCGCAGGTTC‐3′. All constructions were verified by sequencing. Plasmid HA‐p300 expression was obtained from Millipore (Bedford, MA, USA). pGEX‐p300 HAT has been described previously.14
For purification of GST‐Snail, the plasmid pGEX‐Snail was transformed into E.coli strain BL21 (DE3). Recombinant Snail expression was induced by 0.5 mmol/L isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) for four hours at 37°C. The cell lysate was then loaded on a Glutathione Sepharose 4B column (Amersham Biosciences) and GST‐Snail was purified by elute buffer (10 mmol/L reduced glutathione, 50 mmol/L Tris‐hydrochloride [HCl], pH 8.0). Protein concentrations were determined using bicinchoninic acid assay.
Immunoprecipitation assay
Forty‐eight hours post‐transfection, A549 cells were harvested, washed with pre‐chilled phosphate buffered saline, and lysed in immunoprecipitation protocol (IP) buffer (50 mM Tris‐HCl, pH 8.0, 150 mM NaCl, 1% NP‐40, and 1 mM phenylmethylsulfonyl fluoride). The cells were sonicated on ice, followed by centrifugation at 10 000 g for 15 minutes at 4°C. The cell supernatant was incubated with rabbit anti‐Snail antibody at 4°C for two hours. Protein A Sepharose (50% slurry; Sigma‐Aldrich, St. Louis, MO, USA) was added to the protein‐antibody complex. After incubation at 4°C for another two hours, the immune precipitates were washed with IP buffer six times and boiled in 2 × Laemmli buffer for Western blot assay.
In vitro acetylation assay
In vitro acetylation assays were carried out using 1 μg GST or GST‐Snail protein, 500 ng purified p300 HAT domain, and 10 μM Ac‐CoA (Sigma‐Aldrich) in HAT buffer (50 mM Tris‐HCl, pH 7.5, 10% glycerol, 0.1 mM ethylene‐diamine‐tetraacetic acid, 50 mM KCl, 10 mM sodium butyrate, 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride). Acetylation was performed at 37°C for one hour. Samples were electrophoresed on sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue or subjected to Western blotting.
Antibodies and Western blot
The antibody against Snail was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), and anti‐acetylated lysine antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). Samples from IP or in vitro acetylation assay were fractionated with 10–15% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. The membranes were blocked with 5% skimmed milk in phosphate buffered saline‐Tween (0.1%) for one hour, followed by probing with anti‐Snail or anti‐acetylated lysine antibody at a dilution of 1:1000. After incubation at 4°C overnight, the membrane was probed with horseradish peroxidase‐conjugated secondary antibody for one hour, while the blots were visualized with Chemiluminescent HRP Substrate (Millipore).
Results
p300 regulates Snail and E‐cadherin expression in lung cancer cells
According to previous studies, EMT transcriptional factors are closely related with HATs in tumor growth. CBP activates Twist1 transcription in breast cancer, and p300 interaction with Twist1 protein facilitates human gastric cancer progression.15, 16 Recent evidence has revealed that Snail and p300 are upregulated during cardiac EMT.17 In an attempt to verify the correlation between Snail and p300 in lung cancer, we examined Snail expression in A549 (adenocarcinoma), YTMLC‐9 (squamous carcinoma), NL9980 (low metastasis potential), and L9981 (high metastasis potential) cells upon p300 knockdown. RT‐PCR results suggested that p300 silence resulted in the suppression of Snail transcription in all four lung cancer cell lines (Fig 1a). Notably, the most significant inhibition of Snail was observed in A549 cells. We then analyzed CDH1 expression by transfection of p300 siRNA in A549. As shown in Figure 1b, the Snail level was reduced, consistent with previous findings. In addition, CDH1 expression was promoted in a dose‐dependent fashion by the transfection of increasing amounts of p300 siRNA (50, 100, and 200 nM). These results demonstrate that p300 plays a potential role in modulating Snail transcription, as well as E‐cadherin expression and EMT in lung cancer.
Figure 1.
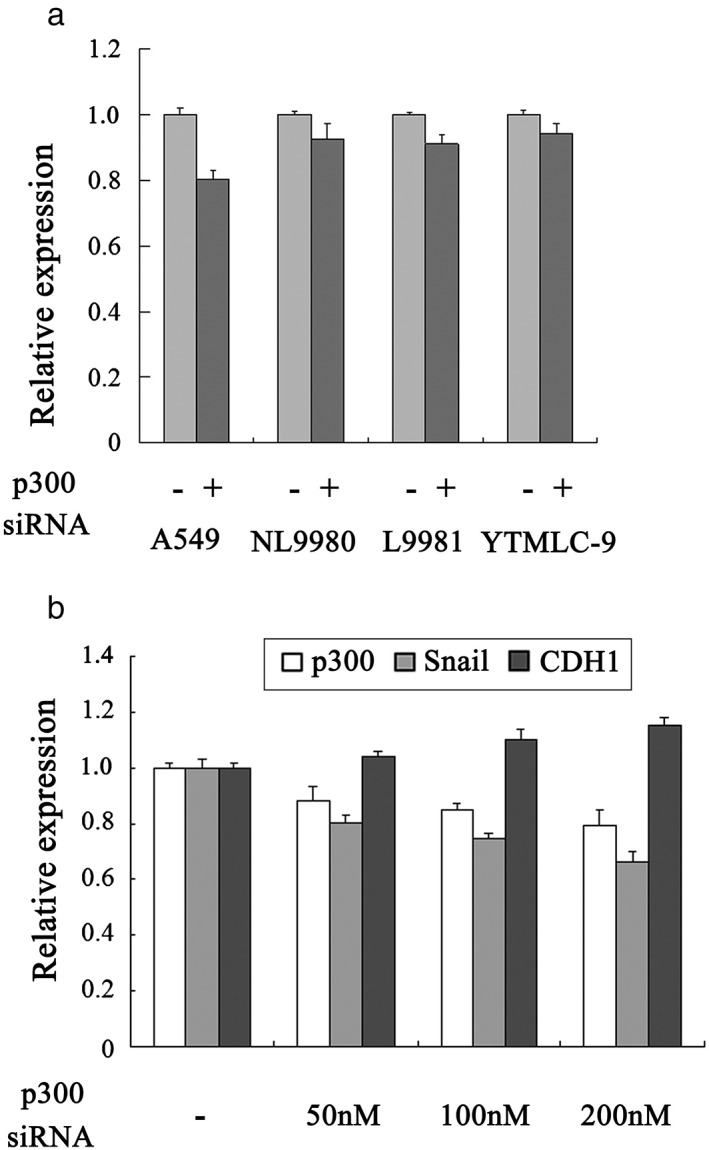
Snail and E‐cadherin (CDH1) expression were regulated by p300 inhibition in lung cancer cells. (a) A549, NL9980, L9981 and YTMLC‐9 cells were transfected by p300 small interfering RNA (50 nM). Forty‐eight hours post transfection the relative Snail expression level was measured by quantitative real‐time‐PCR. (b) A549 cells were transfected by 50, 100 or 200 nM p300 small interfering RNA. Forty‐eight hours post transfection the relative p300, Snail and CDH1 expression levels were measured by quantitative real‐time‐PCR. siRNA, small interfering RNA.
Snail acetylation by p300 in vivo
Feng et al. and Hsu et al. determined that the HDAC inhibitor and CBP was involved in Snail acetylation.11, 12 To confirm whether p300, a member of the HAT family related structurally and functionally with CBP, could acetylate Snail in lung cancer, A549 cells were transfected with eukaryotic plasmids expressing Snail with p300 or vector. Immunoprecipitation assay was conducted to enrich Snail protein and the Snail acetylation level was detected by Western blot using anti‐acetyl lysine antibody. The data revealed an increase in Snail acetylation compared with the basal acetylation level of Snail in the control group (Fig 2). This result implied that p300 was required to acetylate Snail, which was in line with our inference.
Figure 2.
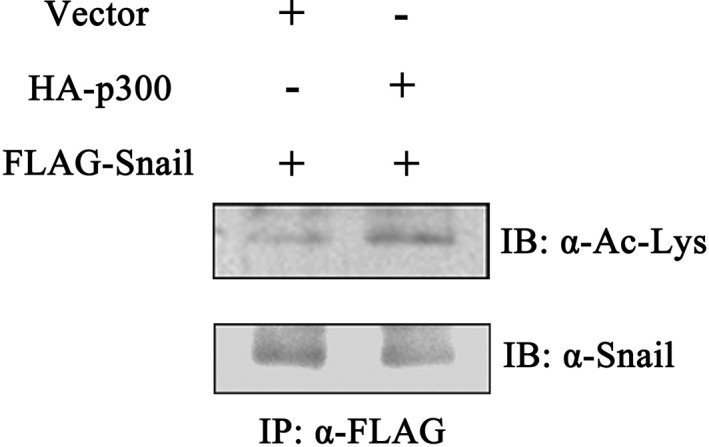
Snail acetylation by p300 in vivo. The plasmid pCMV‐Tag2B‐Snail were transfected into A549 cells with p300 or an empty vector. Cells were harvested and lysed 48 hours post transfection, and Snail protein was immunoprecipitated with antibody against FLAG and blotted with anti‐acetylated lysine antibody (upper panel) or anti‐Snail antibody (lower panel).
Snail acetylation by p300 in vitro
To explore whether Snail is a substrate for p300‐mediated acetylation in vitro, we performed in vitro acetylation assay. A fragment of 795 bp corresponding to the Snail‐coding sequence was amplified by PCR and cloned into pGEX‐6p‐1 vector (Fig 3a). Plasmid correction was verified by sequencing. The pGEX‐Snail construct was then transformed into the E.coli BL21 (DE3) strain. For purification of the recombinant protein, IPTG was applied to induce GST‐tagged Snail expression. The bacterial expressed protein, which has a molecular weight of 56kD, was affinity purified by Glutathione Sepharose 4B (Fig 3b, lane 6).
Figure 3.
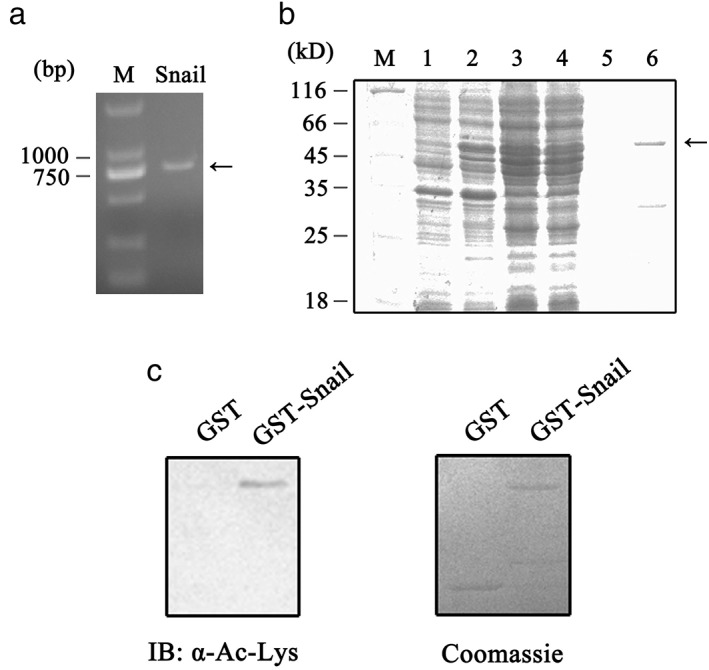
Snail acetylation by p300 in vitro. (a) Amplification of Snail fragment using pCMV‐Tag2B‐Snail as template by PCR. (b) Purification of recombinant glycogen synthase kinase‐Snail protein. The pGEX‐Snail construct was transformed into E . coli BL21 (DE3) and induced with isopropyl β‐D‐1‐thiogalactopyranoside. The destined proteins were purified using Glutathione Sepharose 4B.M: Marker. Lane 1: total proteins from uninduced E . coli BL21 (DE3) transformed with pGEX‐Snail; Lane 2: total proteins from induced E .coli BL21 (DE3) transformed with pGEX‐Snail; Lanes 3 and 4: soluble proteins and pellets from induced E . coli BL21 (DE3); Lane 5: wash buffer treated; Lane 6: purified recombinant glycogen synthase kinase (GST)‐Snail (56 kD). (c) In vitro acetylation of Snail. GST or GST‐Snail were incubated with p300 histone acetyltransferase domain and acetyl coenzyme A at 37°C for one hour. (left panel) The samples were analyzed by Western bolt using anti‐acetylated lysine antibody. (right panel) Proteins used in the assay were detected by Coomassie blue staining.
Glycogen synthase kinase or GST‐Snail fusion protein was then incubated with the HAT domain of p300 (1077‐1718aa) and acetyl coenzyme A for in vitro acetylation assay. No GST acetylation occurred, but GST‐tagged Snail was acetylated by HAT assay (Fig 3c, left panel). Equivalent amounts of substrate proteins were applied, as shown by Coomassie blue staining (Fig 3c, right panel). Together, these results suggest that Snail is a target for p300‐mediated acetylation in vitro.
Lysine 187 mutation affects Snail acetylation
Further, we sought to identify which lysine(s) in Snail is a substrate for acetylation by p300 in lung cancer. In a recent report, K146 and K187 of Snail were recognized as acetylated residues via CBP‐regulated acetylation.12 Lysine 187 matched the “G/SK motif” as the lysine in this domain may be acetylated by p300 HAT. Hence, lysine 187 in wild‐type Snail was replaced by employing arginine site‐directed mutagenesis to construct the K187R mutation (Fig 4a). A549 cells were transfected with wild type Snail or K187R mutation. Proteins were then immunoprecipitated and subjected to Western blot using anti‐acetyl lysine antibody. The data in Figure 4b show a weak reduction of acetylation status in K187R mutation. Therefore, Snail acetylation may be affected by a mutation at K187, which may be assumed as a putative site for p300 acetylation in lung cancer.
Figure 4.
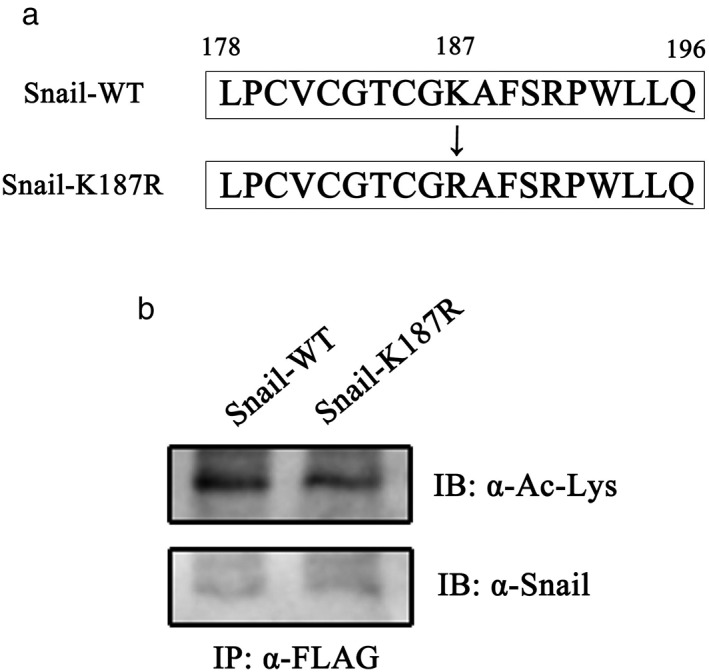
Lysine 187 mutation affected Snail acetylation. (a) A schematic representation of wild‐type and K187R Snail mutation. (b) pCMV‐Tag2B‐Snail or pCMV‐Tag2B‐Snail‐K187R were transfected into A549 cells and harvested 48 hours post transfection. The Snail protein was immunoprecipitated with antibodies against FLAG and blotted with (upper panel) anti‐acetylated lysine antibody or (lower panel) anti‐Snail antibody. IP, immunoprecipitation protocol; WT, wild‐type.
Discussion
Lung cancer is one of the most frequently occurring types of tumor and the death rate from lung cancer has increased significantly in the last few years. As a large number of studies have suggested, cancer metastasis is characterized as a multi‐step behavior associated with the increased invasive and migratory capacity of cancer cells. Although EMT regulation and several markers have been elucidated in lung tumor progression, the key roles of EMT transcriptional factors in mediating the EMT process require further exploration. Given that Snail appears necessary to promote cancer metastasis, defining the regulatory mechanism of Snail function via different pathways is crucial.
In the current study, we investigated the potential involvement of HATs in the Snail‐dependent EMT process, as Snail acetylation modification has not been reported in lung cancer. Firstly, we detected Snail and E‐cadherin expression levels by transfecting p300 siRNA into different lung cancer cells. The results demonstrated Snail reduction and E‐cadherin induction in A549. These results were consistent with previous findings, in that p300 was correlated with EMT marker and transcriptional factors in other cancer cells, and plays a key role in cell progression.7, 10 In consideration of Snail acetylation by CBP or HDAC inhibitor, we co‐transfected Snail and p300 expression plasmids into A549 cells and observed the Snail acetylation status by immunoprecipitation assay.11, 12 Furthermore, a GST‐tagged plasmid harboring Snail coding sequence was constructed, which was purified for in vitro acetylation. By incubation of recombinant Snail with p300 HAT acetyltransferase, we demonstrated Snail acetylation in vitro. In addition, lysine 187 mutation led to a reduction in the Snail acetylation level in comparison with the wild type. These results implied the possibility that Snail acetylation is a requisite for its function as an EMT mediator.
The acetyltransferase p300 belongs to the HAT family and participates in many physiological processes, including development, proliferation, and apoptosis. Of note, p300 gene mutation is related to various human cancers. Functions of numerous cellular regulators are substrates for acetylation through p300, such as histones, viral proteins, and EMT transcriptional factors. Twist was acetylated by p300/CBP‐associated factor (PCAF) at K73, K76, and K77, which promoted its nuclear import and transcription activation.18 It has been reported that Tip 60 acetylates Twist in basal‐like breast cancer.19 ZEB1 expression is positively regulated by PCAF‐involved acetylation at three lysines following the C‐terminal binding protein interaction domain.20 By contrast, both substrate residue and function of Snail acetylation is still poorly understood. Evidence has shown that glycine, serine, or lysine are often followed by acetylated lysine in substrate proteins, known as the “G/SK” motif. There are 14 lysines in Snail, and three lysines correspond with this rule. Although we detected reduced acetylation after K187 mutation, further analysis of Snail truncation and point mutation of potential sites is necessary to identify the acetylated lysine(s) of Snail by p300 in lung cancer cells. We also cannot exclude the probability that HATs other than p300 (e.g. PCAF and GCN5) acetylate Snail in lung cancer cells. Similar to phosphorylation, lysine acetylation may affect DNA‐binding affinity, protein interaction, subcellular localization, and the stability of EMT transcriptional factors (Fig 5). Therefore, the Snail acetylation mutant, which cannot be acetylated by HATs, is valuable for the functional study of Snail in regulating tumor progression.
Figure 5.
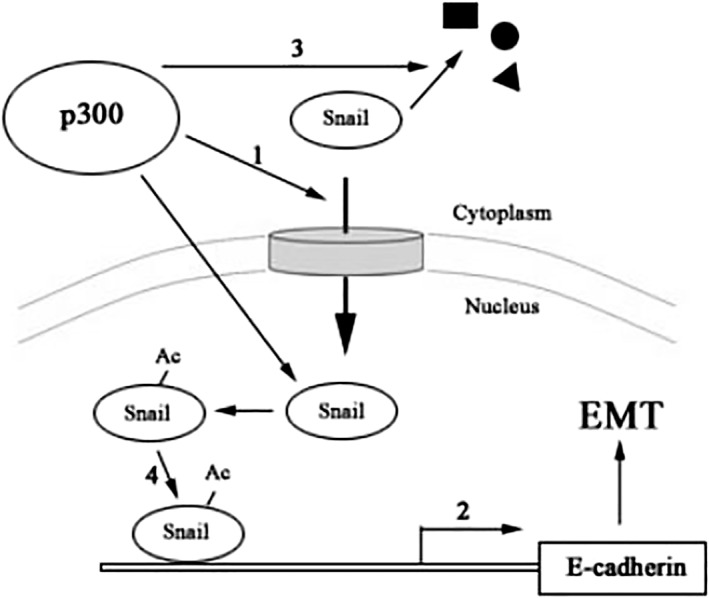
Potential function of Snail acetylation by p300. EMT, epithelial to mesenchymal transition.
In conclusion, our data presented here suggested that Snail was acetylated by p300 in lung cancer, which suggests acetylation as a potential and alternative mechanism during the Snail‐dependent EMT process. A better understanding of the regulatory pathway of Snail will provide critical clues to inhibit EMT and metastasis. Different HATs may acetylate distinct lysines of Snail to perform diverse functions in regulating cancer metastasis, which may be developed as personalized therapeutic targets for clinical lung cancer treatment.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was partly supported by the grants from the National Natural Science Foundation of China (No. 81572288, to, Qinghua Zhou) and TMUGH Funding (No. ZYYFY2014002, to Rui Chang).
Contributor Information
Peng Zhang, Email: zhang_peng1225@sina.com.
Qinghua Zhou, Email: zhouqh135@163.com.
References
- 1. Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial‐mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol 2012; 3: 117–36. [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou BP, Deng J, Xia W et al Dual regulation of Snail by GSK‐3beta‐mediated phosphorylation in control of epithelial‐mesenchymal transition. Nat Cell Biol 2004; 6: 931–40. [DOI] [PubMed] [Google Scholar]
- 3. Boohaker RJ, Cui X, Stackhouse M, Xu B. ATM‐mediated Snail serine 100 phosphorylation regulates cellular radiosensitivity. Radiother Oncol 2013; 108: 403–8. [DOI] [PubMed] [Google Scholar]
- 4. Sun M, Guo X, Qian X et al Activation of the ATM‐Snail pathway promotes breast cancer metastasis. J Mol Cell Biol 2012; 4: 304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang K, Rodriguez‐Aznar E, Yabuta N et al Lats2 kinase potentiates Snail1 activity by promoting nuclear retention upon phosphorylation. EMBO J 2012; 31: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial‐to‐mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res 2005; 65: 3179–84. [DOI] [PubMed] [Google Scholar]
- 7. Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY, Kuo PL. Lung tumor‐associated osteoblast‐derived bone morphogenetic protein‐2 increased epithelial‐to‐mesenchymal transition of cancer by Runx2/Snail signaling pathway. J Biol Chem 2011; 286: 37335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mu Y, Sundar R, Thakur N et al TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun 2011; 2: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokomizo C, Yamaguchi K, Itoh Y et al High expression of p300 in HCC predicts shortened overall survival in association with enhanced epithelial mesenchymal transition of HCC cells. Cancer Lett 2011; 310: 140–7. [DOI] [PubMed] [Google Scholar]
- 10. Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E‐cadherin gene expression. Oncogene 2005; 24: 8277–90. [DOI] [PubMed] [Google Scholar]
- 11. Feng J, Cen J, Li J et al Histone deacetylase inhibitor valproic acid (VPA) promotes the epithelial mesenchymal transition of colorectal cancer cells via up regulation of Snail. Cell Adh Migr 2015; 9: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu DS, Wang HJ, Tai SK et al Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 2014; 26: 534–48. [DOI] [PubMed] [Google Scholar]
- 13. Zhou Q, Wang Y, Che G et al [Establishment and their biological characteristics of clonal cell subpopulations (NL9980 and L9981) from a human lung large cell carcinoma cell line (WCQH‐9801).] Zhongguo Fei Ai Za Zhi 2003; 6: 464–8. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- 14. Chang R, Tan J, Wang RM, Chen QM, Geng YQ, Qiao WT. Expression and application of recombinant p300 histone acetyltransferase domain. Chin J Biochem Mol Biol 2011; 27: 452–8. [Google Scholar]
- 15. Liang Y, Hu J, Li J et al Epigenetic activation of TWIST1 by MTDH promotes cancer stem‐like cell traits in breast cancer. Cancer Res 2015; 75: 3672–80. [DOI] [PubMed] [Google Scholar]
- 16. Qian J, Luo Y, Gu X, Zhan W, Wang X. Twist1 promotes gastric cancer cell proliferation through up‐regulation of FoxM1. PLoS One 2013; 8: e77625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghosh AK, Nagpal V, Covington JW, Michaels MA, Vaughan DE. Molecular basis of cardiac endothelial‐to‐mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell Signal 2012; 24: 1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiota M, Yokomizo A, Tada Y et al P300/CBP‐associated factor regulates Y‐box binding protein‐1 expression and promotes cancer cell growth, cancer invasion and drug resistance. Cancer Sci 2010; 101: 1797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi J, Wang Y, Zeng L et al Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal‐like breast cancer. Cancer Cell 2014; 25: 210–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J 2003; 22: 2453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


