Abstract
Background
The relationship between epidermal growth factor receptor (EGFR) gene mutation status, preoperative computed tomography (CT), and clinical features in patients with small peripheral lung adenocarcinoma (<3 cm) was investigated.
Methods
We included 209 patients who underwent surgical resection for stage I or II lung adenocarcinoma at Nanjing General Hospital between December 2010 and May 2016. 171 cases of patients underwent a pretreatment chest CT. Eleven different CT descriptors were assessed. Multiple logistic regression analyses were performed to identify independent risk factors for the prediction of EGFR mutation. Receiver operating characteristic analysis was used to evaluate the performance of the logistic regression model.
Results
EGFR mutation was determined in 126 patients (60.3%) and appeared more frequently in women (P = 0.025), never‐smokers (P < 0.001), and patients with a carcinoembryonic antigen level <2.6 ng/ml (P = 0.045). Papillary predominant adenocarcinomas (P = 0.014), intermediate/low pathologic grade tumors (P = 0.003), tumors in the upper lobe (P = 0.028), and showing ground‐glass opacity (GGO) or mixed GGO on CT (P = 0.039) also more frequently harbored EGFR mutations. GGO on CT, acinar or papillary predominant adenocarcinoma, and non‐smoker were identified in multivariable analyses as significantly independent risk factors. The multiple logistic regression model showed high predictive power for identifying EGFR mutations. The CT features were similar between the L858R and 19 deletion mutations.
Conclusions
Combined CT and clinical features may be helpful for determining the presence of EGFR mutations in patients with small peripheral lung adenocarcinoma, particularly in patients where mutational profiling is not available or possible.
Keywords: Computed tomography (CT), epidermal growth factor receptor (EGFR), lung adenocarcinoma
Introduction
Lung cancer is one of the leading causes of death in the world. About 85% of all lung cancers are non‐small cell lung carcinoma (NSCLC), and adenocarcinoma is the most common histologic subtype.1 Epidermal growth factor receptor (EGFR) mutations are associated with high sensitivity to EGFR‐tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and afatinib.2 Targeted therapies have significantly improved the survival rates of lung cancer patients harboring EGFR mutations. Determining the EGFR mutation status of patients is therefore crucial for the prediction of response to EGFR‐TKIs and, thereby, choice of treatment regime. However, not all patients can undergo analysis for EGFR mutation status.
Although stage I NSCLC patients have a better prognosis, with five‐year survival rates ranging from 40% to 90%, nearly 30–35% will relapse.3, 4 The American Society of Pathology (CAP), the International Society for Lung Cancer Research (IASLC), and the American Society for Molecular Pathology (AMP) released a guideline for lung cancer gene testing, which recommends that patients with advanced lung cancer or those with disease recurrence or progression should be assessed for EGFR mutation status.5 Most early stage NSCLC patients only undergo surgical resection, and while the guidelines encourage EGFR status testing in such patients, they do not directly recommend testing. Computed tomography (CT) imaging is routinely used in lung cancer. Finding specific CT features that are associated with EGFR mutation might improve treatment and care for early stage NSCLC patients who for various reasons cannot undergo genetic mutation analysis.
Previous studies that have evaluated the relationship between some CT features and EGFR genetic mutations in NSCLC6, 7, 8, 9, 10 have mainly focused on patients with advanced adenocarcinomas (stages IIIB and IV), and only a few have investigated the correlation between CT features and EGFR mutation status in stage I or II adenocarcinoma patients.6, 11 This might be because EGFR mutation status is not routinely analyzed in early stage lung adenocarcinoma. In this study, we retrospectively surveyed the EGFR mutation status of stage I/II lung adenocarcinoma patients with tumor lesions <3 cm. The aim of the present study was to identify the relationship between EGFR mutation status, clinical features, and CT characteristics in surgically resected lung adenocarcinomas in a cohort of Chinese patients.
Methods
Patient selection
The study population was retrospectively selected from patients who underwent surgical resection of their lung adenocarcinoma at Nanjing General Hospital between December 2010 and May 2016. All medical records were reviewed to extract the patients’ clinical characteristics. Their EGFR mutation status was recorded. A total of 827 patients were identified. Patients who did not undergo EGFR mutation testing (n = 465); were pathologically diagnosed with stage III/IV lung cancer (n = 52); with a tumor >3 cm (n = 41); and who received preoperative treatment, such as radiation therapy or chemotherapy (n = 60), were excluded. Finally, the data of 209 patients was analyzed for any association between clinical characteristics and EGFR mutation status. Of the 209 patients, 171 underwent chest CT and were analyzed for an association between CT characteristics and EGFR mutation status. The study design was approved by the ethics committee of Nanjing General Hospital, who waived the need for informed consent because of the non‐invasive nature of the study and patient anonymity.
Computed tomography (CT) scanning protocol and image interpretation
All evaluations were performed using a multi‐slice CT scanner (Somatom Sensation 64, Siemens, Erlangen, Germany). Scanning parameters were: tube voltage 120 kVp, tube current 150–200 mA, rotation time 0.5 seconds, and 2 mm reconstruction thickness with a 1 mm reconstruction interval. Two radiologists with 15 and 20 years’ experience in chest image interpretation assessed CT images using both mediastinal (width, 360 HU; level, 60 HU) and lung window settings (width, 1600 HU; level, −600 HU). The radiologists were blinded to the pathological findings. When their interpretations of the CT images differed, discussion was conducted to reach a final consensus. Each CT corresponded to a single patient, and data were recorded on an Excel spreadsheet (Microsoft Office Excel 2007, Richmond, VA, USA).
The CT descriptors that were assessed are shown in Table S1.
Histologic evaluation and epidermal growth factor receptor (EGFR) mutation analysis
Adenocarcinoma was classified according to the 2011 IASLC/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification system. DNA was extracted from five pieces of formalin‐fixed, paraffin‐embedded tumor tissue using the QIAamp FFPE Tissue Kit (Qiagen, Valencia, CA, USA). Molecular analysis of the mutation status of EGFR exons 18, 19, 20, and 21 was examined using the Human EGFR Gene Mutations Detection Kit (AmoyDx, Xiamen, China), which is a PCR‐based amplification‐refractory mutation system.
Statistical analysis
Unpaired t‐tests were used to compare two continuous variables. Categorical variables were analyzed by chi‐square tests, except where a small sample size (<5) required the use of Fisher’s exact test. Before performing multiple logistic regression analysis, variables were selected by a stepwise method. Receiver operating characteristic (ROC) analysis was performed to determine cut‐off values and to evaluate the performance of the logistic regression model. All reported P values were two‐tailed, and P values <0.05 were considered statistically significant. Statistical analyses of the data was performed using SPSS version 21 (IBM Corp., Armonk, NY, USA).
Results
Patient demographics and EGFR mutation status
The demographic and pathological data of the study population are presented in Table 1. All 209 of the enrolled patients were surgically treated: lobectomy in 181 (86.6%) patients, wedge resection in 22 (10.5%), and segmentectomy in six (2.9%) patients. There were 96 (45.9%) men and 113 (54.1%) women, with a median age of 60.1 years (range 27–81). Tumor node metastasis stage distribution was: IA in 163 patients (77.9%), IB in eight (3.8%), IIA in 30 (14.4%), and IIB in eight patients (3.8%). Most of the tumors were stage I (171, 81.8%). All cases were invasive lung adenocarcinomas and the most common histologic subtype was acinar predominant (113, 54.1%), followed by lepidic predominant (38, 18.2%), which included five cases of minimally invasive adenocarcinoma (MIA) and two adenocarcinoma in situ (AIS). In the tumors with an EGFR mutation, 67 (53.2%) had an L858R mutation and 50 (39.6%) had a 19 deletion mutation.
Table 1.
Patient demographics and tumor characteristics
| Characteristics | Number (%) |
|---|---|
| Age (years) | 60.11 ± 9.62 |
| Gender | |
| Male | 96/209 (45.9%) |
| Female | 113/209 (54.1%) |
| Family tumor history | |
| None | 194/209 (92.8%) |
| Lung cancer | 9/209 (4.3%) |
| Gastrointestinal cancer | 3/209 (1.4%) |
| Other† | 3/209 (1.4%) |
| Clinical symptoms | |
| Asymptomatic | 75/209 (35.9%) |
| Symptomatic | 134/209 (64.1%) |
| Lobe | |
| RUL | 73/209 (34.9%) |
| ML | 19/209 (9.1%) |
| RLL | 38/209 (18.2%) |
| LUL | 45/209 (21.5%) |
| LLL | 34/209 (16.3%) |
| TNM stage | |
| IA | 163/209 (77.9%) |
| IB | 8/209 (3.8%) |
| IIA | 30/209 (14.4%) |
| IIB | 8/209 (3.8%) |
| Surgical method | |
| VATS | 140/209 (67.0%) |
| Conventional thoracotomy | 45/209 (21.5%) |
| Da Vinci surgical robotic system | 24/209 (11.5%) |
| Operation selection | |
| Lobectomy | 181/209 (86.6%) |
| Segmentectomy | 6/209 (2.9%) |
| Wedge resection | 22/209 (10.5%) |
| Histologic subtype | |
| Lepidic | 38/209 (18.2%) |
| Acinar | 113/209 (54.1%) |
| Papillary | 36/209 (17.2%) |
| Micropapillary | 1/209 (0.5%) |
| Solid | 21/209 (10.0%) |
| EGFR status | |
| EGFR+ | 126/209 (60.3%) |
| L858R | 67/126 (53.2%) |
| 19 deletion | 50/126 (39.6%) |
| L858R/T790M | 1/126 (0.8%) |
| L858R /19 deletion | 1/126 (0.8%) |
| Exon21 L861Q | 2/126 (1.6%) |
| Exon18 G719X | 3/126 (2.4%) |
| Exon20 S768I | 2/126 (1.6%) |
| EGFR− | 83/209 (39.7%) |
Other includes bladder cancer, gynecological oncology. EGFR, epidermal growth factor receptor; LLL, left lower lobe; LUL, left upper lobe; ML, middle lobe; RLL, right lower lobe; RUL, right upper lobe; TNM, tumor node metastasis; VATS, video‐assisted thoracoscopic surgery.
Correlation of EGFR mutation status with clinical features
There were significant differences in gender, smoking status, pathologic grade, serum carcinoembryonic antigen (CEA) level, and histologic subtype between the EGFR wild type and EGFR mutant groups (Table 2). EGFR mutation rates were significantly higher in women than in men (76/113, 67.2% vs. 50/113, 52.1%, odds ratio [OR] 1.890, 95% confidence interval [CI] 1.078, 3.312; P = 0.025). Significantly more non‐smokers (110/160, 68.7%) harbored EGFR mutations than smokers (16/49, 32.6%, OR 4.537, 95% CI 2.289, 8.995; P < 0.001). EGFR mutations were also significantly more frequent in patients with intermediate (97/149, 65.1%) or low (23/38, 60.5%) pathologic grade (OR 4.974, 95% CI 1.836, 13.480; and OR 4.089, 95% CI 1.305, 12.807, respectively; P = 0.003). Patients with EGFR mutations were more likely to have lower serum CEA levels (3.75 ± 5.34 ng/ml) than patients with wild‐type EGFR (7.39 ± 15.59 ng/ml) (P = 0.021). The cut‐off value of 2.6 ng/ml for CEA level was determined by ROC analysis (Fig 1). The group of patients with a CEA level <2.6 ng/ml had a higher rate of EGFR mutation (OR 1.769, 95% CI 1.011, 3.096; P = 0.045). Considering tumor histology, EGFR mutations were most commonly found in papillary predominant subtypes (25/36, 69.4%, OR 5.682, 95% CI 1.741, 18.544; P = 0.014), followed by acinar (72/113, 63.7%, OR 4.390, 95% CI 1.581, 12.193) and lepidic (23/38, 60.5%, OR 3.833, 95% CI 1.215, 12.090). EGFR mutations were less frequently found in the solid predominant subtype (6/21, 28.6%). There were also no differences in stage distribution, differentiation, family tumor history, clinical symptoms or median age between EGFR mutant and wild‐type groups.
Table 2.
Association between clinical characteristics with EGFR mutation status
| Variable | All patients | EGFR mutation status | P | Univariate OR | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Number of patients | 209 | 126 (60.3%) | 83 (39.7%) | NA | NA |
| Median age | 60.11 ± 9.62 | 60.22 ± 9.42 | 60.02 ± 9.79 | 0.901 | NA |
| Gender | |||||
| Female | 113 | 76 | 37 | 0.025 | Reference |
| Male | 96 | 50 | 46 | 1.890 (1.078, 3.312) | |
| Smoking history | |||||
| Yes | 49 | 16 | 33 | <0.001 | Reference |
| No | 160 | 110 | 50 | 4.537 (2.289, 8.995) | |
| Histologic subtype† | |||||
| Lepidic‡ | 38 | 23 | 15 | 0.014 | 3.833 (1.215, 12.090) |
| Acinar | 113 | 72 | 41 | 4.390 (1.581, 12.193) | |
| Papillary | 36 | 25 | 11 | 5.682 (1.741, 18.544) | |
| Solid | 21 | 6 | 15 | NA | |
| Differentiation | |||||
| High | 50 | 30 | 20 | 0.324 | 2.250 (0.563, 8.996) |
| Intermediate | 100 | 64 | 36 | 2.667 (0.706, 10.077) | |
| Low | 10 | 4 | 6 | Reference | |
| Stage | |||||
| I | 171 | 107 | 64 | 0.152 | Reference |
| II | 38 | 19 | 19 | 0.598 (0. 295, 1.213) | |
| Pathologic grade | |||||
| High | 22 | 6 | 16 | 0.003 | Reference |
| Intermediate | 149 | 97 | 52 | 4.974 (1.836, 13.480) | |
| Low | 38 | 23 | 15 | 4.089 (1.305, 12.807) | |
| Clinical symptoms | |||||
| + | 75 | 48 | 27 | Reference | |
| − | 134 | 78 | 56 | 0.412 | 1.276 (0.712, 2.287) |
| Family tumor history | |||||
| Yes | 15 | 9 | 6 | 0.981 | Reference |
| No | 194 | 117 | 77 | 1.013 (0.347, 2.960) | |
| CEA level (ng/ml) | 5.15 ± 10.64 | 3.75 ± 5.34 | 7.39 ± 15.59 | 0.021 | NA |
| CEA (ng/ml) | |||||
| ≤2.6 | 111 | 74 | 37 | 0.045 | 1.769 (1.011, 3.096) |
| >2.6 | 98 | 52 | 46 | Reference | |
Histologic subtype was categorized according to the 2011 International Society for Lung Cancer Research/American Thoracic Society/European Respiratory Society classification system.
Histologic subtype was categorized as lepidic predominant adenocarcinoma (adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic predominant invasive adenocarcinoma) and other subtypes of dominant histologic findings (acinar, papillary, micropapillary, and solid predominant).
CEA, carcinoembryonic antigen; CI, confidence interval; EGFR, epidermal growth factor receptor; OR, odds ratio.
Figure 1.
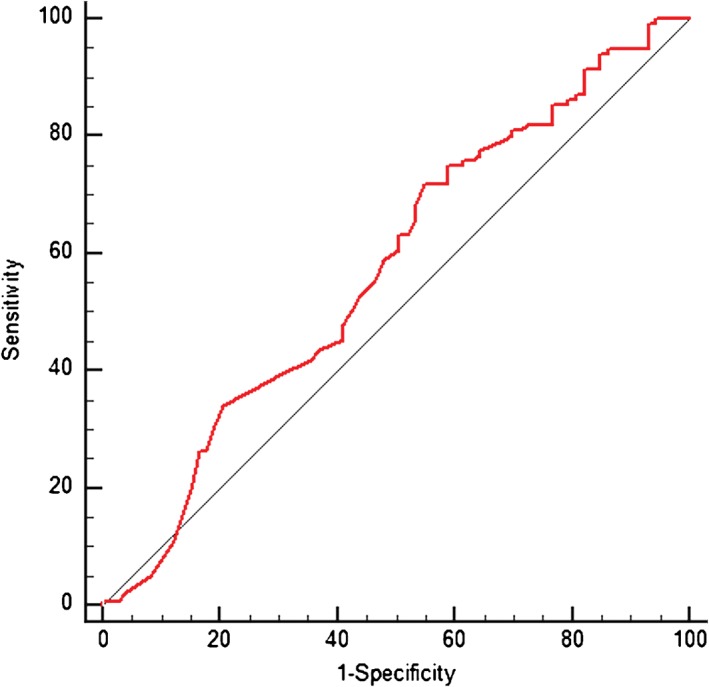
Receiver operating characteristic curve used to predict epidermal growth factor receptor mutation status (area under the curve 0.575; 95% confidence interval 0.501, 0.646; cut‐off value of 2.6 ng/ml for carcinoembryonic antigen level; sensitivity 63.25; specificity 49.32).
EGFR mutation and CT features
Computed tomography features of the lung adenocarcinomas according to EGFR mutation status are summarized in Table 3. No significant differences were observed in any of the studied CT features except the proportion of ground‐glass opacity (GGO), which was significantly higher in tumors with EGFR mutations than in EGFR wild type. When tumors were classified according to GGO proportion, EGFR mutations were significantly more frequent in tumors with GGO categorized as 0% < GGO ≤ 50% (OR 2.346, 95% CI 1.040, 5.292; P = 0.039). EGFR mutations were significantly more frequent in tumors with any GGO (0% < GGO ≤ 50% and 50% < GGO ≤ 100%) than in solid tumors (OR 2.607, 95% CI 0.888, 7.652; P < 0.039). There was a higher frequency of EGFR mutations in upper lobes compared with lower lobes (OR 1.670, 95% CI 1.008, 2.766; P < 0.046). In multivariate logistic regression analysis, tumors with any GGO were identified as an independent predictor of EGFR mutation (OR 2.746, 95% CI 1.101, 6.849; P = 0.030) (Table 4). CT images of GGOs and EGFR mutations are shown in Figure 2.
Table 3.
Association between CT characteristics and EGFR mutation status
| Variable | EGFR mutation status | P | Univariate OR | |
|---|---|---|---|---|
| Positive | Negative | |||
| Diameter (mm) | 19.35 ± 6.15 | 20.65 ± 7.33 | 0.215 | NA |
| Shape | ||||
| Round/oval | 78 | 55 | 0.428 | Reference |
| Irregular | 25 | 13 | 1.356 (0.638, 2.882) | |
| Border definition | ||||
| Well defined | 57 | 46 | 0.108 | Reference |
| Poorly defined | 46 | 22 | 1.687 (0.890, 3.199) | |
| Margins | ||||
| Smooth | 38 | 22 | 0.543 | Reference |
| Lobulated/spiculated | 65 | 46 | 0.818 (0.428, 1.562) | |
| Cavitation/bubble‐like lucency | ||||
| + | 25 | 16 | 0.911 | 1.042 (0.508, 2.138) |
| − | 78 | 52 | Reference | |
| Air bronchogram† | ||||
| + | 37 | 19 | 0.276 | 1.466 (0.743, 2.812) |
| − | 66 | 49 | Reference | |
| Thickening of the adjacent pleura | ||||
| + | 15 | 15 | 0.207 | 0.602 (0.273, 1.331) |
| − | 88 | 53 | Reference | |
| Pleural retraction | ||||
| + | 55 | 29 | 0.169 | 1.541 (0.831, 2.856) |
| − | 48 | 39 | Reference | |
| Vascular convergence | ||||
| + | 17 | 11 | 0.955 | 1.024 (0.447, 2.347) |
| − | 86 | 57 | Reference | |
| Lymphadenopathy | ||||
| + | 15 | 8 | 0.600 | 1.278 (0.510, 3.204) |
| − | 88 | 60 | Reference | |
| GGO proportion | ||||
| GGO negative | 61 | 53 | 0.039 | Reference |
| 0% < GGO ≤ 50% | 27 | 10 | — | 2.346 (1.040, 5.292) |
| 50% < GGO ≤ 100% | 15 | 5 | — | 2.607 (0.888, 7.652) |
| GGO presence | ||||
| GGO negative | 61 | 53 | 0.011 | Reference |
| Any GGO | 42 | 15 | 2.433 (1.214, 4.875) | |
| Lobe | ||||
| Upper lobes | 90 | 47 | 0.028 | 1.915 (1.071, 3.424) |
| Lower lobes | 36 | 36 | Reference | |
+ Air bronchogram present, − air bronchogram absent.
CI, confidence interval; CT, computed tomography; EGFR, epidermal growth factor receptor; GGO, ground‐glass opacity; NA, not applicable; OR, odds ratio.
Table 4.
Multivariable logistic regression analyses of CT features predicting the presence of EGFR mutation in lung adenocarcinoma
| Variable | P | Odds ratio | 95% CI |
|---|---|---|---|
| Shape | |||
| Round/oval | — | Reference | NA |
| Irregular | 0.807 | 1.3 | 0.366, 2.187 |
| Border definition | |||
| Well defined | — | Reference | NA |
| Poorly defined | 0.865 | 1.083 | 0.434, 2.700 |
| Margins | |||
| Smooth | — | Reference | NA |
| Lobulated/spiculated | 0.838 | 1.091 | 0.472, 2.520 |
| Cavitation/bubble‐like lucency | |||
| + | 0.843 | 1.008 | 0.474, 2.496 |
| − | — | Reference | NA |
| Air bronchogram | |||
| + | 0.260 | 1.584 | 0.711, 3.528 |
| − | — | Reference | NA |
| Thickening of the adjacent pleura | |||
| + | 0.443 | 0.715 | 0.303, 1.687 |
| − | — | Reference | NA |
| Pleural retraction | |||
| + | 0.176 | 1.607 | 0.809, 3.192 |
| − | — | Reference | NA |
| Vascular convergence | |||
| + | 0.771 | 1.145 | 0.461, 2.841 |
| − | — | Reference | NA |
| Lymphadenopathy | |||
| + | 0.298 | 1.696 | 0.627, 4.590 |
| − | — | Reference | NA |
| GGO proportion | |||
| GGO negative | — | Reference | NA |
| Any GGO | 0.030 | 2.746 | 1.101, 6.849 |
| Lobe | |||
| Upper lobes | 0.096 | 1.806 | 0.900, 3.624 |
| Lower lobes | — | Reference | NA |
CI, confidence interval; CT, computed tomography; EGFR, epidermal growth factor receptor; GGO, ground‐glass opacity; NA, not applicable.
Figure 2.
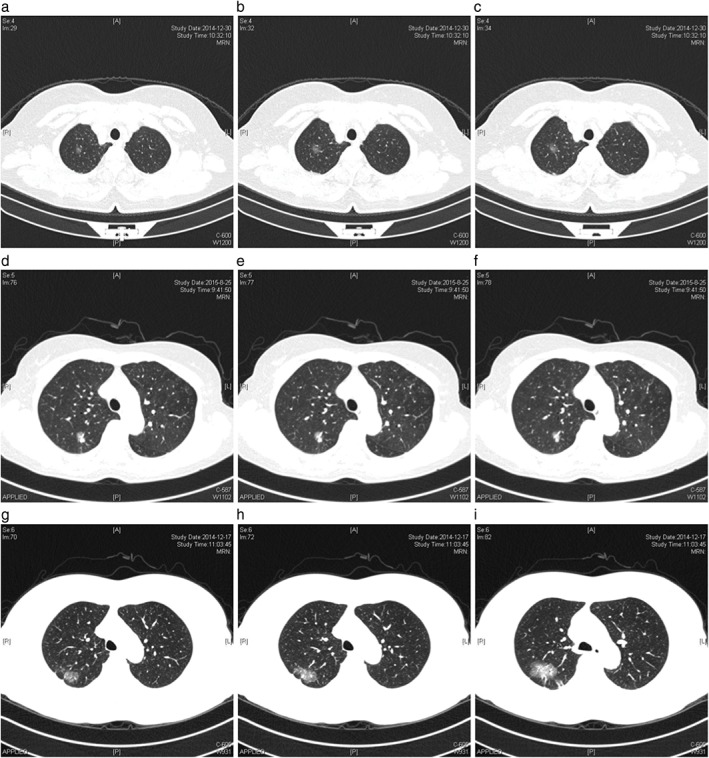
Chest computed tomography images of patients with ground glass opacity. (a–c) Patient #1 with lepidic predominant subtype and L858R mutation; (d–f) patient #2 with acinar predominant subtype and L858R mutation; (g–i) patient #3 with acinar predominant subtype and 19 deletion mutation.
Multivariable analyses of prognostic factors for EGFR mutation and receiver operating characteristic curve analysis
To construct a model with both clinical variables and CT features, four clinical features (gender, smoking history, histologic subtype, and CEA) found to be statistically significant in univariate analysis were kept in the model (Table 5). The most significant independent prognostic factors in the multivariable logistic regression analysis for harboring an EGFR mutation were: never‐smokers (OR 4.039, 95% CI, 1.572, 10.377; P = 0.004), tumors with GGO (OR 2.731, 95% CI 1.147, 6.503; P = 0.023), and acinar (OR 5.110, 95% CI 1.430, 18.256; P = 0.012) or papillary (OR 5.227, 95% CI 1.223, 22.333; P = 0.026) predominant adenocarcinomas. The multiple logistic regression model produced from both clinical and radiological features showed a predictive power of 0.737 (95% CI 0.661, 0.814) for identifying EGFR mutant status by ROC analysis (Fig 3).
Table 5.
Multivariable logistic regression analyses of CT features combined with clinical variables predicting the presence of EGFR mutation in lung adenocarcinoma
| Variable | P | Odds ratio | 95% CI |
|---|---|---|---|
| Gender | |||
| Female | — | Reference | NA |
| Male | 0.648 | 1.207 | 0.538, 2.710 |
| Smoking | |||
| Yes | — | Reference | NA |
| No | 0.004 | 4.039 | 1.572, 10.377 |
| Histologic subtype | |||
| Lepidic | 0.266 | 2.279 | 0.533, 9.744 |
| Acinar | 0.012 | 5.110 | 1.430, 18.256 |
| Papillary | 0.026 | 5.227 | 1.223, 22.333 |
| Solid | — | Reference | NA |
| CEA | |||
| ≤2.6 | 0.318 | 1.434 | 0.706, 2.913 |
| >2.6 | — | Reference | NA |
| GGO proportion | |||
| GGO negative | — | Reference | NA |
| Any GGO | 0.023 | 2.731 | 1.147, 6.503 |
CI, confidence interval; CEA, carcinoembryonic antigen; CT, computed tomography; EGFR, epidermal growth factor receptor; GGO, ground‐glass opacity; NA, not applicable.
Figure 3.
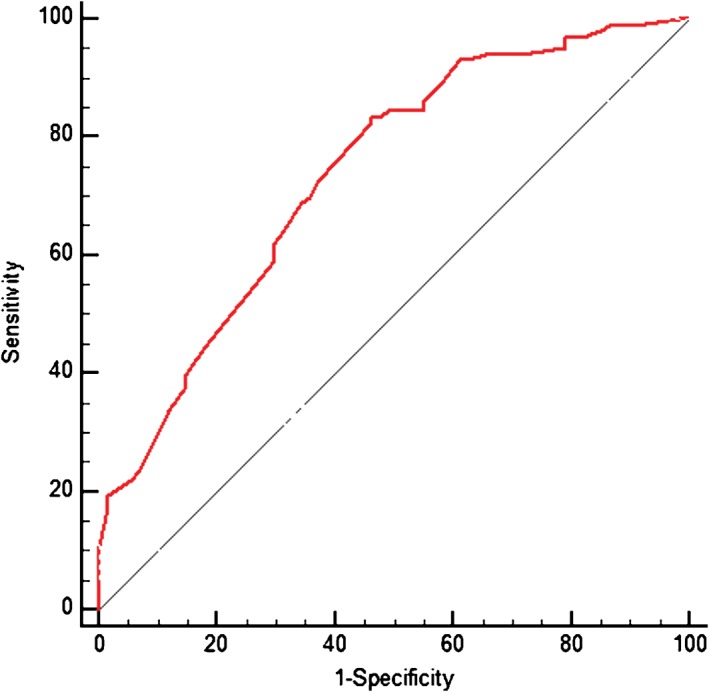
Predicting epidermal growth factor receptor mutation status with clinical variables and computed tomography features by receiver operating characteristic curve (area under the curve 0.737; 95% confidence interval 0.661, 0.814).
Differences in CT features between 19 deletion and L858R EGFR mutations
Patients with 19 deletion and L858R EGFR mutations had statistically similar tumor size, shape, border, thickening of the adjacent pleura, pleural retraction, vascular convergence, lymphadenopathy, degree of enhancement, presence or absence of air‐bronchogram, speculated/lobulated, and cavitation/bubble‐like lucency on CT scan (Table 6). The GGO proportion in tumors with L858R mutation was also similar to tumors with 19 deletion mutation (P = 0.866).
Table 6.
Comparison of EGFR exon mutations based on CT findings
| Variable | EGFR mutation status | P | |
|---|---|---|---|
| 19 deletion (n = 41) | L858R (n = 54) | ||
| Diameter (mm) | 19.46 ± 5.61 | 19.51 ± 6.43 | 0.965 |
| Shape | |||
| Round/oval | 8 | 16 | 0.261 |
| Irregular | 33 | 38 | |
| Border definition | |||
| Well defined | 23 | 29 | 0.816 |
| Poorly defined | 18 | 25 | |
| Margins | |||
| Smooth | 12 | 25 | 0.092 |
| Lobulated/spiculated | 29 | 29 | |
| Cavitation/bubble‐like lucency | |||
| + | 10 | 13 | 0.972 |
| − | 31 | 41 | |
| Air bronchogram | |||
| + | 12 | 21 | 0.329 |
| − | 29 | 33 | |
| Thickening of the adjacent pleura | |||
| + | 5 | 8 | 0.713 |
| − | 36 | 46 | |
| Pleural retraction | |||
| + | 25 | 26 | 0.214 |
| − | 16 | 28 | |
| Vascular convergence | |||
| + | 8 | 7 | 0.386 |
| − | 33 | 47 | |
| Lymphadenopathy | |||
| + | 8 | 6 | 0.253 |
| − | 33 | 48 | |
| GGO proportion | |||
| GGO negative | 25 | 32 | 0.866 |
| Any GGO | 16 | 22 | |
| Lobe | |||
| Upper lobes | 33 | 51 | 0.229 |
| Lower lobes | 17 | 16 | |
CT, computed tomography; EGFR, epidermal growth factor receptor; GGO, ground‐glass opacity.
Discussion
Lung cancer is the leading cause of cancer death worldwide. In recent years, EGFR‐TKI therapy has significantly delayed disease progression in patients with EGFR mutations and as a result, TKIs are now considered front‐line therapy for patients with advanced adenocarcinoma harboring EGFR mutations.12 Detecting EGFR mutations in lung adenocarcinomas is therefore important for determining treatment strategy. Unfortunately, EGFR mutation status cannot always be examined in patients because of inoperability, insufficient pathological material or the cost of the molecular examination. Previous studies have reported that EGFR mutations are more often observed in adenocarcinomas, particularly among female patients and in Asian populations.13 Our study investigated the association of EGFR status with a comprehensive set of clinical characteristics and imaging features in peripheral small lung adenocarcinoma. We found a significant correlation between EGFR mutation status and papillary predominant histological subtype. Moreover, there was a significant association between never‐smokers and EGFR mutation. The presence of GGO in tumors was the only significant CT feature predictive of EGFR mutation. In multivariable logistic regression analysis, the presence of GGO was closely related to EGFR mutation status.
Although EGFR mutations are frequently observed in never‐smoker females with invasive adenocarcinoma with a predominant lepidic pattern, a significant percentage have also been noted in acinar and papillary variants of adenocarcinoma.14, 15, 16, 17, 18 Few studies have reported correlations between the predominant subtype in lung adenocarcinomas and EGFR mutations. Liu et al. examined 385 surgically resected lung adenocarcinomas in Chinese patients and found that EGFR mutations occurred significantly more frequently in lepidic predominant subtypes.10 Song et al. reported that EGFR mutations occurred significantly more frequently in micropapillary and lepidic predominant subtypes and were less common in the solid predominant subtype.19 Villa et al. found that the lepidic predominant subtype was more common in EGFR‐mutant lung cancers compared with acinar in EGFR wild‐type lung cancers.18 In a cohort of 69 surgical resection patients with stage III (N2) lung cancer, Russell et al. showed that EGFR mutations were associated with acinar and micropapillary predominant tumors.20 Previous research has also reported that EGFR exon 21 mutations are commonly associated with lepidic predominant adenocarcinomas and EGFR exon 20 mutations with solid histology.14, 21 Our results indicate that EGFR mutations are associated with a higher frequency of papillary and acinar predominant subtypes, and are uncommon in the solid predominant subtype. The discrepancy in outcome between previous literature and our results regarding EGFR mutations and histologic subtypes may be related to the study sample size and the distribution of histologic type. Conflicting results may also be attributed to differences in ethnicity of the study population and the diagnostic procedures that were studied.
Several studies have explored the association between GGO on CT and EGFR‐mutated lung cancer.11, 14, 22, 23, 24, 25, 26 Glynn et al. investigated the association of imaging characteristics with EGFR and KRAS mutations in patients with lung adenocarcinoma with bronchoalveolar carcinoma (BAC) features.23 The presence of GGO on CT scan was not significantly associated with EGFR mutation (P = 0.44). Hsu et al. explored EGFR mutation status with different image patterns in a cohort of 162 patients with stage I lung adenocarcinoma with tumor lesions <3 cm, and EGFR mutation was detected less frequently in pure GGO lesions than in lesions with a solid component, especially L858R.11 A higher incidence of EGFR mutation occurs in invasive adenocarcinomas, such as tumors with part‐solid and solid patterns. In contrast, Lee et al. reported that the percentage of the GGO component on CT scan was significantly higher in lepidic predominant adenocarcinoma, which contains a higher frequency of exon 21 missense mutations compared with exon 19 mutations.14 Hong et al. also found that the GGO proportion in adenocarcinomas with EGFR mutation was significantly higher than in EGFR wild‐type tumors, and their results showed that exon 19 deletion was the most common EGFR mutation in lepidic predominant adenocarcinomas, while no difference in GGO proportion was observed between tumors with exon 19 and 21 mutations.24
We found that GGO was an independent predictor of EGFR mutation and that the GGO proportion was similar in L858R and 19 deletion mutations (P = 0.866) (Table 6). Hsu et al. also focused on the correlation between image patterns and EGFR mutation in stage I lung adenocarcinoma, but reported that EGFR mutations were detected less frequently in pure GGO lesions than in lesions with a solid component, especially L858R.11 Glynn et al. also reported that GGO on CT imaging was not significantly associated with the presence of EGFR mutation, and there was no characteristic CT feature that could predict EGFR mutation status.23 An explanation for the difference between our results and previous studies may lie in the fact that small peripheral adenocarcinoma or BAC may present with a high ratio of GGO components on CT scans, and EGFR mutations are less frequently detected in atypical adenomatous hyperplasia (AAH) and BAC lesions compared with invasive adenocarcinoma.6, 27, 28 In the new IASLC/ATS/ERS classification guidelines, AIS and MIA were proposed as substitutes for BAC to define non‐invasive adenocarcinomas. Glynn et al. used a relatively small sample and we assume that the histological type was mainly BAC.23 Hsu et al. did not provide detailed information of the histologic subtypes of their study population, but reported that a pure GGO pattern tended to be correlated with tumors < 2 cm with less typical EGFR mutation, while AIS/MIA tend to appear radiologically as pure GGO.11 The histological subtypes in our study population mainly consisted of invasive adenocarcinoma rather than AIS or MIA, and EGFR mutations are less frequently observed in non‐invasive lesions (AIS/MIA) compared with invasive adenocarcinoma, which may lead to the different results.
Our study is different from previous publications studying the relationship between radiogenomics and lung adenocarcinomas with EGFR mutation.8, 9, 10, 11, 23 Firstly, we focused mainly on peripheral small lung adenocarcinoma <3 cm, and most of our patients were stage I (171/209, 81.8%). Secondly, the histological subtype in our study population was invasive adenocarcinoma, which was further classified as low to intermediate (lepidic, acinar, and papillary) and high growth patterns, such as solid or micropapillary components. Therefore, our study population may present a more accurate example of histological subtypes of invasive adenocarcinomas and their imaging features, according to the new the IASLC/ATS/ERS guidelines. We evaluated 209 cases of consecutive patients with surgically resected lung adenocarcinomas and EGFR mutation who did not undergo preoperative chemotherapy intervention, which may give a more precise picture of the correlation between radiogenomics and EGFR mutation status in lung adenocarcinoma.
However, there are still a number of limitations to our study. Firstly, the final study population was considerably smaller than the initial identified group because preoperative imaging for many patients was not available at our institution. Secondly, CT images were interpreted by consensus, and inter‐observer variability was not assessed. Thirdly, the maximum one‐dimensional diameter on CT images was used to estimate the GGO proportion rather than using a two‐dimensional measurement or dedicated software for volumetric estimation of the GGO component. This measurement strategy was chosen because it is faster and easier to implement in daily clinical practice. Fourthly, we did not check for KRAS mutations, and it has been reported that EGFR‐TKI therapy is unsuitable for such mutations.29, 30 Further studies are necessary to elucidate this issue. Finally, the correlation between CT imaging and progression‐free and overall survival was not addressed.
In conclusion, in stage I/II lung adenocarcinoma with tumor size <3 cm, the GGO proportion in adenocarcinomas with EGFR mutation was significantly higher than in adenocarcinomas without EGFR mutation. GGO proportion was identified as an independent predictor of positive EGFR mutation, and papillary predominant subtype has the highest EGFR mutation rate. Combined CT findings and clinical features, which include never‐smoking, may be helpful for determining the presence of EGFR mutations in patients with peripheral small lung adenocarcinoma, particularly in patients whose mutational profiling is not available or not possible.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1 Definition of computed tomography descriptors.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81602015, No. 81602636, No. 81401903, No. 81572937, No. 81572273) and the Natural Science Foundation of Jiangsu province (No. BK20161386, No. BK20140736).
Contributor Information
Yong Song, Email: yong_song6310@yahoo.com.
Hongbing Liu, Email: netlhb@126.com.
References
- 1. Fan TW, Lane AN, Higashi RM, Bousamra M II, Kloecker G, Miller DM. Metabolic profiling identifies lung tumor responsiveness to erlotinib. Exp Mol Pathol 2009; 87: 83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jorge SE, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: Preclinical and clinical data. Braz J Med Biol Res 2014; 47: 929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early‐stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013; 145: 75–81. [DOI] [PubMed] [Google Scholar]
- 4. Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Risk factors for tumor recurrence in patients with early‐stage (stage I and II) non‐small cell lung cancer: Patient selection criteria for adjuvant chemotherapy according to the seventh edition TNM classification. Chest 2011; 140: 1494–502. [DOI] [PubMed] [Google Scholar]
- 5. Rosell R, Moran T, Queralt C et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361: 958–67. [DOI] [PubMed] [Google Scholar]
- 6. Dai J, Shi J, Soodeen‐Lalloo AK et al. Air bronchogram: A potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer 2016; 98: 22–8. [DOI] [PubMed] [Google Scholar]
- 7. Gevaert O, Xu J, Hoang CD et al. Non‐small cell lung cancer: Identifying prognostic imaging biomarkers by leveraging public gene expression microarray data‐‐methods and preliminary results. Radiology 2012; 264: 387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasegawa M, Sakai F, Ishikawa R, Kimura F, Ishida H, Kobayashi K. CT features of epidermal growth factor receptor‐mutated adenocarcinoma of the lung: Comparison with nonmutated adenocarcinoma. J Thorac Oncol 2016; 11: 819–26. [DOI] [PubMed] [Google Scholar]
- 9. Hsu JS, Huang MS, Chen CY et al. Correlation between EGFR mutation status and computed tomography features in patients with advanced pulmonary adenocarcinoma. J Thorac Imaging 2014; 29: 357–63. [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Kim J, Qu F et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology 2016; 280: 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu KH, Chen KC, Yang TY et al. Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol 2011; 6: 1066–72. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Kang S, Fang W et al. Impact of smoking status on EGFR‐TKI efficacy for advanced non‐small‐cell lung cancer in EGFR mutants: A meta‐analysis. Clin Lung Cancer 2015; 16: 144–51.e1. [DOI] [PubMed] [Google Scholar]
- 13. Pao W, Miller VA. Epidermal growth factor receptor mutations, small‐molecule kinase inhibitors, and non‐small‐cell lung cancer: Current knowledge and future directions. J Clin Oncol 2005; 23: 2556–68. [DOI] [PubMed] [Google Scholar]
- 14. Lee HJ, Kim YT, Kang CH et al. Epidermal growth factor receptor mutation in lung adenocarcinomas: Relationship with CT characteristics and histologic subtypes. Radiology 2013; 268: 254–64. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura H, Saji H, Shinmyo T et al. Association of IASLC/ATS/ERS histologic subtypes of lung adenocarcinoma with epidermal growth factor receptor mutations in 320 resected cases. Clin Lung Cancer 2015; 16: 209–15. [DOI] [PubMed] [Google Scholar]
- 16. Shim HS, Lee DH, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011; 135: 1329–34. [DOI] [PubMed] [Google Scholar]
- 17. Sun PL, Seol H, Lee HJ et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: Correlation with histologic subtypes, EGFR/TTF‐1 expressions, and clinical features. J Thorac Oncol 2012; 7: 323–30. [DOI] [PubMed] [Google Scholar]
- 18. Villa C, Cagle PT, Johnson M et al. Correlation of EGFR mutation status with predominant histologic subtype of adenocarcinoma according to the new lung adenocarcinoma classification of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society. Arch Pathol Lab Med 2014; 138: 1353–7. [DOI] [PubMed] [Google Scholar]
- 19. Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013; 30: 645. [DOI] [PubMed] [Google Scholar]
- 20. Russell PA, Barnett SA, Walkiewicz M et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013; 8: 461–8. [DOI] [PubMed] [Google Scholar]
- 21. Arcila ME, Nafa K, Chaft JE et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: Prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013; 12: 220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aoki T, Hanamiya M, Uramoto H, Hisaoka M, Yamashita Y, Korogi Y. Adenocarcinomas with predominant ground‐glass opacity: Correlation of morphology and molecular biomarkers. Radiology 2012; 264: 590–6. [DOI] [PubMed] [Google Scholar]
- 23. Glynn C, Zakowski MF, Ginsberg MS. Are there imaging characteristics associated with epidermal growth factor receptor and KRAS mutations in patients with adenocarcinoma of the lung with bronchioloalveolar features? J Thorac Oncol 2010; 5: 344–8. [DOI] [PubMed] [Google Scholar]
- 24. Hong SJ, Kim TJ, Choi YW, Park JS, Chung JH, Lee KW. Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: Imaging features and histological subtypes. Eur Radiol 2016; 26: 3660–8. [DOI] [PubMed] [Google Scholar]
- 25. Sugano M, Shimizu K, Nakano T et al. Correlation between computed tomography findings and epidermal growth factor receptor and KRAS gene mutations in patients with pulmonary adenocarcinoma. Oncol Rep 2011; 26: 1205–11. [DOI] [PubMed] [Google Scholar]
- 26. Yano M, Sasaki H, Kobayashi Y et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol 2006; 1: 413–6. [PubMed] [Google Scholar]
- 27. Soh J, Toyooka S, Ichihara S et al. Sequential molecular changes during multistage pathogenesis of small peripheral adenocarcinomas of the lung. J Thorac Oncol 2008; 3: 340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshida Y, Shibata T, Kokubu A et al. Mutations of the epidermal growth factor receptor gene in atypical adenomatous hyperplasia and bronchioloalveolar carcinoma of the lung. Lung Cancer 2005; 50: 1–8. [DOI] [PubMed] [Google Scholar]
- 29. Rotella V, Fornaro L, Vasile E et al. EGFR and K‐Ras mutations in women with lung adenocarcinoma: Implications for treatment strategy definition. J Exp Clin Cancer Res 2014; 33: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Zandwijk N, Mathy A, Boerrigter L et al. EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: Retro‐ and prospective observations in non‐small‐cell lung cancer. Ann Oncol 2007; 18: 99–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Definition of computed tomography descriptors.


