Abstract
Aims/Introduction
The purpose of the present study was to investigate the severity of glucose profiles and β‐cell function associated with diabetic cardiovascular autonomic neuropathy (DCAN) in a Chinese sample.
Materials and Methods
A community‐based, cross‐sectional study to analyze the risk factors of DCAN was carried out with 455 individuals recruited from a Chinese population. The glucose profile risk score was calculated to identify the association between the severity of the glucose profiles and DCAN. The associations of the severity of the glucose profiles and β‐cell function with DCAN were analyzed using multivariable logistic regression.
Results
Univariate analysis showed that the glucose profiles and homeostatic model assessment of insulin resistance were significantly associated with the DCAN outcome, respectively. Multivariable logistic regression showed that significant associations exist between glucose profile indices and DCAN, after controlling for potential confounding factors (P < 0.01 for all) in both models. Multivariable logistic regression also showed that parameters of β‐cell function were associated with the DCAN outcome in the category model (P < 0.1 for all). The glucose profile risk score was independently and significantly associated with the DCAN outcome after controlling for confounding factors (P < 0.001 and P for a trend <0.001).
Conclusions
Our observations suggest that parameters of glucose profile indices and β‐cell function are significantly and independently associated with DCAN, respectively. There was a tendency toward increased glucose profile risk score with increasing prevalence of DCAN.
Keywords: β‐Cell function, Diabetic cardiovascular autonomic neuropathy, Glucose profile
Introduction
Diabetes mellitus is a global health problem. The disease is characterized by high blood glucose, insulin resistance and relative insulin insufficiency. Fasting plasma glucose (FPG), plasma blood glucose (PBG) and hemoglobin A1c (HbA1c) are vital tests for the diagnosis of diabetes mellitus. These parameters are also useful for monitoring and controlling glucose levels. β‐Cell function contributes to regulating blood glucose, and it is necessary for calculating the homeostatic model assessment‐index (HOMA‐I). The accuracy and the precision of the HOMA methods were compared with independent estimates of insulin resistance1. In diabetic patients, the prevalence of cardiovascular autonomic neuropathy (CAN) was found to be 30–60%2. However, the significance of CAN has not been fully appreciated. Individuals with previously undiagnosed CAN dysfunction have an unfavorable cardiovascular risk profile, especially in terms of sudden death, indicating a higher risk of cardiovascular disease3, 4.
Glucose profile and β‐cell function are associated with common human diseases. Hyperglycemia and insulin resistance are major risk factors of diabetic distal sensorimotor polyneuropathy5. Poor glycemic control has been detected in CAN patients who have a high risk of cardiovascular disease and high rates of mortality6. Our earlier study showed that diabetes mellitus and insulin resistance are associated with CAN in a general Chinese population7, 8. In diabetic patients, our previous study investigated the associations of blood pressure profiles and their severity with diabetic cardiovascular autonomic neuropathy (DCAN) in a Chinese sample9. Additionally, the lipid profile and its severity associated with DCAN was also reported10. Other studies have shown that diabetes mellitus, duration of diabetes mellitus and poor blood glucose control are associated with the progression of DCAN in diabetes patients11, 12, 13.
However, these studies only focused on analyzing the association between separate risk factors and outcomes without addressing or systematically analyzing the association between glucose profiles and DCAN. β‐Cell function is strongly correlated with glucose profiles, and regulates FPG and PBG. The associations between β‐cell function and DCAN, and glucose profiles and DCAN should be investigated simultaneously. However, little is known about the association between β‐cell function and DCAN, let alone the association between the severity of glucose profile and β‐cell function in a Chinese population. It is important to clarify the relationship between glucose profiles and β‐cell function and DCAN in diabetes patients, as this information can be useful to clinicians in the prediction, prevention and treatment of DCAN. Thus, the present study aimed to estimate the extent to which the severity of glucose profiles and β‐cell function are associated with DCAN in a Chinese sample.
Methods
Study population
The present study is referred from the data and methods section of our previously published study9, 10, which is also based on the same survey data set and similar methodology. As previously mentioned9, 10, we carried out risk analysis for DCAN in a random sample of a Chinese population. Diabetic participants with undiagnosed DCAN, aged 30–80 years, were included in the present study. A total of 510 participants with diabetes were recruited to a screening visit between 2011 and 2013. As mentioned in our previous study9, exclusion criteria eliminated potential confounding factors to influence cardiovascular autonomic function to include, briefly, a history or findings of arrhythmia and hyperthyroidism or hypothyroidism, pregnancy or lactation, and/or serious hepatic or renal dysfunctions. Of these participants, 455 diabetic participants with complete clinical baseline data were included in this DCAN risk factor analysis.
Ethics statement
The present study was reviewed and approved by the ethics committee at the Fudan University Huashan Hospital and Shanghai Tongji Hospital. Permission to carry out the study was granted by the Fudan University Huashan Hospital and Shanghai Tongji Hospital. Written informed consent was obtained from all study participants. All procedures carried out in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Measurement and definition
As previously mentioned in an earlier study9, we interviewed participants to obtain documentation of demographic information and their medical histories. All participants underwent a complete clinical baseline evaluation, after an 8‐h fast. The demographic information, blood pressure profiles, glucose profiles, lipid profiles, renal function parameters and medical history were previously detailed in the earlier study9. For all analyses, the day‐to‐day and interassay coefficients of variation at Huashan Hospital's (Shanghai, China) central laboratory ranged between 1% and 3%. The homeostatic model assessment of insulin resistance (HOMA‐IR) estimate was calculated as FPG (mmol/L) multiplied by fasting blood insulin (FINS) (mU/L) divided by 22.5. The HOMA insulin sensitivity index (HOMA‐ISI) was calculated as 1/(FPG × FINS). The HOMA β‐cell function (HOMA‐β) was calculated as 20 × FINS/(FPG−3.5). The definition of hypertension (HTN), body mass index (BMI), diabetes mellitus and metabolic syndrome (MetS) was detailed earlier9, 14.
Study outcome
As mentioned in an earlier study9, short‐term HRV was used to evaluate cardiovascular autonomic function. Short‐term HRV analysis was carried out for all participants using a computer‐aided examination and evaluation system for spectral analysis to investigate changes in autonomic regulation. In the present study, CAN was diagnosed based on at least two abnormal cardiovascular autonomic reflex test results based on the short‐term HRV tests15, 16.
Statistical analysis
The results are described as mean ± standard deviation, unless stated otherwise. The between‐group differences in variables and in properties were accessed using t‐test and χ2 analysis, respectively. For data analysis, FPG was categorized by trinary variables (code 0: <6.5 mmol/L, code 1: 6.5–11.4 mmol/L and code 2: >11.4 mmol/L); PBG was categorized by binary variables (code 0: <11.4 mmol/L and code 1: ≥11.4 mmol/L); HbA1c was categorized by trinary variables (code 0: <6.5%, code 1: 6.5–9.0% and code 2: >9.0%); and diabetes mellitus duration (DMD) was categorized by code 0: <1 year, code 1: 1–9 years, code 2: 10–19 years and code 3: >19 years. FINS was categorized by trinary variables (<5 mU/L, 5–20 mU/L and >20 mU/L); HOMA‐IR was categorized by binary variables (code 0: <2.6 mmol/L × mU/L and code 1: ≥2.6 mmol/L × mU/L); HOMA‐IR was categorized by binary variables (code 0: <1.9 L/mmol × L/mU, code 1: 1.9–4.5 L/mmol × L/mU and code 2: >4.5 L/mmol × L/mU); and HOMA‐IR was categorized by binary variables (code 0: ≤50 nU/mmol and code 1: >50 mU/mmol). Difference analyses of the prevalence of DCAN among the glucose profile indices and the β‐cell function parameters with category variables were also carried out.
Univariate logistic regression for the glucose profile indices and the β‐cell function parameters with continuous variables was carried out to determine the variables associated with DCAN. The glucose profile risk score (GRS) was calculated to determine the associations between the severity of the glucose profiles and β‐cell function and DCAN. Multiple logistic regression (MLR) was carried out to detect independent associations of parameters of glucose profile and β‐cell function with the outcome, controlling for confounding factors. GRS was derived from the independent variable and their weights. First, a best‐fit model was used to include the significant independent variables generated from the MLR with stepwise methods. Additionally, the weight of each independent variable was determined by the coefficients in the best‐fit model. Finally, GRS was calculated by using the sum of the independent variable and its weights. Tests were two‐sided, and a P‐value of <0.05 was considered to be significant. For MLR analysis, a P‐value of <0.10 was also considered to be significant. The results were analyzed using Statistical Package for Social Sciences for Windows version 16.0 software (SPSS, Chicago, IL, USA).
Results
Clinical characteristics of the study participants
The baseline characteristics of diabetic participants were previously detailed in an earlier study9 and are listed in Table 1. The study samples included 208 men and 247 women. The mean FPG and PBG were 7.34 and 11.98 mmol/L in the total sample, respectively. The mean heart rate was 75.11 b.p.m., and no significant difference in this variable was reported between the two groups (P = 0.634). The low‐frequency and low‐frequency/high‐frequency values were significantly higher in men than in women (P = 0.042 for low frequency and P = 0.006 for low frequency/high frequency), respectively, whereas the high‐frequency values were lower in men (P = 0.046). In the total sample, the mean duration of diabetes mellitus and HTN was 5.24 years and 6.42 years, respectively; and the prevalence of HTN, MetS, and DCAN was 63.96%, 72.53% and 29.01%, respectively.
Table 1.
Clinical baseline characteristics of individuals
| Variable | Total sample | Male | Female | P‐value |
|---|---|---|---|---|
| Demographical information | ||||
| n | 455 | 208 | 247 | – |
| Age (years) | 62.80 ± 8.61 | 63.54 ± 8.84 | 62.17 ± 8.37 | 0.016 |
| Height (cm) | 162.12 ± 8.15 | 167.95 ± 6.33 | 157.20 ± 5.99 | <0.001 |
| Weight (kg) | 66.63 ± 11.65 | 71.05 ± 10.45 | 62.9 ± 11.30 | <0.001 |
| SBP (mmHg) | 134.30 ± 20.30 | 133.55 ± 19.02 | 134.95 ± 21.33 | 0.305 |
| DBP (mmHg) | 81.08 ± 10.12 | 80.93 ± 10.16 | 81.2 ± 10.10 | 0.690 |
| Glucose profile | ||||
| FPG (mmol/L) | 7.34 ± 2.69 | 7.61 ± 2.82 | 7.11 ± 2.56 | 0.006 |
| PBG (mmol/L) | 11.98 ± 4.42 | 12.07 ± 4.62 | 11.9 ± 4.25 | 0.583 |
| FINS (mU/L) | 10.45 ± 24.39 | 9.68 ± 24.23 | 11.09 ± 24.53 | 0.388 |
| Hba1c (%) | 7.17 ± 1.46 | 7.27 ± 1.54 | 7.11 ± 1.49 | 0.315 |
| Laboratory assay | ||||
| TC (mmol/L) | 5.38 ± 1.11 | 5.06 ± 1.07 | 5.64 ± 1.08 | <0.001 |
| TG (mmol/L) | 1.99 ± 1.18 | 1.99 ± 1.34 | 1.99 ± 1.03 | 0.961 |
| HDL (mmol/L) | 1.30 ± 0.31 | 1.19 ± 0.28 | 1.38 ± 0.29 | <0.001 |
| LDL (mmol/L) | 3.28 ± 0.85 | 3.14 ± 0.82 | 3.40 ± 0.86 | <0.001 |
| SCr (μmol/L) | 81.37 ± 24.04 | 90.93 ± 21.54 | 73.35 ± 23.10 | <0.001 |
| UA (μmol/L) | 298.09 ± 85.47 | 319.48 ± 89.66 | 280.17 ± 77.45 | <0.001 |
| HRV indices | ||||
| HR (b.p.m.) | 75.11 ± 10.41 | 75.29 ± 11.27 | 74.96 ± 9.63 | 0.634 |
| TP (ms2) | 747.3 ± 682.53 | 728.25 ± 734.89 | 763.34 ± 635.42 | 0.440 |
| LF (ms2) | 166.57 ± 225.93 | 183.19 ± 293.24 | 152.57 ± 145.95 | 0.042 |
| HF (ms2) | 152.15 ± 188.51 | 138.57 ± 182.1 | 163.58 ± 193.2 | 0.046 |
| LF/HF | 1.84 ± 2.12 | 2.05 ± 2.33 | 1.66 ± 1.91 | 0.006 |
| Medical history | ||||
| Smoking, yes (%) | 89 (19.56) | 85 (40.87) | 4 (1.62) | <0.001 |
| DMD (years) | 5.24 ± 6.45 | 5.73 ± 6.62 | 4.86 ± 6.29 | 0.063 |
| HTN, yes (%) | 291 (63.96) | 132 (63.46) | 159 (64.37) | 0.776 |
| HTND (years) | 6.42 ± 9.99 | 7.41 ± 10.96 | 5.62 ± 9.05 | 0.008 |
| MetS, yes (%) | 330 (72.53) | 143 (68.75) | 187 (75.71) | 0.019 |
| DCAN, yes (%) | 132 (29.01) | 65 (31.25) | 67 (27.13) | 0.172 |
DBP, diastolic blood pressure; DCAN, diabetic cardiovascular autonomic neuropathy; DMD, diabetes duration; FINS, fasting blood insulin; FPG, fasting plasma glucose; HDL, high‐density lipoprotein cholesterol; HF, high frequency; HR, heart rate; HTN, hypertension; HTND, hypertension duration; LDL, low‐density lipoprotein cholesterol; LF, low frequency; MetS, metabolic syndrome; PBG, plasma blood glucose; SBP, systolic blood pressure; SCr, serum creatinine; TC, serum total cholesterol; TG, triglyceride; TP, total power of variance.
Difference analysis of the DCAN prevalence among groups
There were significant differences in the DCAN prevalence among the three FPG groups (21.60% vs 33.33% vs 43.24%, P < 0.001 and P for trend <0.001; Figure 1a). Similarly, significant differences between the PBG groups were reported (18.18% vs 38.27%, P < 0.001; Figure 1b). The DCAN prevalence was 19.54% vs 32.50% vs 47.61% in the three HbA1c groups, respectively. Significant differences among the three groups were reported (P < 0.001 and P for trend <0.001; Figure 1c). Additionally, there was a tendency toward increased duration of diabetes mellitus with increasing DCAN prevalence (18.18% vs 26.34% vs 36.25% vs 51.72%, P < 0.001 and P for a trend <0.001; Figure 1d).
Figure 1.
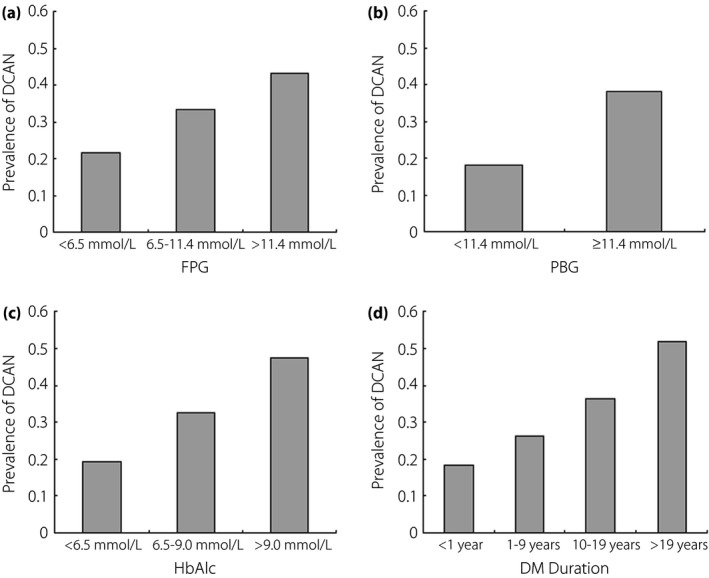
Comparison of the prevalence of diabetic cardiovascular autonomic neuropathy (DCAN) according to glucose profile parameters. (a) Comparison of DCAN prevalence according to fasting plasma glucose (FPG). DCAN prevalence was 21.60%, 33.33% and 43.24% in the three groups, respectively. Significant differences among the three groups were reported (P < 0.001 and P for a trend <0.001). (b) Comparison of DCAN prevalence according to plasma blood glucose (PBG). DCAN prevalence was 18.18% and 38.27% in the two groups, respectively. A significant difference between the two groups was reported (P < 0.001). (c) Comparison of DCAN prevalence according to hemoglobin A1c (HbA1c). DCAN prevalence was 19.54%, 32.50% and 47.61% in the three groups, respectively. Significant differences among the three groups were reported (P < 0.001 and P for a trend <0.001). (d) Comparison of DCAN prevalence according to DMD. DCAN prevalence was 18.18%, 26.34%, 36.25% and 51.72% in the four groups, respectively. Significant differences between the two groups were reported (P < 0.001 and P for a trend <0.001).
Among groups according to FINS, significant differences in the DCAN prevalence were reported (32.33% vs 26.01% vs 52.17%, P < 0.001 and P for a trend <0.001; Figure 2a). For the next data analysis, FINS was coded using code 1: <5 mU/L, code 0: 5–20 mU/L and code 2: >20 mU/L. The DCAN prevalence was 24.39% and 38.25% in the two HOMA‐IR groups, respectively. Significant differences between the two groups were reported (P < 0.001; Figure 2b). Significant differences in the DCAN prevalence among the three HOMA‐ISI groups were also reported (34.49% vs 25.92% vs 20.75%, P = 0.005 and P for a trend = 0.001; Figure 2c). However, no significant differences between the two HOMA‐β groups were reported (28.31% vs 32.86%, P = 0.276; Figure 2d).
Figure 2.
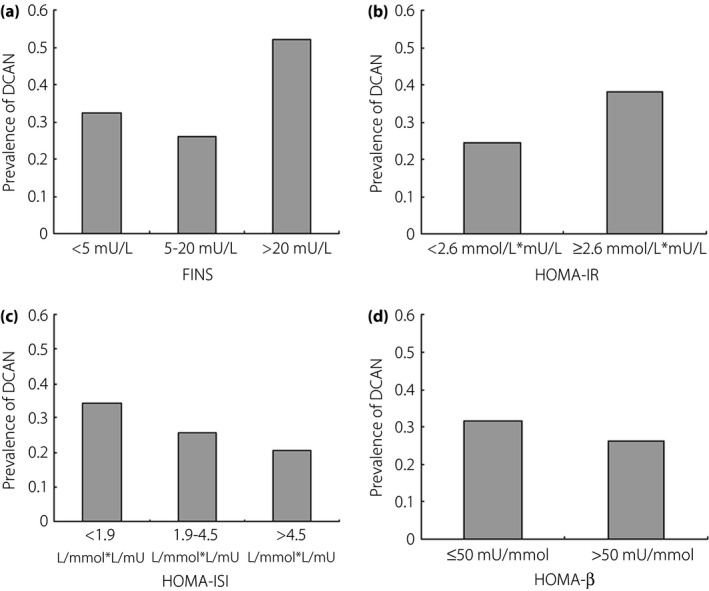
Comparison of prevalence of diabetic cardiovascular autonomic neuropathy (DCAN) according to β‐cell function parameters. (a) Comparison of DCAN prevalence according to fasting insulin resistance (FINS). DCAN prevalence was 32.33%, 26.01% and 52.17% in the three groups, respectively. Significant differences among the three groups were reported (P < 0.001 and P for a trend <0.001). (b) Comparison of DCAN prevalence according to homeostasis model assessment of insulin resistance (HOMA‐IR). DCAN prevalence was 24.39% and 38.25% in the two groups, respectively. Significant differences between the two groups were reported (P < 0.001). (c) Comparison of DCAN prevalence according to homeostasis model assessment of insulin sensitivity index (HOMA‐ISI). DCAN prevalence was 34.49%, 25.92% and 20.75% in the three groups, respectively. There were significant differences among the three groups (P = 0.005 and P for a trend = 0.001). (d) Comparison of DCAN prevalence according to homeostasis model assessment of β –cell function (HOMA‐β). DCAN prevalence was 31.45% and 26.31% in the two groups, respectively. There were no significant differences between the two groups (P = 0.098).
Analysis of the association between the glucose profile indices and DCAN
Similar to the earlier study9, univariate logistic regression models were developed to include glucose profiles, β‐cell function, age, sex, BMI, lipid profiles, renal function and medical history (Table 2). The univariate logistic regression showed that the glucose profiles and HOMA‐IR, age, BMI, triglycerides, hypertension duration, DMD and MetS were significantly associated with DCAN (P < 0.05 for all); however, there were no significant associations between HOMA‐ISI, HOMA‐β, the continuous variables and DCAN (P > 0.05 for all).
Table 2.
Univariate analysis to include independent variables for diabetic cardiovascular autonomic neuropathy
| Variable | β | SE | P‐value | OR | 95% CI |
|---|---|---|---|---|---|
| Glucose profile | |||||
| FPG | 0.098 | 0.026 | <0.001 | 1.103 | 1.048–1.161 |
| PBG | 0.081 | 0.017 | <0.001 | 1.084 | 1.049–1.121 |
| FINS | 0.006 | 0.003 | 0.031 | 1.006 | 1.001–1.012 |
| HbA1c | 0.033 | 0.012 | 0.009 | 1.033 | 1.008–1.058 |
| DMD | 0.031 | 0.012 | 0.010 | 1.031 | 1.007–1.056 |
| HOMA‐IR | 0.033 | 0.012 | 0.009 | 1.033 | 1.008–1058 |
| HOMA‐ISI | −3.088 | 2.738 | 0.259 | 0.046 | 0.001–9.758 |
| HOMA‐β | 0.001 | 0.000 | 0.249 | 1.000 | 1.000–1.001 |
| Covariance | |||||
| Age | 0.035 | 0.009 | <0.001 | 1.036 | 1.018–1.054 |
| Sex | 0.20 | 0.146 | 0.172 | 1.221 | 0.917–1.627 |
| BMI | 0.029 | 0.012 | 0.043 | 1.03 | 1.010–1.070 |
| SBP | 0.004 | 0.004 | 0.242 | 1.004 | 0.997–1.011 |
| DBP | 0.002 | 0.007 | 0.806 | 1.002 | 0.988–1.016 |
| TC | 0.067 | 0.065 | 0.308 | 1.069 | 0.940–1.215 |
| TG | 0.257 | 0.060 | <0.001 | 1.293 | 1.150–1.455 |
| HDL | −0.231 | 0.243 | 0.340 | 0.793 | 0.493–1.277 |
| LDL | −0.04 | 0.086 | 0.645 | 0.961 | 0.811–1.138 |
| SCr | 0.005 | 0.003 | 0.073 | 1.005 | 1.000–1.011 |
| HR | 0.091 | 0.009 | <0.001 | 1.095 | 1.076–1.114 |
| Smoking | 0.210 | 0.180 | 0.242 | 1.234 | 0.868–1.756 |
| HTN | 0.12 | 0.153 | 0.433 | 1.128 | 0.835–1.523 |
| HTND | 0.014 | 0.007 | 0.050 | 1.014 | 1.000–1.028 |
| MetS | 0.527 | 0.175 | 0.003 | 1.694 | 1.202–2.387 |
CI, confidence interval; DBP, diastolic blood pressure; FINS, fasting blood insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein cholesterol; HOMA‐β, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance; HOMA‐ISI, homeostatic model assessment of insulin sensitivity index; HR, heart rate; HTN, hypertension; LDL, low‐density lipoprotein cholesterol; MetS, metabolic syndrome; OR, odds ratio; PBG, plasma blood glucose; SBP, systolic blood pressure; SCr, serum creatinine; SE, standard error; TC, serum total cholesterol; TG, triglyceride.
MLR controlling for potential confounding factors (age, sex, BMI, lipid profiles and medical history) was carried out on the glucose profiles. The results showed that there were significant associations between FPG, HbA1c, and DMD and DCAN in model 1 with the continuous variables, respectively (P < 0.05 for the three variables; Table 3). Furthermore, there were significant associations between all the glucose profile indices and DCAN in model 2 with the category variables, respectively (P < 0.05 for all).
Table 3.
Multiple variable analysis to include glucose profile parameters for diabetic cardiovascular autonomic neuropathy
| Model | Variable | β | SE | P‐value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | FPG | 0.111 | 0.042 | 0.008 | 1.118 | 1.030–1.213 |
| PBG | 0.041 | 0.027 | 0.127 | 1.042 | 0.988–1.098 | |
| HbA1c | 0.945 | 0.227 | <0.001 | 2.573 | 1.650–4.012 | |
| DMD | 0.046 | 0.014 | 0.001 | 1.047 | 1.019–1.076 | |
| Model 2 | FPG | 0.544 | 0.169 | 0.001 | 1.724 | 1.238–2.399 |
| PBG | 0.883 | 0.207 | <0.001 | 2.419 | 1.612–3.632 | |
| HbA1c | 0.931 | 0.226 | <0.001 | 2.538 | 1.631–3.949 | |
| DMD | 0.333 | 0.11 | 0.002 | 1.396 | 1.126–1.731 |
Model 1: independent variables with continuous variables. Model 2: independent variable with category variables. All models adjusted for age, sex, body mass index, blood pressure profiles, lipid profiles, hear rate, serum creatinine, uric acid and medical history. CI, confidence interval; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; LDL, low‐density lipoprotein cholesterol; MetS, metabolic syndrome; OR, odds ratio; PBG, plasma blood glucose; SE, standard error.
Analysis of the association between the β‐cell function parameters and DCAN
After confounding factors of age, sex, BMI, renal function and medical history, the results showed that there was no significant association between the β‐cell function parameters and DCAN, respectively (P > 0.05 for all, Table 4). In contrast, there were significant associations between of all the β‐cell function parameters and DCAN, respectively (P < 0.05 for all).
Table 4.
Multiple variable analysis to include β‐cell function parameters for diabetic cardiovascular autonomic neuropathy separately
| Model | Variable | β | SE | P‐value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | FINS | 0.001 | 0.003 | 0.982 | 1.001 | 0.993–1.007 |
| HOMA‐IR | 0.010 | 0.013 | 0.452 | 1.010 | 0.985–1.035 | |
| HOMA‐ISI | −0.034 | 0.055 | 0.538 | 0.967 | 0.868–1.077 | |
| HOMA‐β | 0.001 | 0.000 | 0.124 | 0.999 | 0.999–1.001 | |
| Model 2 | FINS | 0.401 | 0.159 | 0.011 | 1.494 | 1.095–2.039 |
| HOMA‐IR | 0.601 | 0.195 | 0.002 | 1.824 | 1.245–2.672 | |
| HOMA‐ISI | −0.279 | 0.144 | 0.053 | 0.756 | 0.571–1.004 | |
| HOMA‐β | −0.540 | 0.185 | 0.004 | 0.583 | 0.405–0.838 |
Model 1: independent variables with continuous variables. Model 2: independent variable with category variables. All models adjusted for age, sex, body mass index, blood pressure profiles, lipid profiles, heart rate, serum creatinine, uric acid and medical history. CI, confidence interval; FINS, fasting insulin resistance; HOMA‐IR, homeostasis model assessment of insulin resistance; HOMA‐ISI, homeostasis model assessment of insulin sensitivity index; OR, odds ratio.
Analysis of the association between the severity of GRS and DCAN
The best‐fit model was generated to include PBG, FINS and HOMA‐IR with category variables using MLR with stepwise methods (Table 5). The weights of the PBG, FINS and HOMA‐IR variables were calculated by dividing the regression coefficients (β) by a common factor (0.466) and rounding to the nearest integer. The GRS was derived from the formula: 2 × PBG + FINS + 2 × HOMA‐IR.
Table 5.
Multiple variable analysis to include glucose profile risk score for diabetic cardiovascular autonomic neuropathy
| Model | Variable | β | SE | P‐value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | PBG | 0.977 | 0.259 | <0.001 | 2.657 | 1.598–4.419 |
| FINS | 0.466 | 0.203 | 0.022 | 1.593 | 1.069–2.373 | |
| HOMA‐IR | 1.131 | 0.262 | <0.001 | 3.099 | 1.856–5.176 | |
| Model 2 | GRS | 0.443 | 0.064 | <0.001 | 1.558 | 1.375–1.764 |
All models adjusted for age, sex, body mass index, blood pressure profiles, lipid profile, heart rate, serum creatinine, uric acid and medical history. FINS, fasting insulin resistance; GRS, glucose profile risk score; HOMA‐IR, homeostasis model assessment of insulin resistance; PBG, plasma blood glucose.
There were significant differences among the six GRS groups (14.00% vs 21.43% vs 22.69% vs 38.67% vs 45.65% vs 61.54%, P < 0.001 and P for a trend <0.001; Figure 3a). The receiver operating characteristic analysis showed the area under the receiver operating characteristic curve was 0.671, 95% confidence interval 0.633–0.710, P < 0.001. MLR showed that there were significant associations between the severity of GRS and DCAN (P < 0.001, odds ratio 1.558, 95% confidence interval 1.375–1.764; Table 5).
Figure 3.
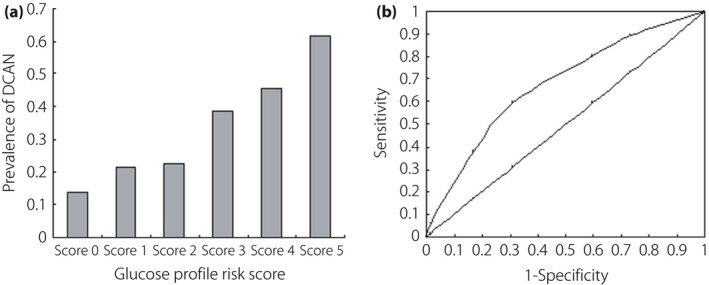
Comparison of prevalence of diabetic cardiovascular autonomic neuropathy (DCAN) according to glucose profile risk score (GRS) and its predictive performance analysis. (a) Comparison of DCAN prevalence according to glucose profile risk score. DCAN prevalence was 14.00%, 21.43%, 22.69%, 38.67%, 45.65% and 61.54% in the six groups, respectively. There were significant differences among these groups (P < 0.001 and P for a trend <0.001). (b) Receiver operating characteristic curves showed the performance of GRS in predicting prevalence of DCAN. Area under the curve 0.671, 95% confidence interval 0.633–0.710, P < 0.001.
Discussion
The associations of glucose profile indices and β‐cell function parameters with DCAN were analyzed among 455 diabetic participants in China. Importantly, in Chinese diabetic patients, we carried out predictive value analysis for DCAN using glucose profile indices and β‐cell function parameters. It is crucial to understand the predictive value of these two type factors on DCAN. This is partly because the prevalence of diabetes mellitus has increased rapidly in China. Clinicians can expect to treat more diabetes mellitus patients having DCAN progression. Furthermore, a better understanding of the predictive value of the two type factors (glucose profile and β‐cell function) will help clinicians prevent and treat DCAN.
An interesting finding was that both glucose profile and β‐cell function had a high value in predicting DCAN in a Chinese population. First, the DCAN prevalence dramatically increased in relation to decreased glucose profile control and increased the duration of diabetes mellitus. Significant differences in DCAN prevalence were also reported among the different HOMA‐IR groups and HOMA‐ISI groups. Furthermore, univariate analysis and association analysis for DCAN showed that the glucose profiles and HOMA‐IR, age, BMI, triglycerides, hypertension duration, DMD, and MetS were strongly and independently associated with DCAN. After adjusting for potential confounding factors, multivariable logistic regression showed that all of the glucose profile indices and all of the β‐cell function parameters were significantly and independently associated with DCAN. These results provide evidence that there is a good association between glucose profiles and β‐cell function and DCAN. Finally, the GRS, which was derived from the PBG, FINS and HOMA‐IR variables, was shown to be significantly associated with DCAN.
Major risk factors, including diabetes duration, hyperglycemia, age, hypertension, dyslipidemia, obesity, smoking, insulin resistance and hypoinsulinemia, have been found to contribute to the progression of DCAN. Suarez et al.12 observed that the reduction of HRV was greater in diabetic patients than in subjects with impaired fasting glycemia. In addition, another study observed that intensive glucose therapy significantly reduced the risk of diabetic peripheral neuropathy and CAN in type 1 diabetes mellitus17. HbA1c variability was independently associated with the presence of CAN in patients with inadequately controlled type 2 diabetes mellitus13. Perciaccante et al.18 carried out a study to explore the association between insulin resistance and sympathetic overactivity. Their results showed that sympathetic overactivity is directly correlated to the grade of insulin resistance calculated according to the HOMA‐I. Furthermore, the participants with type 2 diabetes mellitus had greater autonomic dysfunction than the insulin resistant participants in the normal glucose regulation, the impaired fasting glycemia and the impaired glucose tolerance groups. Several studies have shown sympathetic overactivity in insulin resistant individuals with normoglycemia19, 20. In general, our previous study reported that hyperglycemia and insulin resistance are significantly associated with CAN in a general population21.
Furthermore, in the present study, the predictive GRS for DCAN was evaluated using receiver operating characteristic analysis, and the findings showed that GRS has a high predictive value for DCAN. To our knowledge, this is the first study to have reported that a glucose profile combined with β‐cell function has such a high predictive value for DCAN in a Chinese population. In practice, it is crucial for clinicians to identify and treat DCAN as early as possible. This finding is significant for preventing and treating DCAN in diabetic patients. DCAN is one of the most overlooked of all serious complications of diabetes. A retrospective study showed that CAN patients had poorer glycemic control and a fivefold higher mortality rate than type 1 diabetes mellitus patients without CAN during a 10‐year follow‐up period11. Additionally, DCAN is associated with other diabetic complications. A study including 449 patients with a 13.3‐year follow‐up period showed that the development of diabetic foot ulcers was independently associated with CAN in patients with type 2 diabetes mellitus without diabetic polyneuropathy22.
Basic medical studies have implicated that diabetes‐associated metabolic disturbances, such as hyperglycemia, can lead to DCAN through deregulated cell signaling pathways, direct neuronal damage, reduced blood flow, increased free radical production, increased oxidative stress and altered nitric oxide homeostasis23, 24. Furthermore, the association between β‐cell function and DCAN has been identified. It was shown that an increase in the plasma insulin level was related to increased urinary and plasma norepinephrine25. Ciccacci et al.26 evaluated the possible involvement of genetic polymorphisms in micro ribonucleic acid regions in the susceptibility to CAN, and they found associations between MIR146a and MIR27a single‐nucleotide polymorphisms and CAN susceptibility. However, the exact mechanism underlying the association between DCAN and hyperglycemia or β‐cell function has not been fully elucidated. In the present study, we did not determine the mechanism by which hyperglycemia and β‐cell function induces and accelerates DCAN.
The present study had several limitations. First, the design was cross‐sectional, which is susceptible to selection bias. Therefore the causality of the relationship between the glucose profile and β‐cell function and DCAN could not be evaluated directly. Additionally, it is important to note that because the present study was carried out with Chinese participants, its findings might not be directly applicable to other ethnicities.
Our observations suggest that the glucose profile indices of FPG, PBG, HbA1c and DMD were significantly and independently associated with DCAN, respectively; and that β‐cell function parameters of FINS and HOMA‐IR were significantly and independently associated with DCAN, whereas HOMA‐ISI and HOMA‐β were significantly, independently and negatively correlated with DCAN. There was a tendency toward increased GRS with increasing prevalence of DCAN. These findings provide evidence that both glucose profiles and β‐cell function influence the progression of DCAN, and they also provide insights into biological functions.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank the grant from Fudan University Huashan Hospital and Shanghai Tongji Hospital to support the study. Funding sources: grants from the Medical Science Foundation of Fudan University Huashan Hospital and Clinical Medicine Foundation of Shanghai Tongji Hospital.
J Diabetes Investig 2017; 8: 354–362
References
- 1. Singh JP, Larson MG, O'Donnell CJ, et al Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 2000; 86: 309–312. [DOI] [PubMed] [Google Scholar]
- 2. Spallone V, Ziegler D, Freeman R, et al Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011; 27: 639–653. [DOI] [PubMed] [Google Scholar]
- 3. Ziegler D, Zentai C, Perz S, et al Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp Clin Endocrinol Diabetes 2006; 114: 153–159. [DOI] [PubMed] [Google Scholar]
- 4. Kamphuis MH, Geerlings MI, Dekker JM, et al Autonomic dysfunction: a link between depression and cardiovascular mortality? The FINE Study. Eur J Cardiovasc Prev Rehabil 2007; 14: 796–802. [DOI] [PubMed] [Google Scholar]
- 5. Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabet Stud 2015; 12: 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvin E, Steffes MW, Zhu H, et al Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Z, Tang ZH, Zeng F, et al Associations between the severity of metabolic syndrome and cardiovascular autonomic function in a Chinese population. J Endocrinol Invest 2013; 36: 993–999. [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Tang ZH, Zeng F, et al Artificial neural network models for prediction of cardiovascular autonomic dysfunction in general Chinese population. BMC Med Inform Decis Mak 2013; 13: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge X, Chen H, Zhang K, et al The analysis of blood pressure profiles and their severity in relation to diabetic cardiovascular autonomic neuropathy in the Chinese population: preliminary analysis. J Endocrinol Invest 2016; 39: 891–898. [DOI] [PubMed] [Google Scholar]
- 10. Song L, Zhou L, Tang Z. An association analysis of lipid profile and diabetic cardiovascular autonomic neuropathy in a Chinese sample. Lipids Health Dis 2016; 15: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lacigova S, Brozova J, Cechurova D, et al The influence of cardiovascular autonomic neuropathy on mortality in type 1 diabetic patients; 10‐year follow‐up. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016; 160: 111–117. [DOI] [PubMed] [Google Scholar]
- 12. Suarez GA, Clark VM, Norell JE, et al Sudden cardiac death in diabetes mellitus: risk factors in the Rochester diabetic neuropathy study. J Neurol Neurosurg Psychiatry 2005; 76: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jun JE, Jin SM, Baek J, et al The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol 2015; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grundy SM, Hansen B, Smith SC Jr, et al Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 2004; 109: 551–556. [DOI] [PubMed] [Google Scholar]
- 15. Zeng F, Tang ZH, Li Z, et al Normative reference of short‐term heart rate variability and estimation of cardiovascular autonomic neuropathy prevalence in Chinese people. J Endocrinol Invest 2014; 37: 385–391. [DOI] [PubMed] [Google Scholar]
- 16. Tang ZH, Zeng F, Yu X, et al Bayesian estimation of cardiovascular autonomic neuropathy diagnostic test based on baroreflex sensitivity in the absence of a gold standard. Int J Cardiol 2014; 171: e78–e80. [DOI] [PubMed] [Google Scholar]
- 17. Martin CL, Albers JW, Pop‐Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perciaccante A, Fiorentini A, Paris A, et al Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc Disord 2006; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malliani A, Pagani M, Lombardi F, et al Cardiovascular neural regulation explored in the frequency domain. Circulation 1991; 84: 482–492. [DOI] [PubMed] [Google Scholar]
- 20. Ewing DJ, Neilson JM, Shapiro CM, et al Twenty four hour heart rate variability: effects of posture, sleep, and time of day in healthy controls and comparison with bedside tests of autonomic function in diabetic patients. Br Heart J 1991; 65: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng F, Tang ZH, Li Z, et al Normative reference of short‐term heart rate variability and estimation of cardiovascular autonomic neuropathy prevalence in Chinese people. J Endocrinol Invest 2014; 37: 385–391. [DOI] [PubMed] [Google Scholar]
- 22. Yun JS, Cha SA, Lim TS, et al Cardiovascular autonomic dysfunction predicts diabetic foot ulcers in patients with type 2 diabetes without diabetic polyneuropathy. Medicine 2016; 95: e3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Axelrod S, Lishner M, Oz O, et al Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron 1987; 45: 202–206. [DOI] [PubMed] [Google Scholar]
- 24. Pop‐Busui R, Kirkwood I, Schmid H, et al Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol 2004; 44: 2368–2374. [DOI] [PubMed] [Google Scholar]
- 25. Ward KD, Sparrow D, Landsberg L, et al Influence of insulin, sympathetic nervous system activity, and obesity on blood pressure: the Normative Aging Study. J Hypertens 1996; 14: 301–308. [DOI] [PubMed] [Google Scholar]
- 26. Ciccacci C, Morganti R, Di Fusco D, et al Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol 2014; 51: 663–671. [DOI] [PubMed] [Google Scholar]


