Abstract
Aims/Introduction
Despite being the most common complication of diabetes, the pattern of clinical development of diabetic neuropathy is not well‐known. In the present study, we retrospectively examined sequential changes in nerve conduction studies (NCS) for 4 years to characterize the way neuropathic changes develop in patients with type 2 diabetes.
Materials and Methods
We randomly selected 158 patients with type 2 diabetes who newly visited Naka Memorial Clinic, Ibaraki, Japan, and underwent serial 4‐year NCS. Records of clinical profile, signs and symptoms of neuropathy, and NCS data from median and tibial nerves were extracted to determine the progression of neuropathy. NCS data were represented by motor nerve conduction velocities, amplitudes of compound muscle action potentials (CMAPs) and minimal latencies of F‐wave.
Results
The prevalence of clinical neuropathy in 158 cases was 30% at baseline and 29% at the end of the study, with improvement of glycated hemoglobin (8.6–6.9%). Over 4 years, there were no changes of the signs and symptoms of neuropathy. Motor nerve conduction velocities were slightly improved or consistent at the fourth year compared with those at the beginning (+1.5% in median nerve, P < 0.05; +0.8%, not significant in the tibial nerve). The extent of the glycated hemoglobin correction correlated with the improvement of motor nerve conduction velocity. In contrast, CMAPs of both median and tibial nerves were decreased (−11.6%, P < 0.01; −3.7%, P < 0.05, respectively). For the decrease in CMAPs, no specific risk factors were identified by logistic regression analysis.
Conclusions
The present study showed progressive decline of CMAPs despite improved glycemic controls or the lack of NCV slowing in patients with early type 2 diabetes.
Keywords: Diabetic neuropathy, Natural history, Nerve conduction
Introduction
Despite being an early and common complication of diabetes, the mode and pattern of development of diabetic neuropathy are not well‐known. Neuropathy affects approximately 40–50% of diabetes patients. It is associated with intractable pain, paresthesia and sensory loss, often requiring amputation with resultant shortened life expectancy1, 2. Although long‐term hyperglycemia, hyperlipidemia, increased body mass index (BMI) and hypertension are found to contribute to the progression of neuropathy in type 1 diabetes3, factors for neuropathy in type 2 diabetes appear to be more complicated. In fact, meticulous blood glucose control in type 2 diabetes in the UK Prospective Diabetes Study did not significantly influence the outcome of newly onset neuropathy4. More recently, hyperlipidemia, oxidative stress and various genetic factors have been suggested to be associated with the development of neuropathy by gene microarray analysis5. It is not known, however, how these factors influence the development of neuropathy.
The natural history of neuropathy has yet to be made clear. There have been some attempts to create criteria for the clinical staging and severity of neuropathy in diabetes6. Scores of symptoms and signs for the severity of diabetic neuropathy were also proposed7, 8. These proposals are largely dependent on major symptoms, outlook for feet and examinations of objective signs of neuropathy (ankle jerk test, vibration perception test and sensory test), which are shown to be difficult to correctly detect or often unpredictable, and are not reproducible9.
Dyck et al.10 disclosed that nerve conduction studies are reliable and reproducible markers for the severity and progression of neuropathy. In their studies, slow progression during 7–10 years of conduction delay was found to be characteristic in patients with diabetes. However, there still remain unanswered questions as to how neuropathy develops in the early stage of type 2 diabetes, and which factors influence its development. In the present study, we therefore retrospectively examined the sequential changes of nerve conduction studies for 4 years to characterize the way in which neuropathic changes develop in patients with type 2 diabetes.
Materials and Methods
Among the files of 4,300 patients with type 2 diabetes who newly visited the Naka Memorial Clinic, Ibaraki, Japan, from January 2006 to December 2010, we extracted 158 patients who had a continuous history of annual evaluation of neuropathy by clinical signs and symptoms, neurological examinations, and nerve conduction studies (NCS). Data of clinical profiles and laboratory tests nearest to the NCS studies in each year were extracted for each patient, and serial changes were analyzed in relation to the NCS results. For these patients, we examined the prevalence of neuropathy and background clinical profiles, such as age, BMI, blood pressure (BP), blood lipids and postprandial (causal) blood glucose, as well as glycated hemoglobin (HbA1c). Patients with other causes for peripheral nerve damage, such as spinal disorders, alcoholism, liver cirrhosis, severe cardiovascular disorders, stroke and vitamin deficiencies, were excluded from the present study.
HbA1c values were expressed in accordance with the National Glycohemoglobin Standardization Program defined by the Japan Diabetes Society11. Hypertension was diagnosed if BP was ≥140/90 mmHg, or if the patient was receiving antihypertensive treatment12. Dyslipidemia was diagnosed according to the presence of total cholesterol ≥5.69 mmol/L, triglycerides ≥1.69 mmol/L or high‐density lipoprotein cholesterol <1.03 mmol/L or if the patient was receiving antidyslipidemia treatment12. The presence of retinopathy and nephropathy was defined by the fundoscopic changes with A1 grade or more according to the World health Organization, and a urinary albumin excretion rate exceeding 50 mg per day according to the criteria of the American Diabetes Association, respectively12.
The presence of clinical neuropathy was determined by the simplified diagnostic criteria proposed by the Japanese Study Group of Diabetic Neuropathy13, after exclusion of other causes of neuropathy, and the presence of two or more of the following three factors provides the probable presence of neuropathy: (i) subjective symptoms in bilateral feet as a result of neuropathy; (ii) loss or decrease in ankle jerk responses in bilateral feet; and (iii) decreased vibration perception threshold at the bilateral internal malleoli (less than or equal of 10 s by C128 tuning forks). Confirmation of neuropathy was carried out to disclose two or more abnormalities of measures in the median and tibial nerves by NCS or decreased coefficient of variation of R‐R intervals (CVR‐R) (<1.5) on electrocardiogram studies. Diagnosis of type 2 diabetes was based on the World Health Organization criteria12 and clinical history with the absence of antibodies specific for type 1 diabetes. Possible cases with secondary diabetes, such as pancreatic diabetes or pituitary/thyroid, or adrenal diseases were also excluded.
Clinical staging of diabetic neuropathy was determined by the criteria reported by the Japan Diabetic Neuropathy Study group14: stage I, absence of neuropathy based on the criteria by Japan Diabetic Neuropathy Study group; stage II, asymptomatic (subclinical) neuropathy that fulfilled the criteria of neuropathy, but with no symptoms or without loss of sensation to the Semmes–Weinstein monofilament (3.61; Arkray, Tokyo, Japan); stage III, symptomatic neuropathy (mild) that fulfilled the criteria and presence of sensory loss determined by monofilament (3.61 or over); stage IV, symptomatic neuropathy (moderate) that fulfilled the criteria and presence of sensory loss with autonomic symptoms, such as orthostatic hypotension, or intractable diarrhea or constipation or abnormal sweating in the hands and face; and stage V, symptomatic neuropathy (severe), showing reduced muscle strength and muscle atrophy often with a foot ulcer or gangrene. The validity of these staging criteria was already confirmed by several NCS studies15, 16.
NCS studies were carried out in a temperature controlled air‐conditioned and shielded room on the left median and left tibial nerves. Values of motor nerve conduction velocity (m/s), amplitude of compound muscle action potential (CMAP; mV) and minimal latency of F‐wave (FCV; s) between the wrist/ankle and the spinal cord were obtained from the measurements of the median and tibial nerves. In each patient, NCS studies were carried out by supramaximal stimulation on the nerve at the proximal and distal site five times, and the fastest value was used as the value for each patient17, 18.
Written informed consent was obtained from all the participants. The study was designed in accordance with the principles stated in the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Naka Memorial Clinic (approval number 2014‐C2).
Results
Serial changes of clinical profile are summarized in Table 1. At the beginning, the duration of diabetes in these patients was 5.5 ± 2.4 years (mean ± SD), and the average age was 56.3 ± 4.8 years. The BMI at the end of the study was slightly smaller compared with the value at the baseline, but the difference was not significant. HbA1c and postprandial blood glucose were both significantly decreased during 4 years from 8.8% and 11.3 mmol/L at baseline, to 6.9% and 8.5 mmol/L at the end of the study, respectively (P < 0.01 for both). In a similar manner, systolic BP, total serum total cholesterol, low‐density lipoprotein cholesterol and triglyceride levels were all suppressed compared with the initial values (P < 0.01 for all). In contrast, the prevalence of hypertension was increased; whereas those of retinopathy and nephropathy were not significantly altered. The percentage frequency of each clinical stage of neuropathy was not significantly altered.
Table 1.
Clinical profile of 158 follow‐up cases and the prevalence of complications at the beginning and end
| Attributes | Year 1 | Year 2 | Year 3 | Year 4 |
|---|---|---|---|---|
| Body mass index (kg/m2) | 25.0 ± 4.5 | 24.6 ± 4.0* | 24.4 ± 3.5** | 24.4 ± 3.9** |
| Casual blood glucose (mmol/L) | 11.3 ± 5.0 | 9.1 ± 2.0** | 8.8 ± 2.1** | 8.5 ± 1.8** |
| HbA1c (%) | 8.6 ± 2.2 | 7.2 ± 0.9** | 7.0 ± 0.9** | 6.9 ± 0.8** |
| IFCC‐NGSP (mmol/mol) | 70.5 ± 24.0 | 55.2 ± 9.8** | 53.0 ± 9.8** | 51.9 ± 8.7** |
| Systolic blood pressure (mmHg) | 140 ± 19 | 132 ± 13** | 131 ± 12** | 132 ± 11** |
| Total cholesterol (mmol/L) | 5.15 ± 0.83 | 4.58 ± 0.57* | 4.60 ± 0.64** | 4.60 ± 0.54** |
| LDL cholesterol (mmol/L) | 2.92 ± 0.72 | 2.40 ± 0.49** | 2.43 ± 0.54** | 2.38 ± 0.47** |
| Triglyceride (mmol/L) | 1.66 ± 1.15 | 1.50 ± 0.76 | 1.48 ± 0.78* | 1.49 ± 0.78* |
| CVR‐R (%) | 3.26 ± 0.02 | 3.36 ± 0.03 | 3.32 ± 0.03 | 3.43 ± 0.03 |
| Hypertension | 59.5% | 72.8%* | ||
| Retinopathy | 38.0% | 43.0% | ||
| Arteriosclerosis | 51.3% | 55.1% | ||
| Nephropathy | 27.8% | 27.8% | ||
| Polyneuropathy | 30.4% | 29.1% | ||
| Stage of neuropathy | ||||
| (I/II/III/IV+V) | (83.5/12.7/2.5/1.3%) | (83.5/15.2/1.3/0.0%) |
Duration of diabetes was 5.3 ± 7.2 years (mean ± standard deviation), and age was 56.5 ± 9.4 years at the baseline (first year). *P < 0.05 and **P < 0.01 vs baseline, respectively. CVR‐R, Coefficient of variation of R‐R intervals; HbA1c, glycated hemoglobin; IFCC‐NGSP, International Federation of Clinical Chemistry‐National Glycohemoglobin Standardization Program; LDL, low‐density lipoprotein.
At the baseline, NCS data were correlated with clinical staging of neuropathy proposed by the Japanese Study Group of Diabetic Neuropathy (Figure 1). NCVs of both the median and tibial nerves were step‐wisely slowed with advancing stage of neuropathy from stage I to stage III, although the number of stage IV and V were too small for comparison. In a similar manner, CMAPs of both the median and tibial nerves were also reduced with increased stage of neuropathy, whereas the F‐wave latencies were prolonged.
Figure 1.
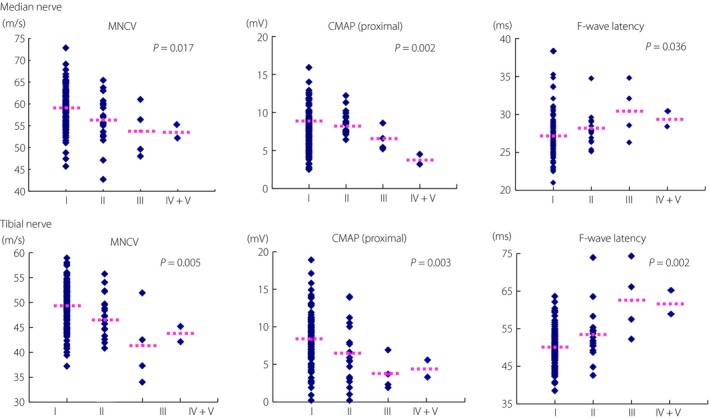
Correlation of clinical staging with nerve conduction data of 158 patients with type 2 diabetes at baseline. At the beginning of the first year, clinical staging of neuropathy was determined based on the criteria proposed by the Japanese Study Group of diabetic polyneuropathy. Because of the small number of patients, stage IV and V were mixed together. Stage I, no neuropathy; stage II, asymptomatic neuropathy; stage III, early symptomatic neuropathy; stage IV, established symptomatic neuropathy; stage V, advanced symptomatic neuropathy. P‐values were evaluated by Spearman's rank sum test. CMAP, compound muscle action potential; MNCV, motor nerve conduction velocity.
During the observation period, the average NCV of the median nerve was slightly improved at the end of the first year, and thereafter consistent (Figure 2a). Tibial NCV was consistent during the 4 years. F‐wave latencies of the median and tibial nerves were slightly shortened at the end of the first year, and were then consistent for the subsequent 3 years. There was a significant inverse correlation between the difference in HbA1c values (ΔHbA1c) and the difference in improved MCV (ΔMCV), FCV of the tibial nerve and improved FCV of the median nerve from baseline to the end of the study (Table 2). The difference in HbA1c values (ΔHbA1c) was also correlated with the difference in F‐wave latencies of the tibial and median nerves. Comparison of MCVs between groups with and without hypertension, or groups with or without hyperlipidemia, or groups with BMI over 25 or BMI below 25 did not yield any significant difference in both the median and tibial nerves (data not shown).
Figure 2.
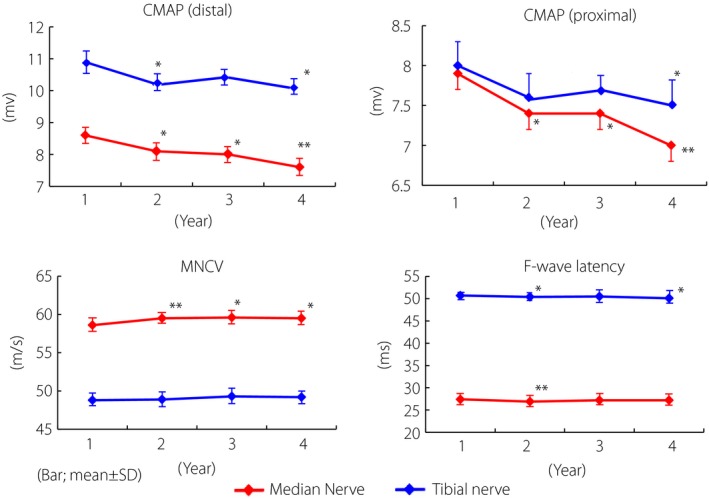
Serial changes of nerve conduction data for 4 years in 158 patients with type 2 diabetes. Although motor nerve conduction velocities, F‐wave nerve conduction or F‐wave latencies of both median and tibial nerves were slightly improved or consistent, not worsened, compound muscle action potentials (CMAP) of the median and tibial nerves were gradually reduced. The differences between the first and fourth year were significant (*P < 0.01, **P < 0.05 vs first year). MNCV, motor nerve conduction velocity.
Table 2.
Correlation of differences of glycated hemoglobin and the changes of nerve conduction velocity data at the baseline and at end
| R | P | |
|---|---|---|
| ΔHbA1c vs ΔNCV of median nerve | −0.282 | NS |
| ΔHbA1c vs ΔF‐wave latency of median nerve | 0.428 | <0.001 |
| ΔHbA1c vs ΔCMAP of median nerve (wrist) | 0.026 | NS |
| ΔHbA1c vs ΔCMAP of median nerve (elbow) | −0.002 | NS |
| ΔHbA1c vs ΔNCV of tibial nerve | −0.313 | <0.01 |
| ΔHbA1c vs ΔF‐wave latency of tibial nerve | 0.431 | <0.001 |
| ΔHbA1c vs ΔCMAP of tibial nerve (ankle) | 0.014 | NS |
| ΔHbA1c vs ΔCMAP of tibial nerve (knee) | 0.054 | NS |
ΔCMAP, compound muscle action potentials; ΔHbA1c, differences of HbA1c; ΔNCV, changes of nerve conduction velocity; NS, not significant.
In contrast to NCV, CMAP was significantly reduced at the fourth year compared with the data at baseline, in either the median or tibial nerve (Figure 2b). Both proximal and distal CMAPs were equally reduced in the median nerve, although transient recovery at the third year was observed in the tibial nerve. To explore the factors relevant to the CMAP decline, we compared the average values of clinical parameters between the participants who showed decline of CMAP in both the median and tibial nerves at 4 years (progressors) compared with the first year and participants whose CMAP was not reduced in either the median or tibial nerves during the 4 years (non‐progressors; Table 3). There were no significant differences in the clinical profile between these two groups, although there was a trend in progressors towards high systolic BP and HbA1c. Multiple logistic regression analysis did not yield any factors for the reduced CMAP (Figure 3). There was no difference among various treatment groups of angiotensin‐receptor blockade, aldose reductase inhibitor, dipeptidyl peptidase 4 inhibitor or other compounds (data not shown), although each group consisted of only small numbers.
Table 3.
Comparison of clinical profile between progressors and non‐progressors
| Progresssors | Non‐progressors | P‐values | |
|---|---|---|---|
| No. cases | 44 | 114 | |
| Age (years) | 57.3 ± 10.2 | 59.2 ± 9.1 | 0.549 |
| Duration of diabetes (years) | 5.3 ± 7.7 | 4.6 ± 6.7 | 0.621 |
| Men/women | 28/16 (63.65/36.4%) | 79/35 (69.3%/30.7%) | 0.492 |
| Body mass index (kg/mm2) | 24.0 ± 3.5 | 24.6 ± 4.0 | 0.354 |
| HbA1c (NGSP) (%) | 6.8 ± 0.7 | 6.9 ± 0.8 | 0.656 |
| IFCC (mmol/mol) | 50.8 ± 7.6 | 51.9 ± 8.7 | |
| Blood glucose (casual) | 8.2 ± 2.6 | 8.3 ± 2.5 | 0.891 |
| Systolic blood pressure (mmHg) | 131 ± 12 | 133 ± 12 | 0.489 |
| Total cholesterol (mmol/L) | 4.5 ± 0.5 | 4.6 ± 0.6 | 0.112 |
| LDL cholesterol (mmol/L) | 2.3 ± 0.4 | 2.4 ± 0.5 | 0.059 |
| Triglyceride (mmol/L) | 1.4 ± 0.8 | 1.5 ± 0.8 | 0.470 |
| Hypertension | 61.0% | 69.3% | 0.341 |
| Arteriosclerosis | 56.8% | 54.4% | 0.786 |
| Retinopathy | 43.2% | 43.9% | 0.937 |
| Nephropathy | 31.8% | 26.3% | 0.489 |
| Neuropathy | 25.0% | 26.3% | 0.480 |
| Stage (I/II/III/IV+V) | 79.5/20.5/0/0% | 85.1/13.2/1.0/0% | 0.252 |
Progressors showed decline of compound muscle action potentials (CMAP) in both median and tibial nerve while non‐progressors did not show decline of CMAP either median or tibial nerves. HbA1c, glycated hemoglobin; IFCC, International Federation of Clinical Chemistry; LDL, low‐density lipoprotein; NGSP, National Glycohemoglobin Standardization Program.
Figure 3.
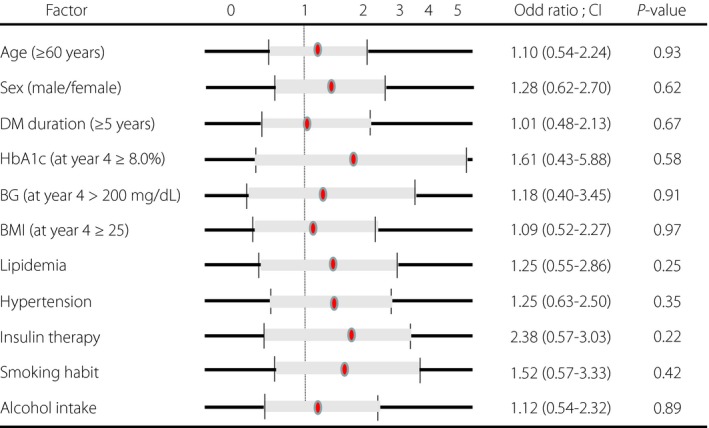
Multiple logistic regression analysis of risk factors for the decline of compound muscle action potentials (CMAP) of median and tibial nerves in patients with type 2 diabetes. Although the odds ratio is relatively high for glycated hemoglobin (HbA1c), insulin therapy and smoking habit, no significant risk factors were identified. BG, blood glucose; BMI, body mass index; DM, diabetes mellitus.
Discussion
In the present study, we found a progressive decline of M‐wave amplitude in both the median and tibial nerve, despite no appreciable deterioration of motor nerve conduction velocity over the 4‐year observation period in patients with well‐controlled type 2 diabetes. The patients were mostly limited to the recently diagnosed and relatively mild stage of neuropathy. The results are in keeping with a recent report that showed slow progression of neuropathy under well controlled diabetes19. Our NCS data were also compatible with recent findings from patients with type 1 and type 2 diabetes, who showed a significant reduction of SNAP of the sural nerve and CMAP (M‐wave) of the peroneal nerve, but not MCV changes, after 3 years under good glycemic control20. As SNAP and CMAP reflect mainly the pathological background of loss of total nerve fibers of the examined nerve, the present results might suggest that perfect protection from the progression of neuropathy was not attained in this 4‐year retrospective study under the condition of 6.9% HbA1c levels.
The effects of glycemic control on NCS in patients with diabetes have been well addressed in previous studies21, 22, 23. Indeed, previous studies showed progressive decline of NCV (−0.5 to −1.0 m/s after 10 years, −0.4 μV after 5 years) in 5–10 years under conventional treatment with 7.5–8.0% HbA1c10. It has not been clear, however, about the length of duration required for meaningful alterations of NCV or CMAP in the progression of neuropathy. NCV correlates with pathological changes of demyelination, loss of the thickest fibers and nerve fiber atrophy24, and also with local metabolic factors, blood flow or temperature18. In contrast, the reduction of CMAP might reflect the reduced nerve fiber density or atrophy of conducting nerve fibers18, 24. Based on this contention, NCV improvement or consistency for 4 years in the present study appears to be in accord with the improved metabolic state or preservation of the fastest nerve fibers in patients with well‐controlled diabetes. Nevertheless, progressive CMAP decline, even if small, might suggest the ongoing loss of smaller nerve fibers and ongoing collateral innervation. Recent findings that there was a progressive decline of the small nerve fibers in the skin (intra‐epidermal nerve fiber density), even in patients with well‐controlled and early diabetes25, 26, also show the difficulty of perfect protection from neuropathy development in diabetes. Hence, it is likely that blood glucose control attained at 6.9% in the present patients might still have been incomplete for the preservation of nerve fiber integrity. As intra‐epidermal nerve fiber density is known to correlate with the severity or clinical stage of neuropathy, future investigations on intra‐epidermal nerve fiber density should be warranted to confirm our speculation.
The natural history of diabetic polyneuropathy is not well‐known in type 2 diabetes, and it is not clear whether there is a point of no return. Signs and symptoms of diabetic neuropathy are difficult to follow because of inadequate reproducibility or inconsistency with pathological backgrounds. In our studies, we could not find apparent deterioration of subjective or objective signs or symptoms of diabetic neuropathy during the 4 years, supporting the difficulty to find markers of severity of neuropathy in clinical symptoms and signs as previously indicated9. The severity of neuropathy in diabetes is generally modest in most of the patients, as shown by the Rochester Diabetic Neuropathy Study6, almost equivalent to the present results. Nevertheless, it should be reinforced that they might eventually develop a more severe stage of neuropathy after 10–20 years. Thus, for better clinical care of patients with diabetes, it should be recommended that a checkup by NCV studies at least every 3 years from the early stages be carried out to determine if the clinical stage of neuropathy has advanced.
The present present study confirmed that the extent of improved glycemic control (ΔHbA1c) correlated well with the improvement of NCV or latencies (Table 2). It is widely accepted that long‐term blood glucose control is crucial for the development of neuropathy, which is well reflected by the decline of NCV10, 27. In fact, the indices for the efficacy of clinical trials are set for the NCV measures28. Balducci et al.29 showed significant decline of NCV, which was effectively suppressed by regular exercise. However, as the present results concord with the data from Gibbons et al.20, only amplitude is sensitive to changes during a 3‐year period in well‐controlled diabetes, showing reduction of SNAP 1 μV in the sural nerve, but no change in velocity (yearly reduction rate 0.33), amplitude or latency of the peroneal nerve. This decline is comparable with that of the present study, in which the annual reduction rate of CMAP in the tibial nerve was −2.5%. The results provide important information on the difficulties in obtaining significant benefits of treatment effects when clinical trials using NCV changes are carried out as a major end‐point. Unfortunately, previous studies mainly denote changes of NCV, but not CMAP or differences between sensory and motor NCV.
It should be noted that NCV data fluctuate even in a single patient dependent on environmental temperature, constraint or reliability in the laboratory. Although the data of NCV can fluctuate even in multiple institutions for NCV 10%, amplitude 30%, F‐wave latencies 5%17, we believe that our data should be meaningful because of reproducibility in a single institution. The enrolled participants in the present study were strictly limited to patients with type 2 diabetes, and patients with disorders that might affect nerve function were carefully excluded. There is a possibility that spinal deformities and subclinical ischemic neuropathies might still be potentially present in some cases. However, an accumulation of cases decreases such confounders or complexities.
Factors influencing progression of DPN as indicated by reduced CMAP20 were not identified in the present study. The average values of clinical data were not different between progressors and non‐progressors. Multiple regression analysis could not detect significant values for the risk factors. These results show that the progression of neuropathy involves a variety of factors including blood glucose control, duration of diabetes, hypertension and lipidemia. The results of the UK Prospective Diabetes Study4 might be consistent with the current results, as blood glucose control did not significantly suppress the progression of neuropathy. It would be intriguing if we examined the progression of neuropathy in patients with type 1 diabetes, and confirmed whether the results would be consistent with Diabetes Control and Complication Trial (DCTT) results.
Several limitations should be mentioned regarding the present study. First, our study was basically retrospective, and there was a potential bias on the patient selection, although we randomly procured the participants from the patient record. There is a possibility that we selected limited well‐controlled patients who followed regular visits with monitoring of NCV and glucose control. Different from a prospective study, we did not have a control group who were not well taken care of by tight glycemic control. In addition, it was not possible to compare the data between diabetic and healthy control aging. It should also be noted that we did not have any data of sensory nerves, such as sural nerve conduction velocity or SNAP of the sural nerve. It is known that most studies examined data of the sural nerve. However, the measurement of the sural nerve is technically more difficult, and the data require careful interpretation because of relatively inadequate reproducibility. In fact, reproducibility in well‐trained groups reported a large spectrum of the data, which yielded difficulty in obtaining a significant difference between the diabetic and control groups. In contrast, measurements of the tibial nerve and median nerve were more consistent with the data, and their reproducibility is known to be satisfactory, suggesting that our data might be comparable with sensory nerve involvement.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors greatly appreciate Mr Kawamata for his help with data recruitment and collection. This study was in part supported by a grant from the Japan Diabetes Foundation to SY. SY was also supported by the Japanese Ministry of Health and Welfare.
J Diabetes Investig 2017; 8: 369–376
References
- 1. Vinik AI, Nevoret ML, Casellini C, et al Diabetic neuropathy. Endocrinol Metab Clin North Am 2013; 42: 747–787. [DOI] [PubMed] [Google Scholar]
- 2. Tesfaye S, Boulton AJ, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tesfaye S, Chaturvedi N, Eaton SE, et al Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 4. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 5. Vincent AM, Callaghan BC, Smith AL, et al Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. [DOI] [PubMed] [Google Scholar]
- 6. Dyck PJ, Karnes JL, O'Brien PC, et al The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology 1992; 42: 1164–1170. [DOI] [PubMed] [Google Scholar]
- 7. Feldman EL, Stevens MJ, Thomas PK, et al A practical two‐step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 8. Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002; 25: 2048–2052. [DOI] [PubMed] [Google Scholar]
- 9. Dyck PJ, Overland CJ, Low PA, et al Cl vs. NPhys Trial Investigators. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve 2010; 42: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dyck PJ, O'Brien PC, Litchy WJ, et al Monotonicity of nerve tests in diabetes: subclinical nerve dysfunction precedes diagnosis of polyneuropathy. Diabetes Care 2005; 28: 2192–2200. [DOI] [PubMed] [Google Scholar]
- 11. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization, Department of Noncommunicable Disease Surveillance Definition, Diagnosis and Classification of Diabetes and its Complications . Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes. Geneva, Switzerland: WHO, 1–59. 1999. [Google Scholar]
- 13. Japan Diabetic Neuropathy Study Group . Simplified diagnostic criteria for diabetic polyneuropathy. Peripheral Nerve 1998; 9: 137–140 (Japanese). [Google Scholar]
- 14. Japan Diabetic Neuropathy Study Group . Proposal for clinical staging of diabetic polyneuropathy. Consensus statement on criteria on staging. Peripheral Nerve 2006; 17: 102–103 (Japanese). [Google Scholar]
- 15. Yasuda H, Sanada M, Kitada K, et al Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract 2007; 77(Suppl 1): S178–S183. [DOI] [PubMed] [Google Scholar]
- 16. Arimura A, Deguchi T, Sugimoto K, et al Intraepidermal nerve fiber density and nerve conduction study parameters correlate with clinical staging of diabetic polyneuropathy. Diabetes Res Clin Pract 2013; 99: 24–29. [DOI] [PubMed] [Google Scholar]
- 17. Kohara N, Kimura J, Kaji R, et al F‐wave latency serves as multicenter analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia 2000; 43: 915–921. [DOI] [PubMed] [Google Scholar]
- 18. Kimura J. Nerve conduction and needle electromyography In: Dyck PJ, Thomas PK. (eds). Peripheral Neuropathy. Philadelphia: Elsevier, 4th edn, Chapter 35, 2005; pp 899–969. [Google Scholar]
- 19. Ziegler D, Behler M, Schroers‐Teuber M, et al Near‐normoglycaemia and development of neuropathy: a 24‐year prospective study from diagnosis of type 1 diabetes. BMJ Open 2015; 5: e006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibbons CH, Freeman R, Tecilazich F, et al The evolving natural history of neurophysiologic function in patients with well‐controlled diabetes. J Periph Nerv Syt 2013; 18: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dahl‐Jorgensen K, Brinchmann‐Hansen O, Hanssen KF, et al Effect of near normoglycemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J 1986; 293: 1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tkac I, Bril V. Glycemic control is related to the electrophysiologic severity of diabetic peripheral sensorimotor polyneuropathy. Diabetes Care 1998; 21: 1749–1752. [DOI] [PubMed] [Google Scholar]
- 23. Dunnigan SK, Ebadi H, Breiner A, et al Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care 2013; 36: 3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Behse F, Buchthal F, Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry 1977; 40: 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quattrini C, Tavakoli M, Jeziorska M, et al Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007; 56: 2148–2154. [DOI] [PubMed] [Google Scholar]
- 26. Løseth S, Stålberg EV, Lindal S, et al Small and large fiber neuropathy in those with type 1 and type 2 diabetes: a 5 year follow‐up study. J Peripher Nerv Syst 2016; 21: 15–21. [DOI] [PubMed] [Google Scholar]
- 27. Litchy WJ, Albers JW, Wolfe J, et al CI. Nphys Trial 4 Investigators. Proficiency of nerve conduction using standard methods and reference values (Cl. NPhys Trial 4). Muscle Nerve 2014; 50: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bril V, Hirose T, Tomioka S, et al, Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care 2009; 32: 1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balducci S, Iacobellis G, Parisi L, et al Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Compl 2006; 20: 216–223. [DOI] [PubMed] [Google Scholar]


