Abstract
Background
Computed tomography (CT) may be useful for increasing the diagnostic yield of transbronchial lung biopsy (TBLB). However, only a few studies with small sample sizes have reported the diagnostic utility of CT‐guided TBLB and the factors affecting the diagnostic accuracy of CT‐guided TBLB are not well known. We evaluated the diagnostic yield of CT‐guided TBLB and associated factors.
Methods
CT‐guided TBLB was performed in 59 patients. Both conventional fluoroscopy and CT were used in all patients for TBLB. The biopsy forceps were advanced toward the lesion under conventional fluoroscopic guidance. CT was used to check whether the forceps were in the correct position.
Results
The average diameter of the lesions was 3.1 ± 1.0 cm. The biopsy forceps correctly reached the lesion in 43 patients by real‐time CT. A diagnosis was made in 42 patients, and the overall diagnostic yield was 71.2%. The sensitivity for malignancy was 85.7%. In multivariate analysis, the only factor associated with diagnostic yield was forceps position assessed by CT scan (adjusted odds ratio 53.31; 95% confidence interval 5.31, 535.27; P = 0.001).
Conclusion
CT‐guided TBLB is a useful diagnostic tool for pulmonary nodules or masses. The correct positioning of biopsy forceps using CT is valuable for successful CT‐guided TBLB.
Keywords: Bronchoscopy, computed tomography, lung biopsy, transbronchial biopsy
Introduction
Bronchoscopic procedures are used to visualize the tracheobronchial tree and obtain specimens of abnormal lesions. Transbronchial lung biopsy (TBLB) by either blind methods or under fluoroscopic guidance is a well‐established method that has been used for more than 30 years. However, small and peripheral lung lesions are difficult to diagnose using these procedures.1, 2 The overall diagnostic yield of conventional fluoroscopic TBLB varies from 18% to 75%.3, 4, 5, 6, 7
Percutaneous transthoracic biopsy with computed tomography (CT) leads to a better diagnostic yield for peripheral lung lesions and pneumothorax is the most common complication.8, 9, 10 Endobronchial ultrasound (EBUS)‐guided TBLB also has a higher diagnostic yield than TBLB with conventional fluoroscopy for peripheral and small lung lesions.11, 12, 13 However, EBUS‐guided TBLB cannot be applied without the use of expensive endoscopic ultrasound systems or skilled operators.
Previous studies have reported a higher yield of CT‐guided TBLB than conventional TBLB.14, 15, 16, 17 However, these studies had small sample sizes, and few have evaluated the factors associated with the diagnostic yield of CT‐guided TBLB. Therefore, we evaluated the diagnostic yield of CT‐guided TBLB and associated factors.
Methods
Study subjects
We retrospectively evaluated a total of 59 patients from January 2013 to April 2015. Chest CTs were performed in all patients before the bronchoscopic procedure. Only patients with measurable nodules or masses in the chest CT were included in the study. We measured lesion size as the longest cross‐sectional diameter. We also determined the location of the lesion in the upper, middle, or lower lobe. Air‐bronchus sign was defined as air‐filled bronchus surrounded by fluid‐filled airspaces in the lesion. Distance from the hilum to the lesion was calculated by Pythagoras theorem. Horizontal distance was measured as the shortest distance from the hilum to the lesion. Vertical distance was calculated as the product of the number of CT slides from the lesion to the hilum and the thickness of the CT scan. Horizontal and vertical distance was applied to Pythagoras theorem to calculate the distance from the hilum to the lesion. Both conventional fluoroscopy and CT were used in all patients scheduled for TBLB. Informed written consent was obtained from all patients before bronchoscopic procedures. The Institutional Review Board of National Health Insurance Service Ilsan Hospital approved this study.
Bronchoscopic procedure
Patients received 0.5 mg of intramuscular atropine and 50 μg of intramuscular fentanyl before procedures. Dexmedetomidine (0.7 mg/kg/hour) was continuously infused during the procedure for sedation. The bronchoscope (240, P240, 40, P40; Olympus; Tokyo, Japan) was inserted through the nose into the trachea and bronchus. Patients received intranasal oxygen and breathed spontaneously during the procedure. Oxygen saturation, blood pressure, and electrocardiography were monitored in all patients.
After bronchoscopic insertion into the tracheobronchial tree, a full inspection was performed from the trachea to the segmental or subsegmental bronchus. The tip of the bronchoscope was placed into the suspected segmental bronchus thought to open through the lung lesions. After the tip of the bronchoscope was positioned, the biopsy forceps were inserted through the selected segmental bronchus under guidance by conventional fluoroscopy. Bronchoscopic procedure was performed in a CT‐equipped angiography room.
Computed tomography procedure
A dynamic CT of the interventional angiography system (Artis Zee, Siemens, Germany) was used for real‐time CT during the procedure. After the biopsy forceps were advanced toward the lung lesions, a brief dynamic CT scan was performed by a radiologist who then transmitted the images to a CT video monitor in real time. The bronchoscopist could check images displayed on a CT video monitor that was placed adjacent to the bronchoscopic video monitor. After confirming via CT images that the biopsy forceps had been placed within the target lesion images (Figs 1, 2), TBLB was performed without additional CT scans. To minimize radiation exposure, a maximum of two CT scans were performed to check forceps position. If the forceps did not reach the lung lesions after two CT scans, TBLB was performed without additional CT confirmation of forceps positioning. Placement of the tip of the biopsy forceps into the lung lesions was considered to indicate successful targeting.
Figure 1.
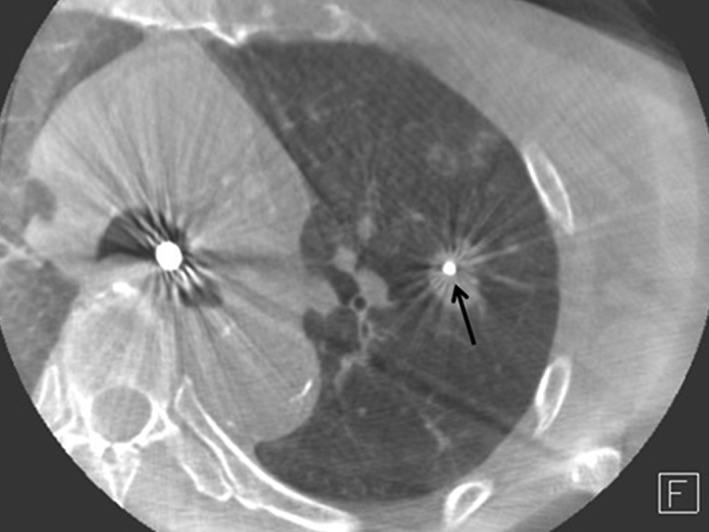
Computed tomography‐guided transbronchial lung biopsy with biopsy forceps in a target lesion (black arrow).
Figure 2.
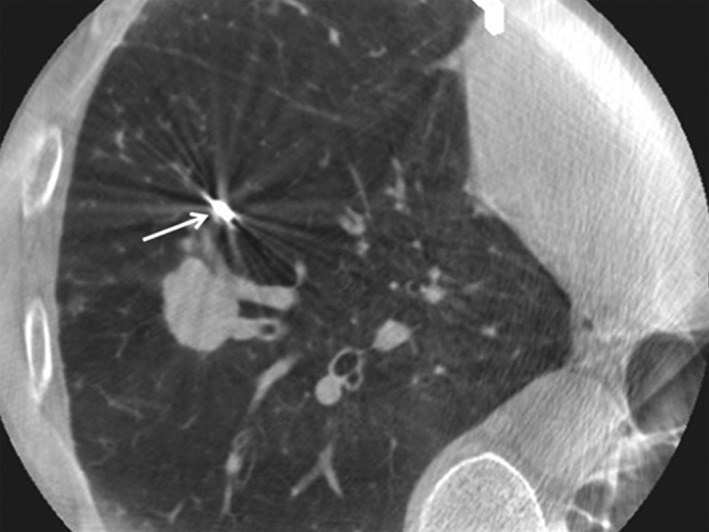
Computed tomography‐guided transbronchial lung biopsy with the biopsy forceps failing to reach a target lesion (white arrow).
Final diagnosis
Biopsy specimens were immediately fixed in formalin and sent to pathology. Pathologic diagnoses were divided into four groups: non‐specific inflammation, tuberculosis, malignancy, and other disease. Tuberculosis, malignancy, and other disease were considered as a definite diagnosis (TBLB positive), while non‐specific inflammation was not (TBLB negative). In cases of non‐specific inflammation, patients underwent additional tests, including transthoracic needle aspiration or video‐assisted thoracoscopic surgery, and lesions that resolved after antibiotic treatment were considered to be pneumonia.
Statistical analysis
Statistical analyses were performed using a SPSS version 21 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as the mean ± standard deviation, and categorical variables were expressed as number and percentage. Independent two‐sample t test and chi‐square or Fisher's exact tests were used to identify differences between groups. Logistic regression analysis was used to analyze the odds ratios of significant factors affecting the diagnostic yield of CT‐guided TBLB. All statistical analyses were two‐tailed and P values less than 0.05 were considered significant.
Results
Baseline characteristics
The enrolled patients included 41 men and 18 women with an average age of 65.5 ± 14.6 years (Table 1). All patients underwent chest CT scans before bronchoscopic procedures. The average size of the lesions was 3.3 ± 1.0 cm. Lung lesions are more frequent in upper than lower lobes.
Table 1.
Baseline characteristics and clinical differences between TBLB results
| Total patients (n = 59) | TBLB positive (n = 42) | TBLB negative (n = 17) | P | |
|---|---|---|---|---|
| Age (years) | 65.5 ± 14.6 | 65.0 ± 15.1 | 66.8 ± 13.8 | 0.673 |
| Male | 41 (69.5) | 29 (69.0) | 12 (70.6) | 0.907 |
| Size (cm) | 3.1 ± 1.0 | 3.3 ± 0.9 | 2.7 ± 0.9 | 0.022 |
| Location | ||||
| Upper lobe | 36 (61.0) | 28 (66.7) | 8 (47.1) | 0.162 |
| Middle or lower lobe | 23 (39.0) | 14 (33.3) | 9 (52.9) | |
| Air‐bronchus sign | 17 (28.8) | 14 (33.3) | 3 (17.6) | 0.344 |
| Distance from hilum (cm) | 5.3 ± 2.0 | 5.1 ± 2.1 | 5.7 ± 1.9 | 0.298 |
| Conventional fluoroscopy | ||||
| Visible | 49 (83.1) | 38 (90.5) | 11 (64.7) | 0.026 |
| Invisible | 10 (16.9) | 4 (9.5) | 6 (35.3) | |
| Number of biopsies | 7.8 ± 2.9 | 7.7 ± 2.5 | 8.1 ± 3.7 | 0.612 |
| Forceps targeting by CT | ||||
| Success | 43 (72.9) | 38 (90.5) | 5 (29.4) | <0.001 |
| Failure | 16 (27.1) | 4 (9.5) | 12 (70.6) | |
| Procedure duration (minutes) | 33.7 ± 7.6 | 33.5 ± 7.8 | 34.2 ± 7.2 | 0.756 |
| Pneumothorax | 2 (3.4) | 1 (2.4) | 1 (5.9) | 0.497 |
CT, computed tomography; TBLB, transbronchial lung biopsy.
Data are shown as the mean ± standard deviation or number (%).
Procedural results and pathologic diagnosis
All patients underwent CT‐guided TBLB and were divided into two groups by pathologic results (Table 1). A total of 10 lung lesions were not visible on conventional fluoroscopy. The biopsy forceps were blindly advanced in these patients. The accuracy of forceps targeting was checked by dynamic CT (Figs 1, 2). The average number of biopsies was 7.8 ± 2.9 and procedure duration was 33.7 ± 7.6 minutes. Successful targeting was confirmed in 43 patients (72.9%) by real‐time CT. In 16 patients, lung lesions were not targeted by the biopsy forceps after a maximum of two CT scans. Pneumothorax occurred in two patients. Other factors, except lesion size, visibility by fluoroscopy, and forceps targeting by CT, were not significantly different between the two groups. Table 2 shows the pathologic diagnoses. The most common finding was malignancy in 30 patients. The second most common finding was non‐specific inflammation in 17 patients. These patients underwent additional tests, including transthoracic needle aspiration or video‐assisted thoracoscopic surgery. Other patients who could not undergo additional biopsies were followed up regularly after antibiotic treatment. Final diagnoses could not be confirmed in two patients.
Table 2.
Pathologic diagnosis by TBLB and final diagnosis
| Number of patients | |
|---|---|
| Pathologic diagnosis by TBLB | |
| Non‐specific inflammation† | 17 |
| Tuberculosis | 11 |
| Adenocarcinoma | 18 |
| Squamous cell carcinoma | 5 |
| Small cell carcinoma | 6 |
| Non‐small cell carcinoma | 1 |
| Aspergilloma | 1 |
| Final diagnosis | |
| Pneumonia | 9 |
| Tuberculosis | 12 |
| Adenocarcinoma | 20 |
| Squamous cell carcinoma | 6 |
| Small cell carcinoma | 7 |
| Non‐small cell carcinoma | 2 |
| Aspergilloma | 1 |
| No final diagnosis | 2 |
Final diagnosis of non‐specific inflammation: pneumonia (9), tuberculosis (1), adenocarcinoma (2), squamous cell carcinoma (1), small cell lung cancer (1), non‐small cell lung cancer (1), no final diagnosis (2).
TBLB, transbronchial lung biopsy.
Diagnostic yield and factors affecting diagnostic yield
The overall diagnostic yield of CT‐guided TBLB was 71.2% (Table 3). For malignancy, the sensitivity of CT‐guided TBLB was 85.7% and the negative predictive value was 81.5%. The diagnostic yield for lung nodules (<3 cm) was 58.6%. The factors associated with diagnostic yield of CT‐guided TBLB are shown in Table 1. Lesion size, visible lesions by fluoroscopy, and proper forceps position confirmed by real‐time CT were associated with diagnostic yield. However, the only significant factor in multivariate analysis was confirmation of forceps position by real‐time CT (Table 4). The diagnostic yield was 53.31 times greater when CT could confirm the position of the biopsy forceps in the lung lesions than when the forceps failed to find the lesions.
Table 3.
Overall diagnostic yield and sensitivity, specificity, PPV, and NPV for malignancy†
| Factor | Result (%) |
|---|---|
| Diagnostic yield | 71.2 |
| Sensitivity | 85.7 |
| Specificity | 100.0 |
| PPV | 100.0 |
| NPV | 81.5 |
Two patients with no final diagnosis were excluded from analyses of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Table 4.
Multivariate analyses of factors associated with diagnostic yield
| Adjusted odds ratio | 95% confidence interval | P | |
|---|---|---|---|
| Forceps targeting | 53.31 | 5.31 –535.27 | 0.001 |
| Visibility by conventional fluoroscopy | 0.62 | 0.06 –6.32 | 0.687 |
| Size | 2.74 | 0.98 –7.63 | 0.054 |
| Air‐bronchus sign | 5.15 | 0.60 –44.47 | 0.136 |
| Distance from hilum | 0.77 | 0.48 –1.23 | 0.272 |
Discussion
Our results suggest that CT‐guided TBLB is suitable for the diagnosis of lung nodules or masses. The overall observed diagnostic yield was 71.2%, and sensitivity for malignancy was 85.7%. The most important factor associated with diagnostic yield was confirmation of the position of the biopsy forceps in lung lesions by real‐time CT.
Most previous studies of CT‐guided TBLB have been small case series, most without control groups. The overall diagnostic yield of CT‐guided TBLB reported in these studies ranged from 62% to 75%.16, 17, 18, 19, 20, 21 Only two studies evaluated CT‐guided TBLB with controls. Tsushima et al. retrospectively compared 82 patients who underwent CT‐guided bronchoscopy to 78 patients treated with fluoroscopy‐guided bronchoscopy.19 The two groups had undergone bronchoscopy during different time periods (2001–2003 vs. 1999–2001) and at different hospitals, but all procedures were performed by the same bronchoscopist. In that study, CT‐guided bronchoscopy demonstrated superior results in terms of lesion size (<15 mm in diameter). Ost et al. prospectively compared 26 patients who underwent CT‐guided bronchoscopy to 24 patients treated with fluoroscopy‐guided bronchoscopy.21 They evaluated CT‐guided bronchoscopic performance for the diagnosis of peripheral lung nodules and mediastinal lymph nodes. Although they failed to demonstrate any benefits of CT‐guided TBLB compared with fluoroscopy, they reported that CT‐guided TBLB might yield superior diagnosis of mediastinal lymphadenopathy compared with conventional bronchoscopy.
Computed tomography‐guided TBLB has shown better diagnostic yield for small peripheral lesions; however, overall diagnostic yield remains 60–70%. Therefore, the identification of factors related to the diagnostic yield of CT‐guided TBLB is important. Shinagawa et al. reported that diagnostic sensitivity was related to the locations of lesions, bronchial generation inserted by bronchoscopy, and the presence of a bronchus close to the lesion.20 In their study, the method had low diagnostic sensitivity for lesions in the left superior segment compared with other locations. An ultrathin bronchoscope that could be inserted into the fifth or higher generation of a bronchus had high diagnostic sensitivity, and lesions connected to a bronchus or an artery could be detected with sensitivity. In our study, targeting of the biopsy forceps by CT scan, lesions visualized by conventional fluoroscopy, and lesion size were correlated with diagnostic yield. Among these, positioning of the biopsy forceps in the lung lesions by CT scan was the most important predictor of accurate diagnosis in multivariate analysis. Predicting the success of biopsies is important because it allows clinicians to prepare another option for evaluation or to explain to patients that the biopsy may fail before results are reported.
The most important problem associated with CT‐guided TBLB is radiation exposure. Previous studies have reported that CT‐guided TBLB causes acceptable levels of radiation exposure. Hautmann et al. found that low‐dose CT guided TBLB had high diagnostic yield and low radiation exposure.17 The average number of CT scans was 4.1, and the mean effective dose of radiation was 0.55 mSv, equivalent to the radiation dose of three chest X‐rays. Another previous study also reported that the radiation exposure associated with real‐time multi‐slide computed tomography (MSCT)‐guided TBLB is low.19 The radiation dose associated with MSCT fluoroscopy is at least double that of conventional fluoroscopy. Unlike conventional fluoroscopy, MSCT fluoroscopy employs a tightly collimated beam that is confined to a narrow area. In our study, a maximum of only two CT scans were performed per procedure to minimize radiation exposure. However, we were unable to calculate exact radiation doses because the necessary program was not in use in our hospital during the study period.
Computed tomography‐guided transthoracic needle aspiration or EBUS‐guided biopsy are alternative options for the diagnosis of lung lesions. However, CT‐guided transthoracic needle aspiration leads to decreased diagnostic accuracy and an increased risk of pneumothorax when the target is distant from the pleura.22 EBUS‐guided biopsies that have higher diagnostic yield do not cause radiation exposure.11, 12, 13 However, such procedures are not widely available and only possible if clinicians receive specialized training. Moreover, small nodules without solid components are difficult to detect with ultrasound‐guided biopsy.23, 24
Our study has several limitations. First, we included a wide range of patients. The higher diagnostic yield in this study may be a result of including relatively large lung lesions. However, separate analyses of small nodules showed an acceptable diagnostic yield, similar to previous studies. Second, this is a single center study. Other institutions may not use real‐time CT‐guided TBLB. However, CT is a widely used diagnostic tool and institutions that perform bronchoscopy and CT may perform this procedure without difficulty. Third, we only retrospectively analyzed patients who underwent CT‐guided TBLB. Therefore, we cannot directly compare diagnostic yield between conventional fluoroscopy‐guided and CT‐guided TBLB. Multicenter studies with large sample sizes are needed to validate the effectiveness of CT‐guided TBLB. Finally, we could not analyze for ground glass nodules because we only enrolled patients with solid nodules or masses. Further studies will be needed to determine the diagnostic yield of CT‐guided TBLB for ground glass nodules.
In conclusion, we found that CT‐guided TBLB provides a good diagnostic yield for pulmonary nodules or masses, with a relatively low risk of radiation exposure and low rate of complications. The positioning of biopsy forceps under the guidance of real‐time CT can predict successful biopsy results. In selected patients, CT‐guided TBLB is a suitable alternative for the diagnosis of lung lesions that are difficult to evaluate by other procedures.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors are indebted to all who participated in this study. We would like to thank the healthcare professionals at National Health Insurance Service Ilsan Hospital for their assistance, specifically those working in the pulmonology units.
References
- 1. Fletcher EC, Levin DC. Flexible fiberoptic bronchoscopy and fluoroscopically guided transbronchial biopsy in the management of solitary pulmonary nodules. West J Med 1982; 136: 477–83. [PMC free article] [PubMed] [Google Scholar]
- 2. Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117: 1049–54. [DOI] [PubMed] [Google Scholar]
- 3. Zavala DC. Diagnostic fiberoptic bronchoscopy: Techniques and results of biopsy in 600 patients. Chest 1975; 68: 12–9. [DOI] [PubMed] [Google Scholar]
- 4. Kvale PA, Bode FR, Kini S. Diagnostic accuracy in lung cancer; comparison of techniques used in association with flexible fiberoptic bronchoscopy. Chest 1976; 69: 752–7. [DOI] [PubMed] [Google Scholar]
- 5. Cortese DA, McDougall JC. Biopsy and brushing of peripheral lung cancer with fluoroscopic guidance. Chest 1979; 75: 141–5. [DOI] [PubMed] [Google Scholar]
- 6. Torrington KG, Kern JD. The utility of fiberoptic bronchoscopy in the evaluation of the solitary pulmonary nodule. Chest 1993; 104: 1021–4. [DOI] [PubMed] [Google Scholar]
- 7. Gasparini S, Ferretti M, Secchi EB, Baldelli S, Zuccatosta L, Gusella P. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest 1995; 108: 131–7. [DOI] [PubMed] [Google Scholar]
- 8. Choi MJ, Kim Y, Hong YS, Shim SS, Lim SM, Lee JK. Transthoracic needle biopsy using a C‐arm cone‐beam CT system: Diagnostic accuracy and safety. Br J Radiol 2012; 85: e182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi JW, Park CM, Goo JM et al. C‐arm cone‐beam CT‐guided percutaneous transthoracic needle biopsy of small (≤ 20 mm) lung nodules: Diagnostic accuracy and complications in 161 patients. AJR Am J Roentgenol 2012; 199: W322–30. [DOI] [PubMed] [Google Scholar]
- 10. Jin KN, Park CM, Goo JM et al. Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C‐arm cone‐beam CT systems. Eur Radiol 2010; 20: 2108–15. [DOI] [PubMed] [Google Scholar]
- 11. Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound‐guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002; 20: 972–4. [DOI] [PubMed] [Google Scholar]
- 12. Shirakawa T, Imamura F, Hamamoto J et al. Usefulness of endobronchial ultrasonography for transbronchial lung biopsies of peripheral lung lesions. Respiration 2004; 71: 260–8. [DOI] [PubMed] [Google Scholar]
- 13. Herth FJ, Eberhardt R, Becker HD, Ernst A. Endobronchial ultrasound‐guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: A prospective trial. Chest 2006; 129: 147–50. [DOI] [PubMed] [Google Scholar]
- 14. Wagner U, Walthers EM, Gelmetti W, Klose KJ, von Wichert P . Computer‐tomographically guided fiberbronchoscopic transbronchial biopsy of small pulmonary lesions: A feasibility study. Respiration 1996; 63: 181–6. [DOI] [PubMed] [Google Scholar]
- 15. Kickuth R, Kirchner J, Laufer U, Sanner B, Haske M, Liermann D. Better visualization of transbronchial biopsy using CT fluoroscopy. Cardiovasc Intervent Radiol 2000; 23: 347–50. [DOI] [PubMed] [Google Scholar]
- 16. Heyer CM, Kagel T, Lemburg SP et al. Transbronchial biopsy guided by low‐dose MDCT: A new approach for assessment of solitary pulmonary nodules. AJR Am J Roentgenol 2006; 187: 933–9. [DOI] [PubMed] [Google Scholar]
- 17. Hautmann H, Henke MO, Bitterling H. High diagnostic yield from transbronchial biopsy of solitary pulmonary nodules using low‐dose CT‐guidance. Respirology 2010; 15: 677–82. [DOI] [PubMed] [Google Scholar]
- 18. Shinagawa N, Yamazaki K, Onodera Y et al. CT‐guided transbronchial biopsy using an ultrathin bronchoscope with virtual bronchoscopic navigation. Chest 2004; 125: 1138–43. [DOI] [PubMed] [Google Scholar]
- 19. Tsushima K, Sone S, Hanaoka T, Takayama F, Honda T, Kubo K. Comparison of bronchoscopic diagnosis for peripheral pulmonary nodule under fluoroscopic guidance with CT guidance. Respir Med 2006; 100: 737–45. [DOI] [PubMed] [Google Scholar]
- 20. Shinagawa N, Yamazaki K, Onodera Y et al. Factors related to diagnostic sensitivity using an ultrathin bronchoscope under CT guidance. Chest 2007; 131: 549–53. [DOI] [PubMed] [Google Scholar]
- 21. Ost D, Shah R, Anasco E et al. A randomized trial of CT fluoroscopic‐guided bronchoscopy vs conventional bronchoscopy in patients with suspected lung cancer. Chest 2008; 134: 507–13. [DOI] [PubMed] [Google Scholar]
- 22. Saji H, Nakamura H, Tsuchida T et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT‐guided lung biopsy: The angle of the needle trajectory is a novel predictor. Chest 2002; 121: 1521–6. [DOI] [PubMed] [Google Scholar]
- 23. Kikuchi E, Yamazaki K, Sukoh N et al. Endobronchial ultrasonography with guide‐sheath for peripheral pulmonary lesions. Eur Respir J 2004; 24: 533–7. [DOI] [PubMed] [Google Scholar]
- 24. Kurimoto N, Miyazawa T, Okimasa S et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004; 126: 959–65. [DOI] [PubMed] [Google Scholar]


