Abstract
Background
The study was conducted to investigate clinical and computed tomography (CT) features in Chinese lung cancer patients with human immunodeficiency virus (HIV).
Methods
Forty consecutive lung cancer patients with HIV were included. Clinical data were collected, and CT features were reviewed and measured. The factors associated with stages of cancer and the CT features with opportunistic pulmonary infections (OPIs) were also analyzed.
Results
Thirty‐four of the patients were men (85%), and the mean age was 57.5 years. The mean CD4 count was 288 cells/μL, and 23 patients received highly active antiretroviral therapy. OPIs were common (50%). The major histological type (85%) was non‐small cell lung cancer (NSCLC), and 15 NSCLC patients (44%) were in stages IIIb and IV. NSCLC patients with an OPI were more common in the advanced stages compared with those without an OPI (P = 0.04). There were no significant differences in advanced and non‐advanced stages in terms of CD4 level, highly active antiretroviral therapy, and smoking (P = 0.31, P = 1.00; P = 0.49, respectively). The average size of tumors was 4.5 cm. Irregularly shaped or larger sized tumors were associated with OPIs (P = 0.03, P = 0.04, respectively).
Conclusions
The persistence of locally irregular and large lesions in middle‐aged men with HIV and a history of OPIs should be an alert for lung cancer, and clinical management is needed.
Keywords: Acquired immunodeficiency syndrome, computed tomography, human immunodeficiency virus, lung cancer
Introduction
Morbidity and mortality associated with opportunistic infection in patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) has dramatically decreased since the introduction and the extensive application of highly active antiretroviral therapy (HAART) in the last two decades.1, 2 Morbidity and mortality has also decreased in AIDS‐defining cancers (ADCs), such as Kaposi's sarcoma (KS), Non‐Hodgkin's lymphoma (NHL), and cervical cancer.3, 4 The life expectancy of these patients has been extended, and their quality of life has been ameliorated. However, the incidence of non‐AIDS‐defining cancers (NADCs), such as lung cancer, melanoma, anal cancer, hepatocellular carcinoma, and Hodgkin's lymphoma,5 has recently increased in these patients6, 7, 8 and is significantly higher than that of the general population.9 It was estimated that half of the cancers of this population were NADCs.10 Lung cancer was the most prevalent type of cancer,7, 10, 11, 12, 13, 14, 15 and the incidence in this population was approximately two to four times higher than that of the general population.9 In addition, lung cancer affects younger patients in this population with more advanced stages and a poorer overall survival rate compared with the general population.16
In China, although relevant data are limited, a retrospective study reported that 7.7% of hospitalized patients had concurrent cancers, and 66.4% of them had NADCs (13% with lung cancer).17 Our center (a designated hospital for HIV/AIDS care of the Shanghai Municipal Commission of Health and Family Planning) has also recently witnessed an increasing trend in NADCs, particularly lung cancer. Moreover, an early diagnosis of lung cancer was mostly delayed in the setting of an opportunistic pulmonary infection (OPI), and low clinical suspicion might be a factor.18 There were 437 000 people living with HIV/AIDS in China by 2014, as a result of the availability of HAART and subsequent prolongation of survival.19 Therefore, our study purpose was to obtain early diagnostic clues by reviewing the clinical and radiological features of lung cancer in the population and, consequently, improve patient prognosis with timely intervention.
Methods
Medical records
We reviewed the medical records of patients with lung cancer and HIV infection confirmed by histology and the local Centers for Disease Control and Prevention from three hospitals during November 2011 and April 2016. A total of 40 consecutive patients were included for further demographic analyses (e.g. age at diagnosis, gender), chief complaints, smoking history, HIV‐1 viral loads, CD4+ T cell counts, HAART duration, OPIs, histological types, and stages.
Staging was based on either the International Association for the Study of Lung Cancer (IASLC, 7th edition) tumor node metastasis (TNM) classification for NSCLC or Veterans Administration Lung Study Group (VALSG) criteria for limited disease and extensive disease for small cell lung cancer (SCLC).20, 21 Pathological staging was utilized in patients who received surgery, while clinical staging was used for patients who had not undergone surgery. Chinese guidelines for the diagnosis and treatment of primary lung cancer were referred to in the management.22
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation in the three hospitals, and with the Helsinki Declaration of 1975 (revised 2008). Informed consent was obtained from all included patients or an immediate family member if they had died.
Chest computed tomography (CT)
Computed tomography scans were performed using various scanners (Siemens Sensation 16, Siemens Medical Systems, Forcheim, Germany; Hitachi Scenaria 64, Hitachi Medical Systems, Tokyo, Japan). The patients were examined in the supine position with their arms extended overhead. Scans were obtained at 5 mm intervals from the thoracic inlet to the inferior of the adrenal gland with a suspension after the end of inspiration. The parameters were as follows: tube voltage 120 or 140 kV; tube currency 160–250 mA; gantry rotation time 0.5 seconds; collimation 16 × 0.75 mm, 64 × 0.625 mm; pitch 1 or 1.0781; field of view (FOV) 350 mm; matrix 512 × 512; and slice thickness/interval 5 mm/5 mm. Contrast‐enhanced scans were performed 40 seconds after iohexol (Shuangbei 100 mL: 35 g [I], Beilu Pharmaceutical Co. Ltd., Beijing, China) was injected via the cubital vein at a rate of 2.5–3 mL/second and a total volume of 70–90 mL. Reconstruction images were obtained in 1 mm slice thicknesses at 0.5 mm intervals and a 150 mm FOV on the targeted region using a lung standard algorithm.
The CT scans were blindly reviewed and interpreted by two thoracic radiologists with seven and 15 years of experience in diagnostic chest imaging, via the Picture Archiving and Communication System (PACS, UniRISC, DJ HealthUnion, Shanghai, China). If there was any disagreement, consensus was achieved through discussion. The evaluation included tumor location (lobar distribution); size (the maximal diameter on the transverse section); shape (irregular or regular [round or oval]); intensity (homogeneous or heterogeneous); adjacent obstructive pneumonia/atelectasis (increased attenuation accompanying a reduced volume of the adjacent affected lung);23 pleural indentation, thickening, or effusion; adenopathy (mediastinal, hilar and axillary nodes with short axes of at least 1 cm);24 and enhancement after the administration of contrast materials.
Clinical impressions from determinate benign, likely benign, indeterminate, likely malignant, to determinate malignant were made. A misdiagnosis was defined as an impression of indeterminacy, likely, or determinate benignity.
Statistical analysis
Statistical analysis was performed by STATA (version 12.0; Statacorp; Houston, TX, USA) using a Mann–Whitney test for CD4+ cell counts, an unpaired t‐test for tumor size, and a Fisher's exact test for the categorical data (i.e. proportion of HAART, smoking, and OPI with different stages; proportion of tumor with clear borders, regular shape, pleural changes, hydrothorax, and adenopathy with OPI). A two‐tailed P value of less than 0.05 indicated a significant difference.
Results
Clinical findings
The clinical data of patients with lung cancer and HIV are displayed in Table 1. Most of the patients were men (85%, 34/40). The mean age at diagnosis was 57.5 years (range 40–77). Seven patients complained of bloody phlegm, seven complained of chest pain, 21 experienced cough (4/21 had accompanied expectoration), five complained of chest tightness, seven had no symptoms, and one patient experienced fatigue only. Eighteen patients (45%, 18/40) had a history of smoking, with a mean consumption of 50 pack‐years (range 15–160), and all were men.
Table 1.
Clinical data of 40 patients with lung cancer and HIV infection
| Case No./Gender/Age | CC | Smoking (pack‐years) | OPI | HIV‐RNA (copies/mL) | CD4 (cells/μL) | HAART (months) | Histology | TNM/stage |
|---|---|---|---|---|---|---|---|---|
| 1/M/40 | Bloody phlegm | 0 | No | NA | 522 | 3 | ADC | pT2bN0M0/IIa |
| 2/M/52 | Cough, chest pain | 60 | No | 83 500 | 223 | 0 | SCLC | ED |
| 3/M/50 | Cough, expectoration | 15 | TB | <40 | 464 | 24 | ADC | cT3N2M1a/IV |
| 4/F/51 | Fatigue | 0 | No | 142 | 319 | NA | ADC | pT2aN0M0/Ib |
| 5/M/62 | Cough, expectoration | 30 | TB | 50.4 | 38 | 9 | ADC | cTxN3M1b/IV |
| 6/M/57 | Cough, polypnea | 0 | TB, NTM | 183 | 127 | 0 | SCLC | LD |
| 7/M/55 | Cough | 0 | No | <40 | 594 | 96 | LCNEC | pT1bN0M0/Ia |
| 8/M/44 | Cough | 60 | TB, F | NA | 98 | 36 | SCC | cT2aN3M0/IIIb |
| 9/M/51 | Chest pain | 60 | TB | NA | 213 | 0 | SCC | cT3N3M1a/IV |
| 10/M/50 | None | 20 | No | NA | 266 | 24 | ADC | pT2aN0M0/Ib |
| 11/M/44 | Bloody phlegm | 0 | NTM, F | <40 | 111 | 36 | ADC | pT2bN2M0/IIIa |
| 12/M/59 | Cough, bloody phlegm | 30 | TB | NA | 263 | 0 | LCC | pT3N0M0/IIb |
| 13/M/77 | Cough, expectoration | 0 | No | 40 | 274 | 0 | SCC | pT1bN0M0/Ia |
| 14/F/59 | Bloody phlegm | 0 | TB | <40 | 373 | 24 | AC | pT3N0M0/IIb |
| 15/M/53 | Chest tightness, polypnea | 0 | No | NA | 281 | 24 | ADC | cT4N2M1a/IV |
| 16/M/67 | Cough | 48 | No | NA | 341 | 0 | SCLC | ED |
| 17/F/62 | Cough | 0 | No | NA | 204 | 0 | ADC | pT1aN0M0/Ia |
| 18/M/60 | Cough, bloody phlegm, chest pain | 60 | No | <40 | 235 | 72 | SCC | cT4N1M0/IIIa |
| 19/M/65 | Cough | 0 | F | NA | 145 | 0 | ADC | cT1bN0M0/Ia |
| 20/M/59 | None | 37.5 | No | 9010 | 160 | 12 | SCC | pT2bN2M0/IIIa |
| 21/M/65 | None | 30 | No | <40 | 253 | 3 | SCC | pT2aN0M0/Ib |
| 22/M/57 | Chest tightness | 36 | No | NA | 174 | 0 | ADC | cT2aN3M1b/IV |
| 23/F/57 | Chest tightness | 0 | TB | NA | 483 | 36 | ADC | cTxN2M1a/IV |
| 24/M/60 | Chest tightness | 0 | No | 4170 | 641 | 0 | ADC | cTxN1M1a/IV |
| 25/M/57 | Cough, bloody phlegm | 160 | No | 90 900 | 422 | 0 | SCC | pT1aN0M0/Ia |
| 26/M/46 | Chest pain | 0 | PCP | <40 | 412 | 32 | ADC | pT1aN0M0/Ia |
| 27/M/51 | None | 0 | No | 7630 | 435 | 0 | ADC | pT2aN1M0/IIa |
| 28/M/66 | Cough, fever | 0 | F | 21 300 | 113 | 0 | SCC | cT4N3M1a/IV |
| 29/M/76 | Cough, chest tightness | 0 | PCP, TB | NA | 325 | 24 | ADC | cTxN0M1b/IV |
| 30/M/58 | Cough, bloody phlegm | 52.5 | No | 10 400 | 210 | 0 | LCC | cT4N2M1a/IV |
| 31/M/63 | Cough, expectoration | 50 | TB | NA | NA | 24 | SCC | cT4NxM1b/IV |
| 32/M/62 | None | 80 | F | <40 | 401 | 168 | ADC | pT1aN0M0/Ia |
| 33/M/69 | None | 0 | No | NA | 574 | 36 | ADC | pT1aN0M0/Ia |
| 34/M/51 | None | 0 | No | NA | 147 | 60 | ADC | pT2aN1M0/IIa |
| 35/F/67 | Cough, fever | 0 | F | NA | 245 | 0 | ADC | cT2aNxM1a/IV |
| 36/M/55 | Chest pain, fever | 0 | F | 3860 | 207 | 0 | ADC | cT3N1M0/IIIa |
| 37/M/52 | Chest pain | 30 | TB | NA | 109 | 2 | ADC | cT3N3M1b/IV |
| 38/M/37 | Chest pain, cough, phlegm | 40 | No | <40 | 679 | 60 | SCLC | LD |
| 39/M/66 | Cough, phlegm | 0 | F, TB | NA | 8 | 20 | SCLC | ED |
| 40/F/68 | Cough, phlegm | 0 | No | NA | 173 | 96 | SCC | cT4N3M1a/IV |
AC, atypical carcinoid; ADC, adenocarcinoma; CC, chief complaint; c, clinical; ED, extensive disease; F, fungus; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; LCC, large cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; LD, limited disease; NA, not available; NTM, non‐tuberculosis mycobacteria; OPI, opportunistic pulmonary infection; p, pathological; PCP, Pneumocystis carinii pneumonia; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; TB, tuberculosis; TNM, tumor node metastasis.
Twenty patients (50%, 20/40) had an OPI before the diagnosis of lung cancer: eight patients had tuberculosis (TB), five had a fungal infection (FI), two had Pneumocystis carinii pneumonia (PCP), one had both non‐tuberculosis mycobacteria (NTM) and TB, two had both TB and an FI, one had both NTM and an FI, and one patient had both TB and PCP.
An HIV‐1 viral load was available for 21 patients ranging from under the lower limits of detection (<40 copies/mL) to 90 900 copies/mL. The mean CD4+ T cell count was 288 cells/μL (range 8–641 cells/μL; normal lower limits of 410 cells/μL in our laboratory) within one month of the diagnosis of lung cancer. Twenty‐three patients received HAART, with a mean duration of 40 months (range 2–168) at diagnosis.
Histological type was based on either tissues or cells from percutaneous fine needle aspiration under CT guidance (21 patients), bronchoscopic biopsy (8 patients) and resected specimen (7 patients), or on hydrothorax (4 patients). Thirty‐four patients (85%, 34/40) were confirmed with NSCLC: 21 with adenocarcinoma, 10 with squamous cell carcinoma, and three with large cell carcinoma (1 patient with neuroendocrine). Five patients had SCLC, and one patient had an atypical carcinoid.
Fifteen patients (44.1%, 15/34) with NSCLC were in advanced stages (14 at stage IV, 1 at stage IIIb), while the remaining 19 patients were in stages I–IIIa (8 at stage Ia, 3 at stage Ib, 3 at stage IIa, 1 at stage IIb, and 4 at stage IIIa). Two patients with SCLC had limited disease, and three had extensive disease.
Six patients received positron emission tomography–CT (PET‐CT) scans before surgery and chemotherapy. The remaining 34 patients were evaluated by other imaging modalities (e.g. ultrasound, contrast‐enhanced magnetic resonance imaging, and CT scan) for possible remote metastases.
Seventeen patients underwent surgery (10 radical resection, 4 lobectomy, 3 wedge resection); 15 patients received chemotherapy (14 in stage IV, 1 with poor pulmonary function in stage IIIa ineligible for surgery). Eight patients were not treated with either surgery or chemotherapy: three had a poor overall general condition, one died from a severe pulmonary infection before treatment, and four patients refused treatment.
Factors associated with stages
Eight NSCLC patients in advanced stages had a history of smoking (53.3%, 8/15), while seven in non‐advanced stages had a history of smoking (36.8%, 7/19). Smoking history did not differ between the advanced and non‐advanced stage groups (P = 0.49). Out of the 15 NSCLC patients at advanced stage, 10 (66.7%, 10/15) had a previous OPI, while six (31.6%, 6/19) with non‐advanced stages had a previous OPI. An OPI was more common in NSCLC patients at advanced stage (P = 0.04). The mean CD4+ T cell count of the advanced NSCLC patients did not differ from that of the non‐advanced NSCLC patients (313 vs. 255 cells/μL; P = 0.31). Of the 20 patients receiving HAART before a lung cancer diagnosis, nine were at advanced stages (45%, 9/20), and no difference was observed between the stages for patients treated with or without HAART (P = 1.00) (Table 2).
Table 2.
Comparisons of associated factors between non‐advanced stages
| Factors | Stages | P | |
|---|---|---|---|
| I–IIIa† | IIIb–IV† | ||
| Smoking (+) | 7 | 8 | 0.49 |
| Smoking (−) | 12 | 7 | |
| OPI (+) | 6 | 10 | 0.04 |
| OPI (−) | 13 | 5 | |
| CD4+ T cell count | 313 | 255 | 0.31 |
| HAART (+) | 11 | 9 | 1.00 |
| HAART (−)‡ | 7 | 6 | |
I–IIIa and advanced stages (IIIb–IV) of non‐small cell lung cancer with HIV infection.
HAART data unavailable in case 4 with stage Ib, thus patient data was excluded.
Bold indicates statistically significant difference.
(+), with; (−), without; CD4+, CD4 positive; HAART, highly active antiretroviral therapy; OPI, opportunistic pulmonary infection; CD4+ T cell count‐using Mann–Whitney test; smoking, OPI, HAART using Fisher's exact text.
CT findings
Most of the lung cancers (80%, 32/40 patients) were in lobar distribution: 10 in the right upper lobes, 10 in the right lower lobes, seven in the left upper lobes, four in the left lower lobes, and one in the right middle lobe. One squamous cell carcinoma was located within the middle segmental bronchus, and one was located in the right hilum. One Pancoast tumor was located in the right apex with adjacent rib destruction. One multiple metastatic adenocarcinoma was scattered in different lobes. The remaining four tumors were indeterminate: one was possibly from the right upper lobe, one might have been from the right hila, and two might have been from the left hila.
The majority of lung cancers with clear borders were round or oval (71%, 22/31 tumors; 16 with lobulation, 13 with spiculation), and the remaining nine tumors manifested as irregular masses (8 tumors) or an irregular thickening cavity (1 tumor). The average size of the lung cancers with clear borders was 4.5 cm (range 0.4–12 cm), and the density of these tumors was mostly heterogeneous (80.6%, 25/31 tumors). Fifteen patients received contrast CT scans with obvious heterogeneous enhancement of the tumor after contrast administration (increased enhancement >15 HU).
Obstructive pneumonia/atelectasis distal to the tumor was frequent (65%, 26/40 patients). Adenopathy was quite common, especially in the mediastinal lymph nodes (75%, 30/40). Thirteen patients with adenopathy showed confluence, and four patients had axillary adenopathy. In the patients with adenopathy, 16 had OPIs (53.3%, 16/30).
Hydrothorax was observed in 13 patients with ipsilateral predominance (84.6%, 11/13), and was mostly in minimum stage (69.2%, 9/13). Irregular pleural thickening was observed in 22 patients (55%, 22/40); seven of these patients were complicated with multiple nodules. Pleural indentation was observed in 14 patients; 11 of these patients had histological adenocarcinoma (78.6%, 11/14).
CT features with opportunistic pulmonary infection
The relationship between certain CT features and OPIs was analyzed (Table 3). An OPI was more frequent in tumors with an irregular shape (75.0%, 9/12 patients) than in those with a round or an oval shape (31.8%, 7/22; P = 0.03) and in tumors larger in size (5.34 ± 0.72 vs. 3.66 ± 0.41; P = 0.04). Other CT features, including a tumor with or without a definite pulmonary lesion, with or without adenopathy, pleural indentation, pleural thickening, and hydrothorax, did not differ with respect to OPIs (P = 0.66, 0.72, 0.21, 0.34, and 0.20, respectively).
Table 3.
Comparison of CT features of lung tumors between HIV patients with and without OPI
| CT features | OPI | P | |
|---|---|---|---|
| + | − | ||
| TD | 16 | 18 | 0.66 |
| TI | 4 | 2 | |
| Round/oval | 7 | 15 | 0.03 |
| Irregular† | 9 | 3 | |
| Size (cm) | 5.34 ± 0.72 | 3.66 ± 0.41 | 0.04 |
| Adenopathy (+) | 16 | 14 | 0.72 |
| Adenopathy (−) | 4 | 6 | |
| PI (+) | 8 | 6 | 0.21 |
| PI (−) | 10 | 16 | |
| PT (+) | 14 | 8 | 0.34 |
| PT (−) | 8 | 10 | |
| Hydrothorax (+) | 9 | 4 | 0.20 |
| Hydrothorax (−) | 11 | 16 | |
Four patients with opportunistic pulmonary infection (OPI) had indeterminate lung cancers (e.g. tumors mixed with infection or atelectasis, tumors without concise origin), and two patients without OPI.
(+), with; (−), without; PI, pleural indentation; PT, pleural thickening; TD, tumor with determinate boarder; TI, tumor with indeterminate boarder.
Bold indicates statistically significant difference.
Radiological impression
Twenty‐seven patients were diagnosed with great confidence (13 with determinate malignancy, 14 with likely malignancy). In addition, six patients were diagnosed with dilemma, while seven were misdiagnosed with an infection (2 with interpretation of a TB infection, 4 with interpretation of an FI [Fig 1], and 1 with interpretation of pleural malignancy for its broad base adjacent to the pleura).
Figure 1.
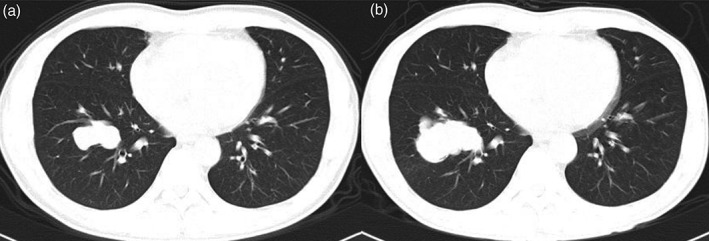
A 44‐year‐old male treated with highly active antiretroviral therapy for 36 months complained of occasional bloody phlegm. (a) An irregular mass with a maximal diameter of 4.4 cm in the transverse section was located in the right lower lobe. He received antibiotic treatment, as the mass was interpreted as an infection and non‐tuberculosis mycobacteria was proven by sputum culture. (b) Eighteen months later, the mass had grown to 5.9 cm. Mucinous adenocarcinoma was histologically confirmed after radical resection (stage IIIa).
Discussion
Similar to a previous review, our study also determined that lung cancer was common in male patients with HIV.13 This may be because of the high frequency of HIV in men as a result of high‐risk sexual behavior, particularly men who have sex with men, and heavy tobacco consumption in this population. The mean age was 57.5 years old, which was somewhat younger than the general population and slightly older than that reported by Winstone et al.13 Our study sample included lung cancer patients likely HIV infected at a relatively older age and younger HIV patients who died from serious OPIs before lung cancer genesis, compared with the sample in Winstone et al.'s review. OPIs were common in our patients. Inflammation caused by an OPI, notably the Pneumocystis carinii infection (a common subtype of an OPI), might promote lung tumor genesis though the activation of inflammation and coagulation of the inflammatory cytokines,25, 26, 27 as some studies found high rates of such pathogen colonization were detected in lung cancer.28, 29 Two of the patients in our sample developed lung adenocarcinoma two years after PCP (Fig 2).
Figure 2.
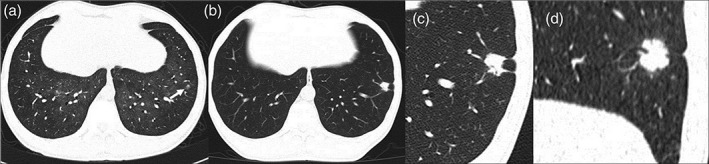
A 46‐year‐old male treated with highly active antiretroviral therapy for 32 months complained of chest pain for two months. (b–d) A solid nodule with lobulation and pleural indentation in the left lower lobe was noticed in the lung window on chest computed tomography scan, which was more prominent and larger than it was two years before (a), when it was inconspicuous (white arrow) in the set of Pneumocystis carinii pneumonia. Adenocarcinoma was confirmed by wedge resection (stage Ia).
Similar to the results of other reports, our study also found that NSCLC was the most common histological type of lung cancer, and adenocarcinoma was the most predominant subtype.30, 31, 32 Forty‐four percent of the NSCLC patients were in advanced stages. We analyzed the possible risk factors (including smoking, OPIs, CD4+ T cell counts, and HAART) associated with lung cancer staging) and found that the patients with OPIs were more frequently in advanced stage compared with other risk factors. This may be a result of the delayed diagnosis of lung cancer in patients with OPIs. Preventive medication for OPIs and clinical suspicion of lung cancer of a persistent pulmonary lesion after regular and adequate antibiotic treatment might help to make an earlier diagnosis, and ultimately, improve survival.
Computed tomography scan manifestations of lung cancer in patients with HIV infection have previously been reported in North America and Europe;33, 34, 35 however, few studies have been reported in the Chinese literature thus far. Our study found that all of the lung cancers with prominent nodules or masses on CT scan were in a lobar distribution. Of these 32 lung cancers, 65.6% were in the right lung, similar to previous reports.33, 35 Lobulation and spiculation were common, mirroring similar manifestations in the general population. The mean size of the tumors with clear borders on CT scan was 4.5 cm; therefore, the intensity was usually heterogeneous, and they were generally classified as T2 stage. Secondary manifestations, including OPIs, hydrothorax, pleural thickening or indentation, and adenopathy, were also quite frequent in our group. It is worth mentioning that adenopathy is common in the era of HAART, which is mainly caused by OPIs and malignancy. A recent investigation found that the prevalence of adenopathy caused by NADCs, such as lung cancer, had increased.36 In view of this, an early biopsy of enlarged lymph nodes should be conducted in such patients without overt pulmonary lesions when necessary.
Opportunistic pulmonary infections are common in China, which might result from a lack of awareness of HIV infection status and poor compliance with HAART. In view of this, we compared CT scan manifestations of lung cancer with and without OPIs and found that lung cancers with OPIs tended to be irregular and larger. However, there was no significant statistically difference in the latter, indicating that aggressive follow‐up of persistently suspected OPIs, especially mass‐like and irregular pulmonary lesions, is warranted for the early detection of lung cancers.
In our clinical practice, the misdiagnosis rate was 32.5% (13/40 patients), and the major misdiagnosis was infectious disease (53.8%, 7/13). This may primarily be because of low suspicion, as pulmonary infection is common, and the laboratory confirmed the specific pathogens. Furthermore, there was an overlap of CT manifestations between infections and tumors. Secondly, some of the imaging interpreters had limited experience in the diagnosis of lung cancer. Thirdly, there was lack of other referential examinations, such as a PET‐CT scan, for economic reasons.
Admittedly, there are several limitations to our study. Firstly, some selection bias is introduced in a retrospective study, and further prospective study is needed. Secondly, measurement bias may have occurred, as data were collected retrospectively; however, the pulmonary lesions were measured and reviewed blindly. Thirdly, PET‐CT scans were not generally used for TNM staging, because of economic considerations, but also because of the lack of such a modality in our center. Generally, an infectious disease hospital is not equipped to perform PET‐CT scans in China. Discrimination usually occurs if a patient attends a general hospital. Nevertheless, we used other compensative imaging modalities to evaluate and determine TNM stages according to Chinese guidelines for the diagnosis and treatment of primary lung cancer. Finally, a single‐center study with a relatively small sample size makes generalization of the findings difficult; therefore, a multicenter study with a larger sample size is warranted.
Lung cancer at advanced stage is more common in middle‐aged men with HIV and OPIs. HIV patients with CT manifestation of irregular and large pulmonary lesions and a history of OPIs should be suspected of lung cancer, and receive a biopsy or regular follow up, if necessary.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We sincerely thank Dr. Yinzhong Shen for his help in clinical data assessment and the editors from Elsevier Webshop for language editing.
References
- 1. Mocroft A, Ledergerber B, Katlama C et al. Decline in the AIDS and death rates in the EuroSIDA study: An observational study. Lancet 2003; 362: 22–9. [DOI] [PubMed] [Google Scholar]
- 2. Palella FJ Jr, Baker RK, Moorman AC et al. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43: 27–34. [DOI] [PubMed] [Google Scholar]
- 3. Hleyhel M, Belot A, Bouvier AM et al. Trends in survival after cancer diagnosis among HIV‐infected individuals between 1992 and 2009. Results from the FHDH‐ANRS CO4 cohort. Int J Cancer 2015; 137: 2443–53. [DOI] [PubMed] [Google Scholar]
- 4. Seaberg EC, Wiley D, Martinez‐Maza O et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer 2010; 116: 5507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sigel K, Dubrow R, Silverberg M, Crothers K, Braithwaite S, Justice A. Cancer screening in patients infected with HIV. Curr HIV/AIDS Rep 2011; 8: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powles T, Robinson D, Stebbing J et al. Highly active antiretroviral therapy and the incidence of non‐AIDS‐defining cancers in people with HIV infection. J Clin Oncol 2009; 27: 884–90. [DOI] [PubMed] [Google Scholar]
- 7. Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV‐infected individuals. AIDS 2008; 22: 489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowalski J, Cholewinska G, Pyziak‐Kowalska K et al. The spectrum of malignancies among adult HIV cohort in Poland between 1995 and 2012: A retrospective analysis of 288 cases. Contemp Oncol (Pozn) 2015; 19: 226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel P, Hanson DL, Sullivan PS et al. Incidence of types of cancer among HIV‐infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148: 728–36. [DOI] [PubMed] [Google Scholar]
- 10. Alshafie MT, Donaldson B, Oluwole SF. Human immunodeficiency virus and lung cancer. Br J Surg 1997; 84: 1068–71. [PubMed] [Google Scholar]
- 11. Shiels MS, Cole SR, Kirk GD, Poole C. A meta‐analysis of the incidence of non‐AIDS cancers in HIV‐infected individuals. J Acquir Immune Defic Syndr 2009; 52: 611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frisch M, Biggar RJ, Engels EA, Goedert JJ, AIDS‐Cancer Match Registry Study Group . Association of cancer with AIDS‐related immunosuppression in adults. JAMA 2001; 285: 1736–45. [DOI] [PubMed] [Google Scholar]
- 13. Winstone TA, Man SF, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest 2013; 143: 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brugnaro P, Morelli E, Cattelan F et al. Non‐AIDS definings malignancies among human immunodeficiency virus‐positive subjects: Epidemiology and outcome after two decades of HAART era. World J Virol 2015; 4: 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petoumenos K, Hui E, Kumarasamy N et al. Cancers in the TREAT Asia HIV Observational Database (TAHOD): A retrospective analysis of risk factors. J Int AIDS Soc 2010; 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pakkala S, Ramalingam SS. Lung cancer in HIV‐positive patients. J Thorac Oncol 2010; 5: 1864–71. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Su S, Zhao H et al. Prevalence and mortality of cancer among HIV‐infected inpatients in Beijing, China. BMC Infect Dis 2016; 16 (1): 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brock MV, Hooker CM, Engels EA et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: Implications for patient care. J Acquir Immune Defic Syndr 2006; 43: 47–55. [DOI] [PubMed] [Google Scholar]
- 19. UNAIDS . 2014 China AIDS Response Progress Report. National Health and Family Planning Commission of the People's Republic of China 2014. [Cited 24 Feb 2017.] Available from URL: http://www.unaids.org.cn/en/index/Document_view.asp?id=859.
- 20. Shepherd FA, Crowley J, Van Houtte P et al The International Association for the Study of Lung Cancer lung cancer staging project: Proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007; 2: 1067–77. [DOI] [PubMed] [Google Scholar]
- 21. Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 1973; 4 (2): 31–42. [PubMed] [Google Scholar]
- 22. Zhi X, Wu Y, Ma S et al [Chinese guidelines on the diagnosis and treatment of primary lung cancer (2011 version). ]Zhongguo Fei Ai Za Zhi 2012; 15: 677–88. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. [DOI] [PubMed] [Google Scholar]
- 24. Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: Number and size according to American Thoracic Society mapping. AJR Am J Roentgenol 1985; 144: 261–5. [DOI] [PubMed] [Google Scholar]
- 25. Kawabata S, Heredia A, Gills J, Redfield RR, Dennis PA, Bryant J. Impact of HIV on lung tumorigenesis in an animal model. AIDS 2015; 29: 633–5. [DOI] [PubMed] [Google Scholar]
- 26. Shiels MS, Pfeiffer RM, Hildesheim A et al Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013; 105: 1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borges AH, Silverberg MJ, Wentworth D et al Predicting risk of cancer during HIV infection: The role of inflammatory and coagulation biomarkers. AIDS 2013; 27: 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 2012; 25: 297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossouw TM, Anderson R, Feldman C. Impact of HIV infection and smoking on lung immunity and related disorders. Eur Respir J 2015; 46: 1781–95. [DOI] [PubMed] [Google Scholar]
- 30. Powles T, Nelson M, Bower M. HIV‐related lung cancer ‐‐ a growing concern? Int J STD AIDS 2003; 14: 647–51. [DOI] [PubMed] [Google Scholar]
- 31. Hakimian R, Fang H, Thomas L, Edelman MJ. Lung cancer in HIV‐infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol 2007; 2: 268–72. [DOI] [PubMed] [Google Scholar]
- 32. D'Jaen GA, Pantanowitz L, Bower M et al Human immunodeficiency virus‐associated primary lung cancer in the era of highly active antiretroviral therapy: A multi‐institutional collaboration. Clin Lung Cancer 2010; 11: 396–404. [DOI] [PubMed] [Google Scholar]
- 33. Fishman JE, Schwartz DS, Sais GJ, Flores MR, Sridhar KS. Bronchogenic carcinoma in HIV‐positive patients: Findings on chest radiographs and CT scans. AJR Am J Roentgenol 1995; 164: 57–61. [DOI] [PubMed] [Google Scholar]
- 34. White CS, Haramati LB, Elder KH, Karp J, Belani CP. Carcinoma of the lung in HIV‐positive patients: Findings on chest radiographs and CT scans. AJR Am J Roentgenol 1995; 164: 593–7. [DOI] [PubMed] [Google Scholar]
- 35. Bazot M, Cadranel J, Khalil A et al Computed tomographic diagnosis of bronchogenic carcinoma in HIV‐infected patients. Lung Cancer 2000; 28: 203–9. [DOI] [PubMed] [Google Scholar]
- 36. Alcada J, Taylor MN, Shaw PJ, Janes SM, Navani N, Miller RF. High prevalence of malignancy in HIV‐positive patients with mediastinal lymphadenopathy: A study in the era of antiretroviral therapy. Respirology 2014; 19: 339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


