Abstract
Background
This study aimed to evaluate mutations of the epidermal growth factor receptor (EGFR) and K‐ras genes and their clinicopathological and prognostic features in patients with resected pathological stage I adenocarcinoma.
Methods
We examined 224 patients with surgically resected lung adenocarcinoma and analyzed the prognostic and predictive value of these mutations in 162 patients with pathological stage I adenocarcinoma.
Results
Mutations of the EGFR and K‐ras genes were detected in 100 (44.6%) and 19 (8.5%) of all tumors, and in 81 (50.0%) and 17 (10.5%) of the pathological stage I tumors, respectively. EGFR mutations were significantly associated with female gender, smoking habit (never smoker), and low grade. By contrast, K‐ras mutations were significantly associated with male gender, smoking habit (ever smoker), and the presence of mucinous components. No significant differences were observed in recurrence‐free or overall survival between the EGFR‐mutant, K‐ras‐mutant, and wild‐type groups (five‐year recurrence‐free survival 77.8% vs. 87.8% vs. 79.5%; five‐year overall survival 82.8% vs. 82.4% vs. 79.2%, respectively). Multivariate analysis showed that neither EGFR nor K‐ras mutation was an independent prognostic factor.
Conclusions
The present study demonstrated that pathological stage I adenocarcinoma harboring EGFR and K‐ras gene mutations have distinct clinicopathological features. The presence of these mutations alone were not prognostic factors in patients with resected pathological stage I adenocarcinoma.
Keywords: Adenocarcinoma, epidermal growth factor receptor, K‐ras
Introduction
Lung cancer remains the leading cause of death among all cancers, and a relationship between tumor node metastasis (TNM) stage and survival has been reported.1 Over the past decade, the overall survival (OS) of lung cancer patients has greatly improved.2 This progress is largely a result of the introduction of new drugs and individualized therapy based on different histological subtypes and driver mutations that determine the biology of lung cancers and can be used to predict drug efficacy.3 The epidermal growth factor receptor (EGFR) gene is currently the most promising and “druggable” oncogene in non‐small cell lung cancer (NSCLC). The targeting of EGFRs, especially by using EGFR‐tyrosine kinase inhibitors (TKIs), has played a central role in advancing NSCLC research, treatment, and outcome prediction. Recently, EGFR‐TKIs have also been shown to improve OS in certain EGFR mutations.4 Some specific EGFR mutations are associated with sensitivity to EGFR‐TKIs. Small exon 19 deletion (del 19) and exon 21‐point mutation (L858R) are the two most common mutations associated with improved outcomes after EGFR‐TKI therapy.5, 6, 7 K‐ras is another oncogene, in which mutations occur more frequently in smokers. Compared with an approximate 50% mutation rate of the gene encoding EGFR in Asian patients, the mutation rate of EGFR is only 10–15% in white populations.8 , 9 K‐ras is the most commonly mutated oncogene in lung cancers in Western countries, with activating point mutations in 15–20% of all NSCLCs10, 11 and 25–35% of all adenocarcinomas.12, 13 Many studies have suggested that mutated K‐ras is associated with poorer OS in patients with NSCLC.14 Anti‐EGFR therapies are ineffective for K‐ras mutant tumors, which are associated with a lack of sensitivity and poorer clinical outcomes when treated with EGFR‐TKIs or chemotherapy.15, 16, 17 It is worth noting that EGFR and K‐ras mutations are rarely found in the same tumor, suggesting that they may drive functionally different carcinogenetic processes. Direct targeting of K‐ras has recently raised some concern, as this represents a key transduction pathway in both normal and tumor tissues. Moreover, several parallel escape mechanisms have been identified.18 Moving from these considerations, alternative targeting of K‐ras is currently under evaluation.
The aims of the present study were to evaluate mutations of the EGFR and K‐ras genes at the time of surgery and to analyze the clinical significance of these mutations in terms of their prognostic and predictive value in pathological stage I adenocarcinoma patients.
Methods
Patient eligibility
Between April 2007 and December 2013, 332 consecutive patients underwent pulmonary resection for lung cancer at the Sagamihara Kyodo Hospital, Kanagawa, Japan. We reviewed the data of 162 of these patients who were diagnosed with pathological stage I adenocarcinoma according to the seventh edition of the TNM Staging Classification for Lung Cancer. Patients who underwent incomplete resection or neoadjuvant chemotherapy/radiotherapy were excluded.
We reviewed the medical records of each patient for the following clinicopathological information: age, gender, smoking habit, serum carcinoembryonic antigen (CEA), extent of pulmonary resection, tumor location, maximum standardized uptake value (SUVmax) of the primary tumor, tumor size (cm), grade, pleural invasion, mucinous components, EGFR mutation status, K‐ras mutation status, and pathological stage. All clinical, intraoperative, radiological, and pathological findings from two hospitals in Kanagawa, Japan (Sagamihara Kyodo Hospital and Yuai Clinic) were reviewed. The patients’ characteristics and preoperative and postoperative tumor evaluations are shown in Table 1. Histological classification of NSCLC was based on the World Health Organization classification.19 Preoperative and postoperative staging were based on the TNM staging system.20 Data collection and analyses were approved, and the need to obtain written informed consent from each patient was waived by the first author's institutional review board.
Table 1.
Clinicopathological characteristics of 162 patients with pathological stage I lung adenocarcinoma
| Variables | N (%) or mean ± SD |
|---|---|
| Age at operation (year) | 68.9 ± 9.7 |
| Gender | |
| Female | 79 (48.8%) |
| Male | 83 (51.2%) |
| Smoking habit | |
| Never smoker | 82 (50.6%) |
| Ever smoker | 80 (49.4%) |
| Serum CEA (ng/mL) | |
| ≤5 | 128 (70.0%) |
| >5 | 34 (30.0%) |
| Extent of pulmonary resection | |
| Sublobar resection | 51 (31.5%) |
| Lobectomy or more | 111 (68.5%) |
| Tumor location | |
| Central | 8 (4.9%) |
| Non‐central | 154 (95.1%) |
| SUVmax of primary tumor | 3.2 ± 2.8 |
| Tumor size (cm) | 2.7 ± 1.7 |
| Grade | |
| 1 | 121 (74.7%) |
| 2–4 | 41 (25.3%) |
| Pleural invasion | |
| Absent | 145 (89.5%) |
| Present | 17 (10.5%) |
| Mucinous components | |
| Absent | 138 (85.2%) |
| Present | 24 (14.8%) |
| EGFR mutation | |
| Absent | 81 (50.0%) |
| Present (exon 19) | 41 (25.3%) |
| Present (exon 21) | 40 (24.7%) |
| K‐ras mutation | |
| Absent | 145 (89.5%) |
| Present (codon 12) | 17 (10.5%) |
| Present (codon 13) | 0 (0.0%) |
| Pathological stage | |
| Stage IA | 103 (63.6%) |
| Stage IB | 59 (36.4%) |
CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; SD, standard deviation; SUVmax, maximum standardized uptake value.
Computed tomography
Diagnostic quality contrast‐enhanced computed tomography (CT) of the chest with a slice thickness of 5 mm was performed for all patients. A tumor was deemed central if its center was located in the inner one‐third of the lung parenchyma (adjacent to the mediastinum) on transverse CT. Peripherally located tumors were identified as those centered in the outer two‐thirds of the lung parenchyma on transverse CT. The maximal diameter of the lung nodules was measured on contrast‐enhanced chest CT. All imaging was performed within four weeks of surgery.
Integrated 18 F‐fluorodeoxyglucose positron emission tomography imaging
Each patient underwent integrated 18F‐fluorodeoxyglucose positron emission tomography/CT (FDG‐PET/CT) imaging before surgical resection. All integrated FDG‐PET/CT imaging was performed within four weeks of surgery. After fasting for six hours, FDG (3.5 MBq/kg body weight) was intravenously injected if the patient's blood sugar level was lower than 200 mg/dL. Image acquisition commenced 60 minutes after the injection using a single PET/CT combined scanner (Eminence‐SOPHIA; Shimadzu, Kyoto, Japan).21 Image emission data from the eyes to the mid‐thigh area were continuously acquired over a period of approximately 20 minutes. After attenuation corrections were made for the resulting image data, reconstruction was performed using a dynamic row‐action expectation maximization algorithm.22 The reconstructed sectional images were then evaluated both visually and quantitatively using the SUVmax inside a volume of interest (VOI) placed on the lesions. The SUVmax was calculated as follows: ([maximum activity in VOI] / [volume of VOI]) / ([injected FDG dose] / [patient weight]). The quality of radiation measurements of the PET/CT scanner was assured by calibration in accordance with National Electrical Manufacturers Association NU‐2 2001 standards.23
Nodal uptake with an SUVmax > 2.5 was considered positive. To determine the SUV, a cylindrical region of interest (ROI) was placed over the tumor site manually on the hottest transaxial slice. The activity concentration within the ROI was determined and expressed as the SUV, where SUV is the ratio of the activity in the tissue to the decay‐corrected activity injected into the patient. All SUV measurements were normalized for patient body weight. SUVmax within an ROI was used as the reference measurement.24
Three experienced radiologists individually analyzed the integrated FDG‐PET/CT images. Final assessment was made by consensus if the initial assessments differed.
Surgical resection
All patients underwent anatomical lung resection and radical lymphadenectomy or sublobar resection in our hospital. Thoracic surgeons at Sagamihara Kyodo Hospital performed all surgical resections and all techniques were standardized. Systematic lymph node dissection was performed in all patients according to American Thoracic Society criteria, removing at least three hilar and three mediastinal stations.
Pathological examination
Experienced pulmonary pathologists examined all resected tumor specimens. Histological classification of NSCLC was based on the World Health Organization classification. Dissected lymph nodes were histologically examined following hematoxylin and eosin staining.
Epidermal growth factor receptor (EGFR) and K‐ras mutation analysis
Genomic DNA was extracted and purified from tumors embedded in paraffin blocks using the Takara DEXPAT kit (Takara Bio Inc., Kusatsu, Shiga, Japan) from materials macro‐dissected from the paraffin‐embedded sections. Quantification of the extracted nucleic acids and measurement of the A260/A280 ratio were performed using an ultraviolet spectrophotometer (Beckman Coulter DU800, Koto‐ku, Tokyo, Japan). A common fragment analysis was used for screening to detect the deletion in exon 19 of the EGFR gene. Sample DNA was amplified with a FAM‐labeled primer set: 5′‐TGGCACCATCTCACAATTGC‐3′ (forward) and 5′‐AGGATGTGGAGATGAGCAGG‐3′ (reverse). PCR products were separated by electrophoresis using an ABI PRISM 310 (Thermo Fisher Scientific, Yokohama, Kanagawa, Japan). When a deletion mutation was present, PCR was used to amplify the shorter DNA segment, thereby creating a new peak in the electropherogram. The deletion in exon 19 was confirmed using primers constructed to make a 147 bp product when the allele was wild type. The primer sequences were 5′‐TGGCACCATC TCACAATTGC‐3′ (forward) and 5′‐GAAAAGGTGGG CCTGAGGTTC‐3′ (reverse). PCR was carried out in 25 mL reaction mixtures containing 1 mL of genomic DNA using Taq DNA polymerase (Takara Bio Inc.) for 35 cycles at 64°C for annealing. To detect L858R in exon 21, a PCR assay was performed for 35 cycles at an annealing temperature of 60°C using Takara Ex‐Taq (Takara Bio Inc.). The sequencing primer was 5′‐CATGAACTACTTGGAGGACC‐3′ (forward) and 5′‐CAGGAAAATGCTGGCTGACC‐3′ (reverse). A PCR‐based restriction fragment length polymorphism analysis was performed to detect the K‐ras mutations in codons 12 and 13. All direct sequencing was performed to detect K‐ras (codons 12 and 13) mutations according to the manufacturer's protocol for the BigDye v1.1 kit (Applied Biosystems, Foster City, CA, USA). Sequencing was performed using the 310 Genetic Analyzer (Applied Biosystems).
Statistical analysis
Statistical analysis was performed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA). Survival curves were constructed using the Kaplan–Meier method. Recurrence‐free survival (RFS) probabilities and OS rates were compared using the log‐rank test. The Cox proportional hazard model was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the univariate and multivariate analyses. All tests were two‐sided, and P values <0.05 were considered statistically significant. Factors found to be significant in univariate analysis (P < 0.05) were included in multivariate analysis.
Results
Patient characteristics
The clinicopathological features of the 162 patients (79 women, 83 men; mean age, 68.9 years; age range 40–86 years) are listed in Table 1. Eighty‐two of the patients were never smokers. The median tumor size was 2.7 cm, and the median SUVmax of the primary tumor was 2.3. EGFR and K‐ras mutations were detected in 81 (50.0%) and 17 (10.5%) of 162 tumors, respectively. Forty‐one patients with EGFR gene mutations showed an exon 19 deletion, and 40 showed an exon 21‐point mutation. Seventeen patients with K‐ras gene mutations showed a codon 12‐point mutation, while no patients showed a codon 13‐point mutation. The EGFR and K‐ras gene mutations were mutually exclusive.
Correlations between the mutations and clinicopathological features were analyzed (Table 2). EGFR mutations were significantly associated with female gender, smoking habit (never smoker), and low grade. By contrast, K‐ras mutations were significantly associated with male gender, smoking habit (ever smoker), and the presence of mucinous components.
Table 2.
Association between mutation status and clinicopathological characteristics in patients with pathological stage I lung adenocarcinoma
| Variables | EGFR (n = 81) N (%) | K‐ras (n = 17) N (%) | Wild (n = 64) N (%) | P |
|---|---|---|---|---|
| Age at operation (year) | ||||
| <70 | 39 (48.1%) | 8 (47.0%) | 30 (46.9%) | 0.988 |
| ≥70 | 42 (51.9%) | 9 (53.0%) | 34 (53.1%) | |
| Gender | ||||
| Female | 56 (69.1%) | 5 (29.4%) | 22 (34.3%) | <0.001 |
| Male | 25 (30.9%) | 12 (70.6%) | 42 (65.7%) | |
| Smoking habit | ||||
| Never smoker | 54 (66.7%) | 5 (29.4%) | 21 (32.8%) | <0.001 |
| Ever smoker | 27 (33.3%) | 12 (70.6%) | 43 (67.2%) | |
| Serum CEA (ng/mL) | ||||
| ≤5 | 69 (85.2%) | 15 (88.2%) | 44 (68.8%) | 0.033 |
| >5 | 12 (14.8%) | 2 (11.8%) | 20 (31.2%) | |
| Extent of pulmonary resection | ||||
| Sublobar resection | 26 (32.1%) | 4 (23.5%) | 21 (32.8%) | 0.754 |
| Lobectomy or more | 55 (67.9%) | 13 (76.5%) | 43 (67.2%) | |
| Tumor location | ||||
| Central | 4 (4.9%) | 0 (0.0%) | 4 (6.2%) | 0.572 |
| Non‐central | 77 (95.1%) | 17 (100.0%) | 60 (93.8%) | |
| SUVmax of primary tumor | ||||
| ≤2.3 | 46 (56.8%) | 12 (70.6%) | 24 (37.5%) | 0.015 |
| >2.3 | 35 (43.2%) | 5 (29.4%) | 40 (62.5%) | |
| Tumor size (cm) | ||||
| ≤3 | 59 (72.8%) | 14 (82.4%) | 42 (65.7%) | 0.351 |
| >3 | 22 (27.2%) | 3 (17.6%) | 22 (34.3%) | |
| Grade | ||||
| 1 | 72 (88.9%) | 12 (70.6%) | 37 (57.8%) | <0.001 |
| 2–4 | 9 (11.1%) | 5 (29.4%) | 27 (42.2%) | |
| Pleural invasion | ||||
| Absent | 74 (91.4%) | 17 (100.0%) | 54 (84.4%) | 0.130 |
| Present | 7 (8.6%) | 0 (0.0%) | 10 (15.6%) | |
| Mucinous components | ||||
| Absent | 74 (91.4%) | 5 (29.4%) | 59 (92.2%) | <0.001 |
| Present | 7 (8.6%) | 12 (70.6%) | 5 (7.8%) | |
| Pathological stage | ||||
| Stage IA | 55 (67.9%) | 14 (82.4%) | 34 (53.1%) | 0.044 |
| Stage IB | 26 (32.1%) | 3 (17.6%) | 30 (46.9%) | |
CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; SUVmax, maximum standardized uptake value.
Survival analysis of patients with pathological stage I adenocarcinoma after surgical resection
Among the 162 patients, five‐year RFS and OS were 79.6% and 81.3%, respectively. In the survival analyses, the five‐year RFS rates were 77.8% vs. 87.8% vs. 79.2% for patients with an EGFR mutation, K‐ras mutation, and wild‐type status, respectively (Fig 1a). The five‐year OS rates were 82.8 vs. 82.4 vs. 79.2 for patients with an EGFR mutation, K‐ras mutation, and wild‐type status, respectively (Fig 1b). Significant differences were observed in both RFS and OS between patients with an EGFR mutation and those with wild‐type genes (RFS P = 0.903, OS P = 0.883), and between patients with an EGFR mutation and those with a K‐ras mutation (RFS P = 0.317, OS P = 0.952).
Figure 1.
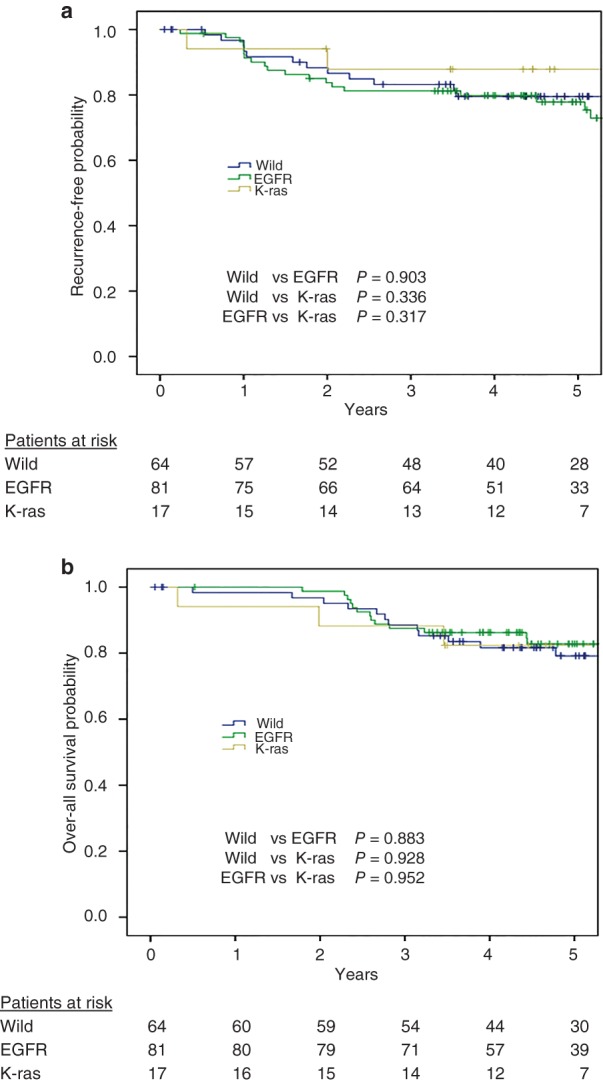
(a) Recurrence‐free survival curves of pathological stage I patients after pulmonary resection. Data are shown for patients with epidermal growth factor receptor (EGFR) and K‐ras mutations and for those who were wild type for both genes. (b) Overall survival curves of pathological stage I patients with EGFR and K‐ras mutations or both wild‐type genes after pulmonary resection.
Univariate analysis showed that serum CEA, SUVmax of the tumor, pleural invasion, and pathological stage were significant unfavorable prognostic factors for RFS (P < 0.05), and that age at operation, serum CEA, and SUVmax of the tumor were significant unfavorable prognostic factors for OS (P < 0.3). In multivariate analysis adjusted for the significant univariate factors, SUVmax of the tumor remained an independent prognostic factor for RFS (P = 0.001), and age at operation and SUVmax of the tumor remained independent prognostic factors for OS (P = 0.029, 0.008; Table 4). EGFR and K‐ras mutations did not affect the prognosis of patients with pathological stage I adenocarcinoma.
Table 4.
Multivariate analyses for RFS and OS in patients with pathological stage I adenocarcinoma
| Variables | RFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at operation (year) | ||||
| <70 | 1 | |||
| ≥70 | — | — | 2.39 (1.09–5.24) | 0.029 |
| Serum CEA (ng/mL) | ||||
| ≤5 | 1 | 1 | ||
| >5 | 1.11 (0.53–2.33) | 0.776 | 1.74 (0.79–3.81) | 0.163 |
| SUVmax of primary tumor | ||||
| ≤2.3 | 1 | 1 | ||
| >2.3 | 5.31 (2.06–13.65) | 0.001 | 3.31 (1.36–8.05) | 0.008 |
| Pleural invasion | ||||
| Absent | 1 | |||
| Present | 1.46 (0.57–3.76) | 0.429 | — | — |
| Pathological stage | ||||
| Stage IA | 1 | |||
| Stage IB | 1.17 (0.53–2.55) | 0.697 | — | — |
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; OS, overall survival; RFS, recurrence‐free survival; SUVmax, maximum standardized uptake value.
Table 3.
Univariate analyses for RFS and OS in patients with pathological stage I adenocarcinoma
| Variables | RFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at operation (year) | ||||
| <70 | 1 | 1 | ||
| ≥70 | 1.11 (0.57–2.15) | 0.767 | 2.33 (1.06–5.09) | 0.034 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.16 (0.59–2.25) | 0.666 | 1.03 (0.72–1.48) | 0.871 |
| Smoking habit | ||||
| Never smoker | 1 | 1 | ||
| Ever smoker | 1.20 (0.86–1.68) | 0.278 | 1.25 (0.87–1.81) | 0.224 |
| Serum CEA (ng/mL) | ||||
| ≤ 5 | 1 | 1 | ||
| > 5 | 2.04 (1.01–4.17) | 0.049 | 2.61 (1.24–5.48) | 0.012 |
| Extent of pulmonary resection | ||||
| Sublobar resection | 1 | 1 | ||
| Lobectomy or more | 0.78 (0.53–1.16) | 0.227 | 0.79 (0.52–1.21) | 0.289 |
| Tumor location | ||||
| Central | 1 | 1 | ||
| Non‐central | 0.93 (0.45–1.89) | 0.833 | 0.57 (0.78–4.21) | 0.584 |
| SUVmax of primary tumor | ||||
| ≤2.3 | 1 | 1 | ||
| 2.3 | 6.08 (3.52–14.65) | 3.85 (1.65–8.98) | 0.002 | |
| Tumor size (cm) | ||||
| ≤3 | 1 | 1 | ||
| >3 | 1.61 (0.81–3.19) | <0.001 | 1.58 (0.75–3.33) | 0.225 |
| Grade | ||||
| 1 | 1 | 0.175 | 1 | |
| 2–4 | 1.31 (0.62–2.71) | 0.482 | 1.18 (0.53–2.66) | 0.687 |
| Pleural invasion | ||||
| Absent | 1 | 1 | ||
| Present | 2.42 (1.06–5.54) | 0.037 | 1.92 (0.73–5.03) | 0.182 |
| Mucinous components | ||||
| Absent | 1 | 1 | ||
| Present | 1.21 (0.71–2.03) | 0.487 | 1.06 (0.62–1.81) | 0.817 |
| EGFR mutation | ||||
| Absent | 1 | 1 | ||
| Present | 1.18 (0.61–2.29) | 0.632 | 1.02 (0.71–1.46) | 0.911 |
| K‐ras mutation | ||||
| Absent | 1 | 1 | ||
| Present | 2.06 (0.49–8.59) | 0.321 | 1.02 (0.56–1.86) | 0.959 |
| Pathological stage | ||||
| Stage IA | 1 | 1 | ||
| Stage IB | 2.31 (1.19–4.51) | 0.014 | 1.69 (0.82–3.46) | 0.153 |
CEA, carcinoembryonic antigen; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival; RFS, recurrence‐free survival; SUVmax, maximum standardized uptake value.
Discussion
We retrospectively evaluated the outcomes of patients with pathological stage I adenocarcinoma. Compared with Western populations, EGFR mutations are detected more frequently in the lung adenocarcinomas of Japanese patients, ranging from 40% to 60%.25, 26, 27, 28, 29, 30, 31 On the other hand, compared with Western populations, K‐ras mutations are detected less frequently in the lung adenocarcinomas of Japanese patients.32 The frequency of K‐ras mutation ranges from about from 7% to 16% in worldwide populations.5, 30, 33, 34 Similarly, the frequency of K‐ras mutations was 10.5% in the current study.
The presence of an EGFR mutation is closely associated with several clinicopathological features, such as gender and smoking habit. This is consistent with previous studies, which reported that EGFR gene mutations are common in lung cancers in never smokers and in women with adenocarcinoma.6, 7, 32 Several reports have described the relationship between K‐ras mutation status and clinicopathological features such as gender, smoking habit, and pathological type.26, 30, 35 Similar to results reported in previous studies, the current series showed a relationship between K‐ras mutation status and gender. Mucinous bronchioloalveolar carcinoma (BAC)/adenocarcinoma with bronchioloalveolar features is found in 48–76% of adenocarcinomas with K‐ras mutations, and K‐ras mutations are found in 28–86% of adenocarcinomas with mucinous BAC.30, 36, 37, 38, 39, 40 In the present study, 12 (70.6%) of the 17 cases with K‐ras mutations were mucinous BAC/adenocarcinoma with bronchioloalveolar features.
In lung adenocarcinoma simultaneously harboring multiple heterogeneous clones of EGFR and K‐ras mutations, the effect of EGFR‐TKIs may be limited to the parts carrying EGFR mutations only.41, 42 Because both EGFR and K‐ras mutations are thought to be early events in lung adenocarcinoma,32 the reported coexistence of EGFR and K‐ras mutations only accounts for about 5% of patients with EGFR mutations.43 Takamochi et al. reported coexisting EGFR and K‐ras mutations in two (2%) of 82 patients with lung adenocarcinomas.6, 41 A previous study reported that all tumors that had responded to gefitinib had wild type K‐ras, 44 thereby suggesting that K‐ras and EGFR mutations are mutually exclusive.45 None of the patients in our series had concomitant EGFR and K‐ras mutations; this result is similar to previous reports, further suggesting that K‐ras and EGFR mutations are mutually exclusive. Accordingly, combined EGFR and K‐ras mutation analyses may be helpful in selecting treatment strategies for patients with lung adenocarcinomas.
We also investigated the effects of EGFR and K‐ras mutation status on survival. Neither EGFR nor K‐ras mutations affected the prognosis of patients with pathological stage I adenocarcinoma. The prognostic role of EGFR mutations in patients with resectable NSCLC has not been established. In their study, Mansuet‐Lupo et al. did not find a significant effect on OS for patients with EGFR mutations compared with those with wild‐type EGFR in their cohort or in a subset with stage I disease.46 Hu et al. found no impact on OS in multivariate analysis when the presence or absence of an EGFR mutation was included.47 On the other hand, in a smaller study, Russell et al. conducted molecular analysis and assessed survival outcomes in 59 patients who had undergone surgical resection of lung adenocarcinoma with N2 nodal involvement.48 Patients with acinar‐predominant adenocarcinoma had significantly better survival than those with micropapillary or solid predominant adenocarcinoma. This trend suggests that patients with resected micropapillary tumors harboring an activating EGFR mutation have similar survival outcomes to patients with acinar predominant tumors, whereas patients with micropapillary predominant tumors with wild‐type EGFR have poorer outcomes.
Yoshizawa et al. did note a statistically and clinically significant improvement in five‐year OS rates in patients with EGFR mutations, but found no difference in five‐year disease‐free survival.49 However, this result was not included in multivariate analysis in our study.
On the other hand, K‐ras mutations have been reported to be prognostic factors in several investigations.10, 26, 32, 34, 50 Kosaka et al. conducted a prognostic analysis of K‐ras mutations in 397 resected adenocarcinomas of Japanese patients and found that patients with K‐ras mutations tended to have a shorter survival period.26 A meta‐analysis of 53 published studies assessing the prognostic value of mutations in the K‐ras gene has also been performed.10 In that analysis, K‐ras mutations were identified as a negative prognostic factor in lung adenocarcinoma. Our findings were not consistent with these previous results, and our multivariate analysis revealed that K‐ras mutations were not a prognostic factor in patients with resected pathological stage I adenocarcinoma.
Our results suggest that EGFR and K‐ras gene mutations are not independent prognostic factors in patients with resected pathological stage I adenocarcinoma. Our findings were further analyzed after the data were restricted to patients with pathological stage I disease. Therefore, the analyzed patients were oncologically equivalent, and the analysis regarding the prognostic value of EGFR and K‐ras gene mutations was valid.
The main limitation of the present study was the retrospective nature of the work. To clarify the true clinicopathological and prognostic features of pathological stage I lung adenocarcinoma harboring EGFR and K‐ras mutations, prospective or randomized trials are warranted. Furthermore, we elected to exclude patients who had received treatment with neoadjuvant chemotherapy or radiotherapy, as these cases can lead to considerable inaccuracy.
In conclusion, the present study demonstrated that surgically resected pathological stage I adenocarcinoma harboring EGFR and K‐ras gene mutations has distinct clinicopathological features. The presence of an EGFR or a K‐ras mutation alone was not a prognostic factor in patients with surgically resected pathological stage I adenocarcinoma.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We acknowledge the assistance of Mr. Tomoyuki Kanno, Yuai Clinic, in the acquisition of the study data.
References
- 1. Sheel AR, McShane J, Poullis MP. Survival of patients with or without symptoms undergoing potentially curative resections for primary lung cancer. Ann Thorac Surg 2013; 95: 276–84. [DOI] [PubMed] [Google Scholar]
- 2. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. (Published erratum appears in N Engl J Med 2007; 356: 318.) N Engl J Med 2006; 355: 2542–50. [DOI] [PubMed] [Google Scholar]
- 3. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non‐small‐cell lung cancer: Recent developments. Lancet 2013; 382: 709–19. [DOI] [PubMed] [Google Scholar]
- 4. Yang JC, Wu YL, Schuler M et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–51. [DOI] [PubMed] [Google Scholar]
- 5. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 6. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 7. Pao W, Miller V, Zakowski M et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004; 101: 13306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Genova C, Rijavec E, Barletta G et al. Afatinib for the treatment of advanced non‐small‐cell lung cancer. Expert Opin Pharmacother 2014; 15: 889–903. [DOI] [PubMed] [Google Scholar]
- 9. Tartarone A, Lazzari C, Lerose R et al. Mechanisms of resistance to EGFR tyrosine kinase inhibitors gefitinib/erlotinib and to ALK inhibitor crizotinib. Lung Cancer 2013; 81: 328–36. [DOI] [PubMed] [Google Scholar]
- 10. Mascaux C, Iannino N, Martin B et al. The role of RAS oncogene in survival of patients with lung cancer: A systematic review of the literature with meta‐analysis. Br J Cancer 2005; 92: 131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shepherd FA, Domerg C, Hainaut P et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early‐stage resected non‐small‐cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol 2013; 31: 2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dogan S, Shen R, Ang DC et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: Higher susceptibility of women to smoking‐related KRAS‐mutant cancers. Clin Cancer Res 2012; 18: 6169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imielinski M, Berger AH, Hammerman PS et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012; 150: 1107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meng D, Yuan M, Li X et al. Prognostic value of K‐RAS mutations in patients with non‐small cell lung cancer: A systematic review with meta‐analysis. Lung Cancer 2013; 81: 1–10. [DOI] [PubMed] [Google Scholar]
- 15. Pao W, Wang TY, Riely GJ et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2005; 2: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Roock W, Claes B, Bernasconi D et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy‐refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol 2010; 11: 753–62. [DOI] [PubMed] [Google Scholar]
- 17. Eberhard DA, Johnson BE, Amler LC et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non‐small‐cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005; 23: 5900–9. [DOI] [PubMed] [Google Scholar]
- 18. Vasan N, Boyer JL, Herbst RS. A RAS renaissance: Emerging targeted therapies for KRAS‐mutated non‐small cell lung cancer. Clin Cancer Res 2014; 20: 3921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Travis WD, Colby TV, Corrin Y, Shimosato Y, Brambilla E. Histological Typing of Lung and Pleural Tumours, 3rd edn. Springer, Berlin: 1999. [Google Scholar]
- 20. Goldstraw P, Crowley J, Chansky K et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. (Published erratum appears in J Thorac Oncol 2007; 2: 985.) J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto K, Kitamura K, Mizuta T et al. Performance characteristics of a new 3‐dimensional continuous‐emission and spiral‐transmission high‐sensitivity and high‐resolution PET camera evaluated with the NEMA NU 2‐2001 standard. J Nucl Med 2006; 47: 83–90. [PubMed] [Google Scholar]
- 22. Kitamura K, Ishikawa A, Mizuta T et al. 3D continuous emission and spiral transmission scanning for high‐throughput whole‐body PET. Nuclear Science Symposium Conference Record, 2004; IEEE 2004, Rome, Italy; Vol 5: 2801–5. [Google Scholar]
- 23. The Association of Electrical Equipment and Medical Imaging Manufacturers . Performance Measurements of Positron Emission Tomographs. NEMA Standards Publication NU 2–2001. NEMA, Rosslyn, VA: 2001. [Google Scholar]
- 24. Nabi HA, Zubeldia JM. Clinical applications of 18F‐FDG in oncology. J Nucl Med Technol 2002; 30: 3–9. [PubMed] [Google Scholar]
- 25. Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007; 98: 1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol 2009; 4: 22–9. [DOI] [PubMed] [Google Scholar]
- 27. Hiramatsu M, Ninomiya H, Inamura K et al. Activation status of receptor tyrosine kinase downstream pathways in primary lung adenocarcinoma with reference of KRAS and EGFR mutations. Lung Cancer 2010; 70: 94–102. [DOI] [PubMed] [Google Scholar]
- 28. Tomizawa K, Suda K, Onozato R et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer 2011; 74: 139–44. [DOI] [PubMed] [Google Scholar]
- 29. Sasaki H, Shimizu S, Endo K et al. EGFR and erbB2 mutation status in Japanese lung cancer patients. Int J Cancer 2006; 118: 180–4. [DOI] [PubMed] [Google Scholar]
- 30. Kakegawa S, Shimizu K, Sugano M et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer 2011; 117: 4257–66. [DOI] [PubMed] [Google Scholar]
- 31. Takano T, Fukui T, Ohe Y et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: A historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 2008; 26: 5589–95. [DOI] [PubMed] [Google Scholar]
- 32. Shigematsu H, Lin L, Takahashi T et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97: 339–46. [DOI] [PubMed] [Google Scholar]
- 33. Sugio K, Ishida T, Yokoyama H, Inoue T, Sugimachi K, Sasazuki T. Ras gene mutations as a prognostic marker in adenocarcinoma of the human lung without lymph node metastasis. Cancer Res 1992; 52: 2903–6. [PubMed] [Google Scholar]
- 34. Yatabe Y, Koga T, Mitsudomi T, Takahashi T. CK20 expression, CDX2 expression, K‐ras mutation, and goblet cell morphology in a subset of lung adenocarcinomas. J Pathol 2004; 203: 645–52. [DOI] [PubMed] [Google Scholar]
- 35. Tam IY, Chung LP, Suen WS et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non‐small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 2006; 12: 1647–53. [DOI] [PubMed] [Google Scholar]
- 36. Sakuma Y, Matsukuma S, Yoshihara M et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K‐ras gene‐mutation analyses for Japanese lung adenocarcinomas: Confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol 2007; 128: 100–8. [DOI] [PubMed] [Google Scholar]
- 37. Finberg KE, Sequist LV, Joshi VA et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn 2007; 9: 320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wislez M, Antoine M, Baudrin L et al. Non‐mucinous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer 2010; 68: 185–91. [DOI] [PubMed] [Google Scholar]
- 39. Marchetti A, Buttitta F, Pellegrini S et al. Bronchioloalveolar lung carcinomas: K‐ras mutations are constant events in the mucinous subtype. J Pathol 1996; 179: 254–9. [DOI] [PubMed] [Google Scholar]
- 40. Casali C, Rossi G, Marchioni A et al. A single institution‐based retrospective study of surgically treated bronchioloalveolar adenocarcinoma of the lung: Clinicopathologic analysis, molecular features, and possible pitfalls in routine practice. J Thorac Oncol 2010; 5: 830–6. [DOI] [PubMed] [Google Scholar]
- 41. Takamochi K, Oh S, Matsuoka J, Suzuki K. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K‐ras. Lung Cancer 2012; 75: 313–20. [DOI] [PubMed] [Google Scholar]
- 42. Massarelli E, Varella‐Garcia M, Tang X et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non‐small‐cell lung cancer. Clin Cancer Res 2007; 13: 2890–6. [DOI] [PubMed] [Google Scholar]
- 43. Takeda M, Okamoto I, Fujita Y et al. De novo resistance to epidermal growth factor receptor‐tyrosine kinase inhibitors in EGFR mutation‐positive patients with non‐small cell lung cancer. J Thorac Oncol 2010; 5: 399–400. [DOI] [PubMed] [Google Scholar]
- 44. Kosaka T, Yatabe Y, Endoh H et al. Analysis of epidermal growth factor receptor gene mutation in patients with non‐small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 2006; 12: 5764–9. [DOI] [PubMed] [Google Scholar]
- 45. Onitsuka T, Uramoto H, Nose N et al. Acquired resistance to gefitinib: The contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer 2010; 68: 198–203. [DOI] [PubMed] [Google Scholar]
- 46. Mansuet‐Lupo A, Bobbio A, Blons H et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: The experience of a French cohort. Chest 2014; 146: 633–43. [DOI] [PubMed] [Google Scholar]
- 47. Hu H, Pan Y, Li Y et al. Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. Onco Targets Ther 2014; 7: 1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russell PA, Barnett SA, Walkiewicz M et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013; 8: 461–8. [DOI] [PubMed] [Google Scholar]
- 49. Yoshizawa A, Sumiyoshi S, Sonobe M et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol 2013; 8: 52–61. [DOI] [PubMed] [Google Scholar]
- 50. Marks JL, Broderick S, Zhou Q et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol 2008; 3: 111–6. [DOI] [PubMed] [Google Scholar]


