Abstract
Aims/Introduction
Adipose tissue‐derived hormones are associated with metabolic disorders including type 2 diabetes mellitus. The present study investigated the levels of adiponectin and pro‐inflammatory cytokines including tumor necrosis factor‐α (TNF‐α), interleukin‐1 beta (IL‐1β) and IL‐10 in Vietnamese patients with type 2 diabetes mellitus, and their correlations with clinical parameters of overweight and type 2 diabetes mellitus.
Materials and Methods
Based on body mass index, 73 patients with type 2 diabetes mellitus were categorized either as overweight or non‐overweight. As healthy controls, 57 overweight and non‐overweight individuals without type 2 diabetes mellitus were included. The adiponectin, TNF‐α, IL‐1β and IL‐10 levels were measured in the sera samples in all study participants by enzyme‐linked immunosorbent assay and were correlated with clinical parameters.
Results
The adiponectin levels were lower in patients with type 2 diabetes mellitus (2.5 ± 1.5 μg/mL) compared with controls (16 ± 18.6 μg/mL; P < 0.0001), and were decreased in overweight individuals compared with those who were not overweight. The TNF‐α and IL‐1β levels were increased, whereas the IL‐10 levels were decreased in patients with type 2 diabetes mellitus and in overweight controls compared with non‐overweight controls (P < 0.0001). The adiponectin levels were correlated with the TNF‐α, IL‐1β, IL‐10 levels, and the clinical parameters of overweight and type 2 diabetes mellitus. The quantitative insulin sensitivity check index and homeostasis model assessment insulin resistance indexes were correlated with the relative ratios of adiponectin/TNF‐α, adiponectin/IL‐1β, adiponectin/IL‐10, TNF‐α/IL‐10 and IL‐1β/IL‐10.
Conclusions
Adiponectin and pro‐inflammatory cytokines are associated with type 2 diabetes mellitus, and might serve as a prognostic marker and a therapeutic intervention for overweight‐related type 2 diabetes mellitus.
Keywords: Cytokines, Overweight, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus is a chronic metabolic disorder with an exponential increase in developing countries. The international diabetes federation reported 382 million diabetes cases in 2013, with a prediction of 592 million cases by 2035, and 80% of these cases are in developing countries1, 2. Diabetes accounts for 5.1 million deaths worldwide every year1, 2. The prevalence of type 2 diabetes mellitus in developed countries is 1.2%, whereas in developing countries the prevalence is assumed to be fourfold higher2, 3. An exponential increase of obesity and type 2 diabetes mellitus in developing countries is well recognized by an increased consumption of energy‐rich food, sedentary lifestyle and urbanization4. In Vietnam, the number of patients with type 2 diabetes mellitus is growing, with estimated 3.3 million diabetes cases reported in 2014. The prevalence of diabetes in the age group of 30–69 years is estimated to be 5.7% across Vietnam and 7% in urban areas5.
Type 2 diabetes mellitus constitutes up to 95% of all diabetes, and is characterized by chronic hyperglycemia resulting from defects in insulin secretion and/or insulin action and metabolic disorders of protein and lipids6, 7. The pathogenesis of type 2 diabetes mellitus consists of two major abnormalities including insulin resistance and dysfunction of insulin production, which lead to the inability to regulate blood glucose level8. The damage to pancreatic β‐cells resulting in insufficient production of insulin and adiponectin, as well as an increased production of pro‐inflammatory cytokines as a result of obesity are the major contributing factors to type 2 diabetes mellitus6, 9, 10. Insulin resistance appeared years before the clinical manifestation of type 2 diabetes mellitus and is significantly associated with obesity, especially with abdominal and visceral obesity with an abnormally increased waist‐to‐hip ratio, dyslipidemia, hypertension and other metabolic disorders11. Therefore, obesity largely contributes to insulin resistance in patients with type 2 diabetes mellitus12.
Adipose tissue has recently been recognized as an organ for the metabolism of sexual steroids and the production of adipsin, which significantly contributes to loss of bodyweight13, 14. Adipose tissue‐derived proteins regulate metabolic functionalities including hormone activities15. Adipose tissue is also known as an endocrine organ that can produce various peptides with bioactivities, namely adipocytokine (or adipokines)16, 17. In addition, adipocytes carry many receptors for hormones of the endocrine and central nervous system; therefore, adipocytes are involved in various biological processes, such as energy metabolism, neuroendocrine function and immune response14.
Increased adipose tissue as a result of obesity, especially deposition of visceral fat, is associated with insulin resistance, increased blood glucose levels, lipid metabolic disorders, hypertension and inflammation15. Those metabolic syndromes eventually lead to obesity‐related type 2 diabetes mellitus. The adipose tissue‐derived hormones, such as adiponectin, leptin, adipsin and resistin, have been shown to be associated with metabolic disorders, and are risk factors for type 2 diabetes mellitus and cardiovascular diseases18, 19, 20. The present study aimed to investigate the levels of adiponectin and different pro‐inflammatory cytokines, such as tumor necrosis factor‐α (TNF‐α), interleukin‐1 beta (IL‐1β) and interleukin‐10 (IL‐10), as well as their correlations with insulin resistance and clinical progressions of overweight and type 2 diabetes mellitus in a Vietnamese study group.
Materials and Methods
Patients and controls
A total of 73 Vietnamese patients with type 2 diabetes mellitus and 57 control individuals were included in the study. The patients were newly diagnosed to have type 2 diabetes mellitus, and some patients (such as the patients who had high levels of glucose) were immediately treated with the diabetes drug, Diamicron MR (gliclazide MR), and/or a low dose of insulin injection. Patients were classified into two subgroups based on their body mass index (BMI) and type 2 diabetes mellitus status. The first subgroup included the patients who were both overweight (BMI ≥25) and had type 2 diabetes mellitus (patients with overweight type 2 diabetes mellitus, n = 20). The second subgroup included patients with type 2 diabetes mellitus, but who were not overweight (BMI <25; patients with non‐overweight type 2 diabetes mellitus, n = 53). At the time of sampling, most of the recruited patients had not been treated with any antidiabetic drugs.
The patients were diagnosed for type 2 diabetes mellitus based on the standard criteria reported by the World Health Organization in 1998 and by the International Diabetes Federation in 200521, 22. The anthropometric indicators, such as height, weight, and waist and hip circumference were measured for all study participants. BMI and waist‐to‐hip ratio were calculated based on their anthropometric indicators. Blood pressure and electrocardiogram were measured and recorded.
The exclusion criteria for the patient group included diabetes complications or more severe complications, acute brain stroke, paroxysmal hypertensive crises, patients with liver cirrhosis, heart and kidney failure or other infectious diseases, such as bacterial infections, hepatitis and tuberculosis. Patients taking certain medications, which might affect the biochemical test results, such as glucocorticoid, drugs for treatment of dyslipidemia, thiazide diuretics, antihypertensives enzyme inhibitors and angiotensin II receptor blockers, must discontinue taking the medications at least a week before sampling (Table 1).
Table 1.
Characteristics of patients with type 2 diabetes mellitus and controls
| Characteristics | Type 2 diabetes mellitus | Without type 2 diabetes mellitus | P‐valuea | ||||
|---|---|---|---|---|---|---|---|
| Overweight T2DM (n = 20) | Non‐overweight T2DM (n = 53) | P‐value | Overweight individuals (n = 24) | Non‐overweight individuals (n = 33) | P‐value | ||
| Age, years (range) | 55 (41–76) | 53 (41–83) | NS | 48 (40–72) | 47 (38–61) | NS | <0.0001 |
| Sex (male/female) | 11/9 | 29/24 | NS | 4/20 | 8/25 | NS | 0.002 |
| Smoking (yes/no) | 4/16 | 27/26 | NS | 0/24 | 1/32 | NS | <0.0001 |
| Alcohol use (yes/no) | 7/13 | 26/27 | NS | 0/24 | 1/32 | NS | <0.0001 |
| BMI (range) | 25.3 (25–28.3) | 19.4 (13.6–21.7) | <0.0001 | 25.5 (25–32.9) | 18.7 (15.8–24.9) | <0.0001 | NS |
| WHR (range) | 0.92 (0.85–0.96) | 0.85 (0.74–0.98) | <0.0001 | 0.87 (0.81–0.93) | 0.83 (0.73–0.92) | <0.0001 | 0.001 |
| Fasting glucose, mmol/L (range) | 16.5 (8.2–25) | 15 (7.1–29.8) | NS | 5.1 (3.8–5.5) | 5 (3.8–5.5) | NS | <0.0001 |
| Blood urea, mmol/L (range) | 5.5 (3.8–8.6) | 5.2 (2.5–9.2) | NS | 4.9 (2.5–51) | 5.2 (2.1–7.4) | NS | NS |
| Blood creatinine, µmol/L (range) | 82 (5.7–127) | 84 (55–121) | NS | 75 (49–110) | 68 (45–110) | NS | 0.001 |
| Triglycerides, mmol/L (range) | 3.9 (1.5–12) | 2.3 (0.5–11.8) | 0.01 | 1.5 (0.6–2.7) | 1.8 (0.7–6.9) | NS | <0.0001 |
| Total cholesterol, mmol/L (range) | 6.6 (4.6–8.7) | 5.7 (3.4–8.1) | 0.0018 | 4 (2.6–7.4) | 4.2 (2.6–6.8) | NS | <0.0001 |
| HDL‐C, mmol/L (range) | 0.9 (0.7–1.1) | 0.97 (0.5–1.9) | 0.016 | 1.7 (0.8–2.3) | 1.2 (0.6–2.2) | NS | <0.0001 |
| LDL‐C, mmol/L (range) | 3.2 (0.9–5.3) | 3.1 (1.4–5.4) | NS | 2.3 (1–3.6) | 2.4 (1.1–3.6) | NS | <0.0001 |
| Total bilirubin, µmol/L (range) | 13 (6.9–20) | 12.3 (2.1–21) | NS | 12.7 (5.7–18) | 14.8 (4.4–21.5) | NS | NS |
| GOT (AST), U/L (range) | 19.5 (8–35) | 19 (10–39) | NS | 25.5 (10–39) | 22 (11–36) | NS | NS |
| GPT (ALT), U/L (range) | 25 (10–39) | 23 (10–37) | NS | 30 (6–39) | 27 (10–39) | NS | NS |
| Glycosylated hemoglobin (%) | 11 (6.8–13.2) | 10.6 (6.6–17) | NS | NA | NA | NA | NA |
| Insulin, pmol/L (range) | 70 (14–133.7) | 40 (6.9–160) | 0.04 | 84 (18–118) | 12 (8–88) | <0.0001 | 0.05 |
| HOMA‐RI (range) | 45.8 (6.3–119.3) | 23 (4.7–125) | 0.028 | 19.7 (3.8–29.5) | 2.6 (2–2.9) | <0.0001 | <0.0001 |
| QUICKI (range) | 0.5 (0.5–0.7) | 0.6 (0.4–0.7) | 0.016 | 0.5 (0.5–0.7) | 0.8 (0.5–0.9) | <0.0001 | <0.0001 |
| HOMA‐β (range) | 124.2 (19–365.5) | 75.8 (8.8–650) | NS | 1147.6 (237.5–4000) | 157.1 (64–1563.6) | <0.0001 | <0.0001 |
Comparison between type 2 diabetes mellitus and non‐diabetes mellitus. BMI, body mass index; GOT, glutamic‐oxaloacetic transaminase (aspartate transaminase [AST]); GPT, glutamic‐pyruvic transaminase (aspartate transaminase [ALT]); HDL‐C, high‐density lipoprotein; LDL‐C, low‐density lipoprotein; HOMA‐β, homeostatic model assessment β‐cell function; HOMA‐IR, homeostasis model assessment ‐insulin resistance; NA, not applicable; NS, not significant; QUICKI, quantitative insulin sensitivity check index; T2DM, type 2 diabetes mellitus; WHR, waist‐to‐hip ratio.
All the individuals in the control group were clinically examined and were considered healthy during sampling. None of them had any chronic infectious diseases or conditions such as hepatitis, liver cirrhosis, obstructive pulmonary disease, gout and/or any infection. The control group was also further divided into two subgroups. The first control subgroup included normal healthy individuals, (non‐overweight control individuals, n = 33), with fasting venous blood glucose test <5.6 mmol/L, blood pressure <130 mmHg and <85 mmHg, electrocardiogram in the normal limits, other tests in the normal range, and BMI 18.5–25. The second control subgroup was healthy individuals with BMI ≥25 (overweight control individuals, n = 24), fasting venous blood glucose test <5.6 mmol/L, blood pressure <130 mmHg and <85 mmHg, and electrocardiogram in the normal limits (Table 1). In addition, a history of type 2 diabetes mellitus, such as diabetes, symptoms associated with diabetes, and personal and family history of diabetes; family history of premature cardiovascular diseases; smoking; alcohol use; and lifestyle were obtained from all participants using the standard study questionnaires.
Ethics statement
Informed written consent was received from all studied participants. The study was approved by the institutional review board of the Vietnam Military Medical University.
Measurement of biochemical parameters
The levels of lipid components including cholesterol, triglycerides, high‐density lipoprotein cholesterol (HDL‐C) and low‐density lipoprotein cholesterol (LDL‐C) were measured by using an automatic biochemical Olympus AU400 Chemistry Analyzer (Olympus, Shinjuku, Japan). Fasting blood glucose levels were measured by measurement of ultraviolet with hexokinase. Insulin levels were measured by the electrochemical immune fluorescence method using the ELESYS‐2010 system (Roche Diagnostics Ltd, Basel, Switzerland). Blood fasting glycosylated hemoglobin levels were quantified by the ion‐exchange method using high‐performance liquid chromatography. The insulin resistance index was evaluated according to the homeostasis model assessment insulin resistance (HOMA‐IR) and the quantitative insulin sensitivity check insulin resistance index (QUICKI). In addition, the β‐cell function (HOMA‐β) and the insulin secretion of β‐cells were evaluated according to Matthews’ method10.
Measurement of TNF‐α, IL‐1β and IL‐10 levels
The levels of TNF‐α, IL‐1β and IL‐10 were measured in the respective serum samples of the study participants by using commercially available enzyme‐linked immunosorbent assay kits including AviBion Human TNF‐α, AviBion Human IL‐1β and AviBion Human IL‐10, respectively according to the manufacturer's instruction (Orgenium, Helsinki, Finland).
Measurement of adiponectin levels
The adiponectin levels were measured in the respective serum samples of the study participants by using a commercial enzyme‐linked immunosorbent assay kit following the manufacturer's instructions (AviBion Human Adiponectin; Orgenium). The detection limit of the kit is 0.185 ng/mL.
Statistical analysis
Clinical and demographic data are presented as median values with a range for continuous variables. Student's t‐tests or one‐way anova were used for comparing the mean of two or more groups, respectively. The χ2‐test or Fisher's exact test were used to compare categorical variables. The Kruskal–Wallis or Mann–Whitney U‐test were used to analyze the serum levels of adiponectin, TNF‐α, IL‐1β and IL‐10 in the patients with type 2 diabetes mellitus and in controls wherever appropriate. Spearman's rank correlation coefficient was used to analyze the correlation of adiponectin serum levels with clinical parameters of overweight and type 2 diabetes mellitus, as well as the correlation of insulin sensitivity/resistance indexes with the relative ratios of studied adiponectin and cytokine levels. All statistical analyses were carried out using IBM Statistics Spss version 19 (IBM Corp, Armonk, New York, USA), and the level of significance was set at a P‐value of <0.05.
Results
Demographic, clinical and biochemical characteristics of the study participants
The demographic, clinical and biochemical characteristics of the patients with type 2 diabetes mellitus and controls are summarized in Table 1. The mean age of the patients with type 2 diabetes mellitus (55.7 ± 11.5 years) was higher than control individuals (without type 2 diabetes mellitus, 48.5 ± 6.8 years; P < 0.001), whereas there was no difference of the mean age between patients with overweight type 2 diabetes mellitus and non‐overweight type 2 diabetes mellitus. The proportion of men among patients with type 2 diabetes mellitus was higher than the control group (P = 0.002). In addition, the proportions of smokers and alcohol users among patients with type 2 diabetes mellitus were significantly higher compared with the control group (P < 0.0001 for both smoking and alcohol use). These results imply that smoking and alcohol use could significantly contribute towards increased risk of type 2 diabetes mellitus.
The levels of fasting glucose, creatinine, triglycerides and total cholesterol were significantly higher in the patients with type 2 diabetes mellitus compared with the control individuals (P < 0.0001). The level of HDL‐C was lower, while the level of LDL‐C was higher in the patients with type 2 diabetes mellitus compared with the control individuals (P < 0.0001 for both HDL‐C and LDL‐C). For the patients with type 2 diabetes mellitus, the levels of triglycerides and total cholesterol were significantly higher, whereas the HDL‐C level was decreased in the patients with overweight type 2 diabetes mellitus compared with those with non‐overweight type 2 diabetes mellitus (P = 0.01 for triglycerides, P = 0.0018 for total cholesterol and P = 0.016 for HDL‐C). The levels of insulin, HOMA‐IR, QUICKI and HOMA‐β were significantly higher in the patients with type 2 diabetes mellitus compared with the control individuals (P = 0.05 for insulin, and P < 0.0001 for HOMA‐RI, QUICKI and HOMA‐β). Among the patients with type 2 diabetes mellitus, the levels of insulin and HOMA‐RI were increased, whereas the level of the QUICKI was decreased in the patients with overweight type 2 diabetes mellitus compared with those with non‐overweight type 2 diabetes mellitus (P = 0.04, 0.028 and 0.016, respectively). Similarly, among the control individuals, the levels of insulin, HOMA‐RI and HOMA‐β were increased, whereas the QUICKI level was decreased in the overweight controls compared with the non‐overweight individuals (P < 0.0001). In addition, no difference in liver enzyme levels (aspartate transaminase, alanine transaminase and total bilirubin) was observed between the patients with type 2 diabetes mellitus and the controls (P > 0.05; Table 1).
Adiponectin and cytokine levels in patients with type 2 diabetes mellitus and in controls
The levels of adiponectin, TNF‐α, IL‐1β and IL‐10 were measured in the sera of the patients with type 2 diabetes mellitus and controls, and were compared among subgroups. We observed that the adiponectin levels were significantly lower in the patients with type 2 diabetes mellitus (2.5 ± 1.5 μg/mL) compared with the controls (16 ± 18.6 μg/mL; P < 0.0001; Figure 1a). Among the patients with type 2 diabetes mellitus, the adiponectin levels were lower in the patients with overweight type 2 diabetes mellitus (2.1 ± 0.9 μg/mL) compared with those with non‐overweight type 2 diabetes mellitus (2.7 ± 1.7 μg/mL). However, the difference did not reach statistical significance (P = 0.38). Among the control group, adiponectin levels were significantly decreased in the overweight individuals (5.7 ± 7.3 μg/mL) compared with the non‐overweight individuals (23.5 ± 20.7 μg/mL; P < 0.0001; Figure 1b).
Figure 1.
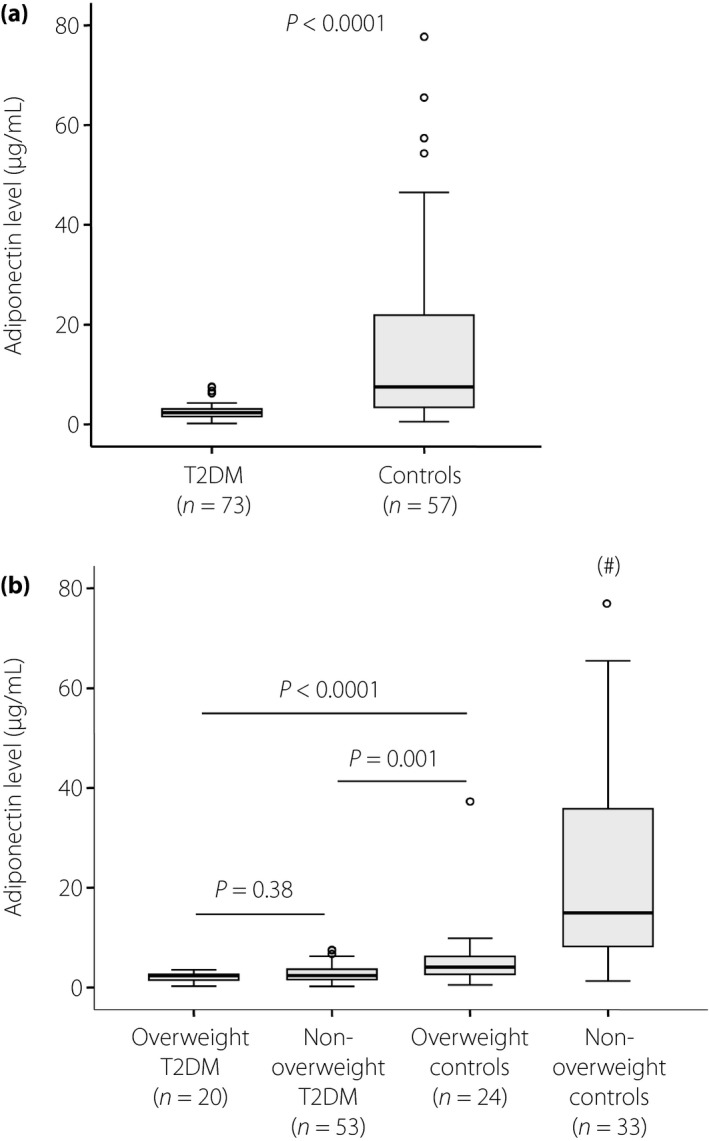
Adiponectin level in type 2 diabetes mellitus (T2DM) patients and controls. Adiponectin level was measured in the sera of patients with type 2 diabetes mellitus and controls, and was compared between groups. (a) Adiponectin levels in patients with type 2 diabetes mellitus and controls. (b) Adiponectin levels in patients with overweight type 2 diabetes mellitus and those with non‐overweight type 2 diabetes mellitus, and in overweight and non‐overweight control individuals. P‐values were calculated by using the Mann–Whitney U‐test. # P < 0.0001 when compared with other groups.
The TNF‐α and IL‐1β levels were significantly increased, whereas the IL‐10 levels were significantly decreased in the patients with type 2 diabetes mellitus and in the overweight individuals compared with the non‐overweight control individuals (P < 0.0001; Figure 2a–c). Among the patients with type 2 diabetes mellitus, the TNF‐α, IL‐1β and IL‐10 levels were not significantly different between the patients with overweight and those with non‐overweight type 2 diabetes mellitus. However, within the control group, the TNF‐α and IL‐1β levels were higher, whereas the IL‐10 levels were lower in the overweight individuals compared wit the non‐overweight individuals (P < 0.0001; Figure 2a–c). In addition, we analyzed the correlations of the adiponectin levels with the TNF‐α, IL‐1β and IL‐10 levels. The adiponectin levels were negatively correlated with the TNF‐α levels (Spearman's rho = −0.56, P < 0.0001) and the IL‐1β levels (Spearman's rho = −0.51, P < 0.0001), whereas they were positively correlated with IL‐10 levels (Spearman's rho = 0.23, P = 0.013; Figure 3a–c).
Figure 2.
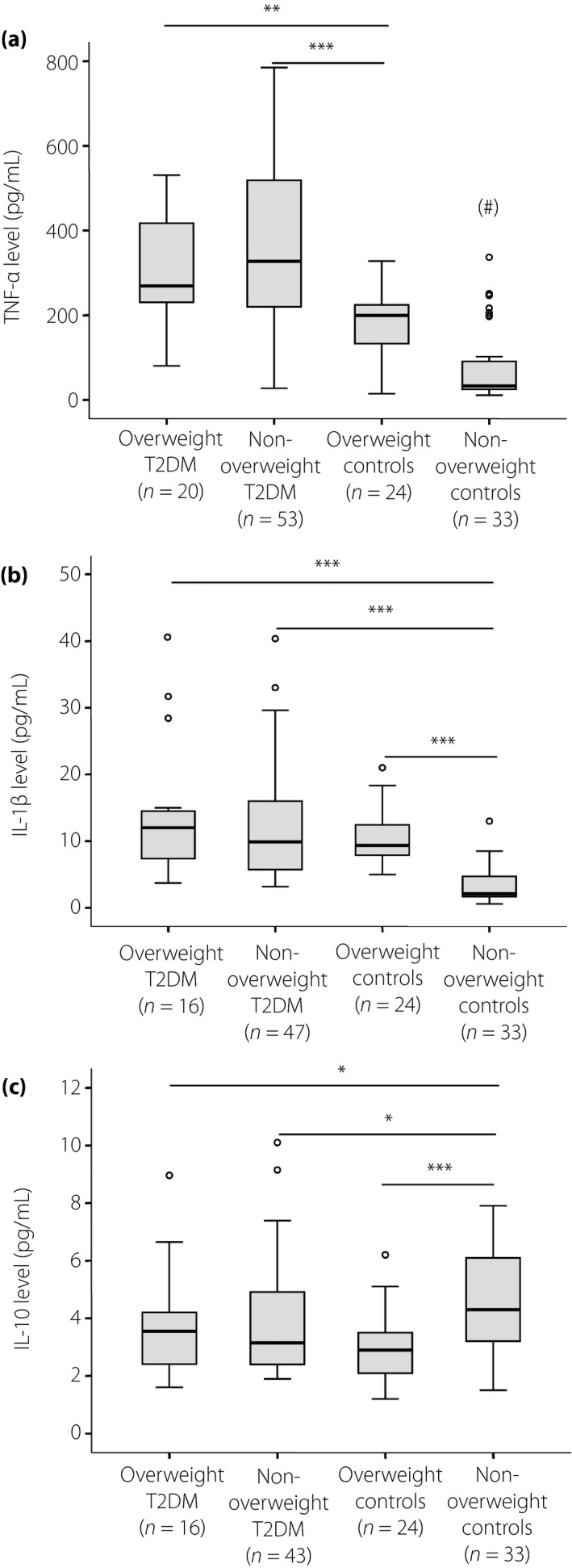
Cytokine levels in type 2 diabetes mellitus (T2DM) patients and controls. The levels of (a) tumor necrosis factor (TNF)‐α, (b) interleukin (IL)‐1β and (c) IL‐10 were measured in the sera of patients with overweight type 2 diabetes mellitus and non‐overweight type 2 diabetes mellitus, and of overweight and non‐overweight control individuals. P‐values were calculated by using the Mann–Whitney U‐test. # P < 0.0001 when compared with other groups; *P < 0.05; **P < 0.005; ***P < 0.0005.
Figure 3.
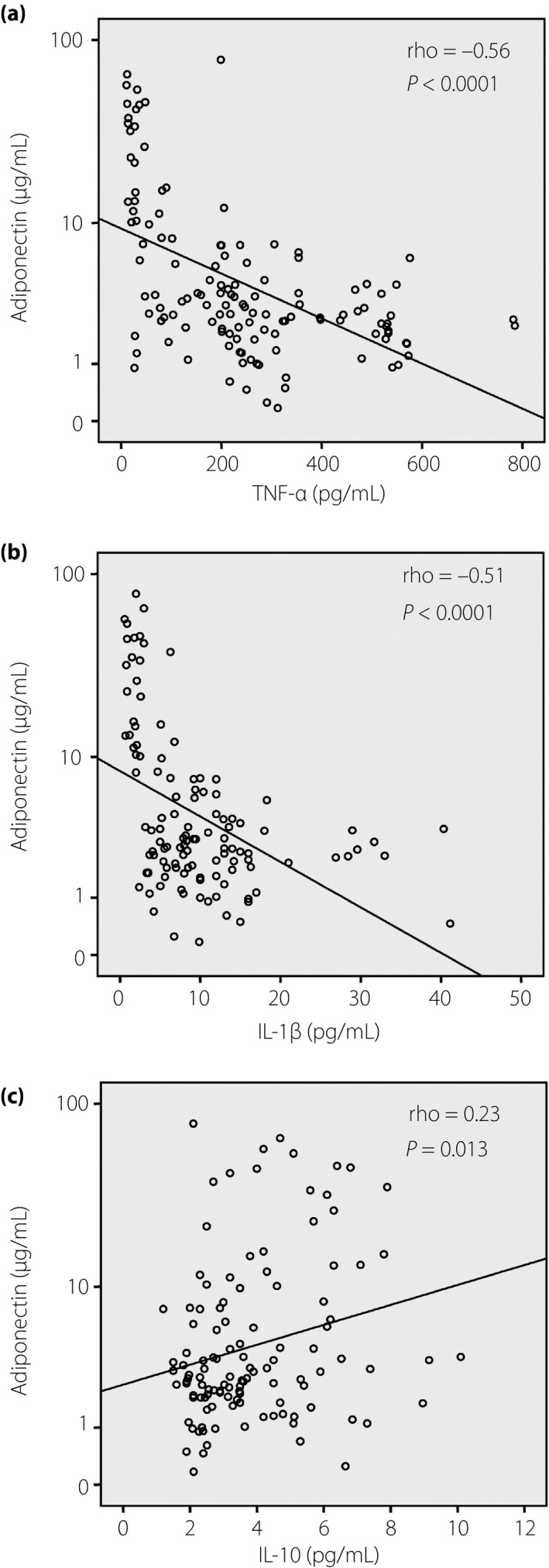
Correlation between adiponectin and cytokine levels. The correlations between adiponectin levels and the levels of (a) tumor necrosis factor (TNF)‐α, (b) interleukin (IL)‐1β and (c) IL‐10 were calculated by using the Spearman's rank correlation coefficient. Spearman's rho and P‐values are presented.
Correlation of adiponectin levels with clinical parameters
We analyzed the correlation of the adiponectin levels with the clinical parameters of overweight and type 2 diabetes mellitus, and observed that the adiponectin levels were negatively correlated with the levels of blood glucose, triglycerides and total cholesterol (Spearman's rho = −0.49, −0.34 and −0.32, respectively). Regarding HDL‐C and LDL‐C, the adiponectin levels were positively correlated with HDL‐C, whereas they were negatively correlated with LDL‐C (Spearman's rho = 0.39 and −0.23, respectively). Regarding the correlation of adiponectin levels with insulin resistance and β‐cell function, adiponectin levels were negatively correlated with insulin levels and HOMA‐IR (Spearman's rho = −0.4 and −0.51, respectively), whereas they were positively correlated with the QUICKI and HOMA‐β (Spearman's rho = 0.43 and 0.19, respectively; Figure 4). However, we did not observe any significant correlation of adiponectin levels with levels of urea, creatinine and liver enzymes (aspartate transaminase, alanine transaminase and total bilirubin).
Figure 4.
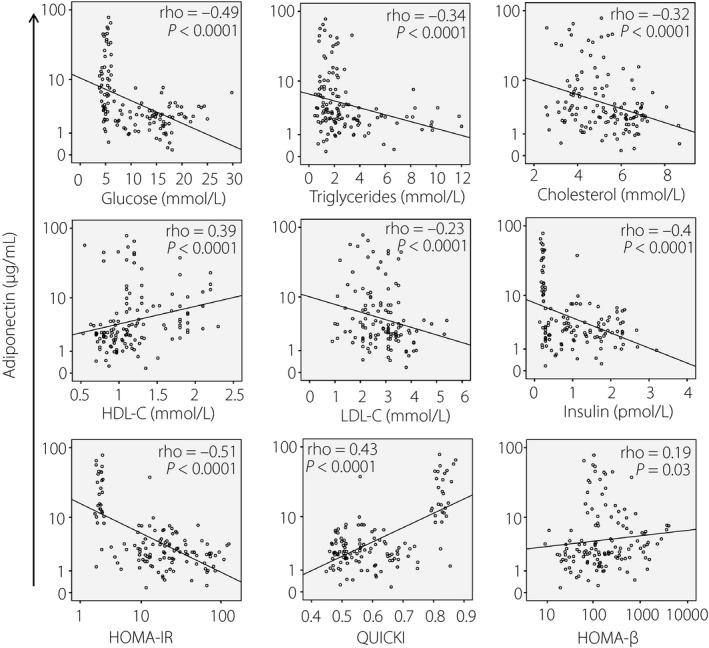
Correlation between adiponectin level and clinical parameters of obesity and type 2 diabetes mellitus. The correlations between adiponectin and numbers of clinical parameters of overweight and type 2 diabetes mellitus were calculated by using Spearman's rank correlation coefficient. Spearman's rho and P‐values are presented. HDL‐C, high‐density lipoprotein cholesterol; HOMA‐β, homeostasis model assessment β‐cell resistance; HOMA‐IR, homeostasis model assessment insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; QUICKI, quantitative insulin sensitivity check insulin resistance index.
Correlation of adiponectin‐to‐cytokine ratios with insulin resistance index
An index based on the relative proportion of adiponectin‐to‐resistin has been proposed to have diagnostic potential for insulin resistance23. Because of the strong correlation of adiponectin levels with the levels of other studied cytokines (TNF‐α, IL‐1β and IL‐10), and the association of these cytokines with insulin resistance and type 2 diabetes mellitus24, the relative proportion of these studied cytokines might also have a potential for the diagnosis of insulin resistance. We calculated the relative ratios of adiponectin/TNF‐α, adiponectin/IL‐1β, adiponectin/IL‐10, TNF‐α/IL‐1β, TNF‐α/IL‐10 and IL‐1β/IL‐10, and analyzed their correlations with the QUICKI and HOMA‐IR indexes. We observed that the QUICKI was positively correlated with the relative ratios of adiponectin/TNF‐α, adiponectin/IL‐1β and adiponectin/IL‐10, whereas it was negatively correlated with the ratios of TNF‐α/IL‐10 and IL‐1β/IL‐10 (Figure 5). Similarly, the HOMA‐IR index was negatively correlated with the relative ratios of adiponectin/TNF‐α, adiponectin/IL‐1β and adiponectin/IL‐10, whereas it was positively correlated with the ratios of TNF‐α/IL‐10 and IL‐1β/IL‐10 (Figure 6). These results show that the relative ratios of adiponectin to studied cytokines (TNF‐α, IL‐1β and IL‐10) might also serve as a potential indicator for insulin resistance.
Figure 5.
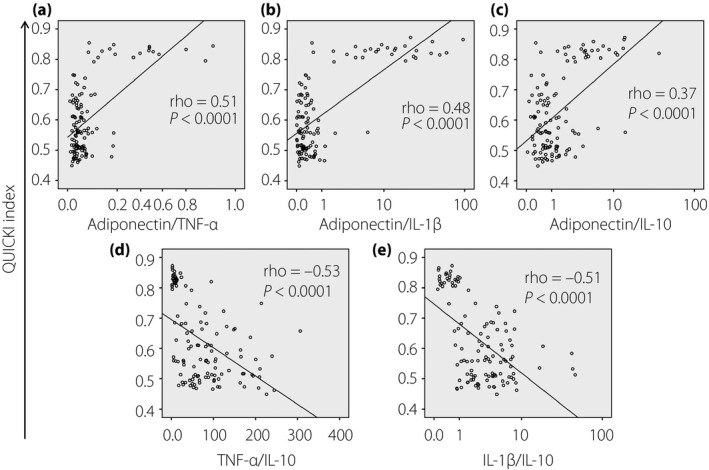
Correlation between the quantitative insulin sensitivity check index (QUICKI) and the relative ratios of studied cytokines. Relative ratios of (a) adiponectin/tumor necrosis factor (TNF)‐α, (b) adiponectin/interleukin (IL)‐1β, (c) adiponectin/IL‐10, (d) TNF‐α/IL‐10 and (e) IL‐1β/IL‐10 were calculated and correlated with the QUICKI. The correlations were calculated by using Spearman's rank correlation coefficient. Spearman's rho and P‐values are presented.
Figure 6.
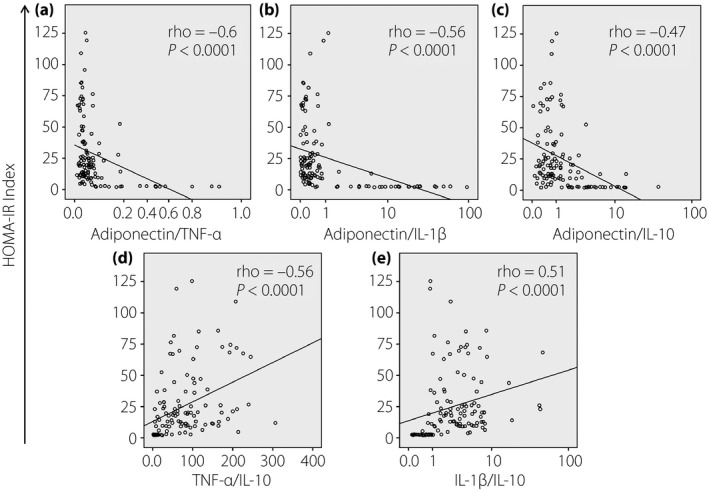
Correlation between the homeostasis model assessment insulin resistance (HOMA‐IR) index and the relative ratios of studied cytokines. Relative ratios of (a) adiponectin/tumor necrosis factor (TNF)‐α, (b) adiponectin/interleukin (IL)‐1β, (c) adiponectin/IL‐10, (d) TNF‐α/IL‐10 and (e) IL‐1β/IL‐10 were calculated and correlated with the HOMA‐IR index. The correlations were calculated by using Spearman's rank correlation coefficient. Spearman's rho and P‐values are presented.
Discussion
Adiponectin plays an important role in the pathogenesis of obesity, type 2 diabetes mellitus, atherosclerosis and inflammation25. The present results showed that adiponectin levels were significantly decreased in patients with type 2 diabetes mellitus and in overweight individuals compared with patients with non‐overweight type 2 diabetes mellitus and healthy individuals, respectively. In addition, our investigations showed that adiponectin levels were correlated with several pro‐inflammatory cytokines, and with clinical markers of overweight and type 2 diabetes mellitus. These results indicate that adiponectin and adipose‐derived cytokines could potentially influence the development of overweight and type 2 diabetes mellitus.
Most of the adipokines are able to regulate metabolism and inflammation, thus they play a vital role in the pathogenesis of metabolic disorders and type 2 diabetes mellitus26, 27. Studies have shown that adipokines are involved in the pathogenesis of obesity, atherosclerosis, inflammation and type 2 diabetes mellitus15. Of those adipokines, adiponectin is considered as a potential marker for the treatment of obesity, type 2 diabetes mellitus, atherosclerosis and inflammation26, 27, 28. In the normal condition, the circulating adiponectin serum levels are high in contrast to the low level of other adipocytokines29. In line with other studies30, 31, 32, 33, 34, 35, the present study also showed that adiponectin levels were significantly decreased in patients with type 2 diabetes mellitus compared with individuals without type 2 diabetes mellitus. In addition, decreased adiponectin levels observed in overweight individuals supports the fact that adiponectin serum levels were inversely correlated with obesity, especially with the accumulation of visceral fat33. Furthermore, decreased adiponectin levels were also associated with the pathogenesis of many other diseases, such as lipid metabolic disorders and cancer36, 37, 38. More directly, our data show that adiponectin levels are significantly correlated with numerous clinical parameters of obesity, such as triglycerides, cholesterol, HDL‐C and LDL‐C. The adiponectin level was also strongly related to type 2 diabetes mellitus clinical parameters, including insulin level and index of insulin sensitivity/resistance (HOMA‐RI and QUICKI) and β‐cell function (HOMA‐β). Those observations indicate that adiponectin levels are involved in the development of type 2 diabetes mellitus.
TNF‐α and IL‐6 are pro‐inflammatory cytokines not only produced mainly by immune cells, but also by adipocytes, and therefore the levels of TNF‐α and IL‐6 are associated with obesity and type 2 diabetes mellitus39. The present study showed that the TNF‐α and IL‐1β levels were increased, whereas the IL‐10 level was decreased in patients with type 2 diabetes mellitus and in overweight individuals compared with non‐overweight individuals. The TNF‐α, IL‐1β and IL‐10 levels were significantly correlated with the adiponectin levels. These results show that pro‐inflammatory cytokines, such as TNF‐α, IL‐1β, IL‐6 and IL‐10, contribute significantly to the pathogenesis of type 2 diabetes mellitus. A previous study has shown that increased TNF‐α, IL‐1β and IL‐6 levels lead to increased insulin resistance in overweight individuals, and subsequently results in type 2 diabetes mellitus24. Adiponectin was shown to induce the expression and secretion of IL‐1040, hence IL‐10 levels were more decreased in patients with type 2 diabetes mellitus than in healthy individuals41, 42. The present results along with previous observations suggest that IL‐10 contributes to influencing the development of type 2 diabetes mellitus, and such an association was further supported by a meta‐analysis showing that the polymorphisms in the promoter region of the IL‐10 gene are associated with type 2 diabetes mellitus risk43. Also, it is likely that decreased adiponectin impairs IL‐10 production, and thus predisposes to metabolic syndrome and type 2 diabetes mellitus.
IL‐1 carries out many functional activities in the regulation of inflammatory responses and metabolism, and can regulate insulin secretion and induce β‐cell apoptosis that can subsequently lead to type 2 diabetes mellitus44, 45. High IL‐1β levels in patients with type 2 diabetes mellitus and in overweight individuals, and their converse correlation with adiponectin levels observed in the present study suggest that IL‐1β might play a key role in the modulation of β‐cell function. Also, high cytokine secretion over a long period of time resulted in impairment of β‐cell function46. Furthermore, IL‐18, another interleukin recently classified as a member of the IL‐1 cytokine super‐family, was associated with obesity and insulin resistance, hypertension, and atherosclerotic disease47, 48, 49.
In contrast to adiponectin, increased insulin sensitivity is improved by reducing bodyweight or treatment with insulin sensitivity affecting drugs. Adiponectin is a hormone derived from adipose tissue that is effective against diabetes and inflammation50, 51, 52. Low adiponectin levels caused by the deficiency of its production were associated with a higher risk of overweight‐related type 2 diabetes mellitus, as observed in the present study. Therefore, adiponectin might be considered as a risk marker for incident prediabetes53, 54, and a promising therapeutic intervention for type 2 diabetes mellitus in overweight individuals28, 55, 56. Increasing the adiponectin serum levels could be one of the strategies for treatment of overweight‐related type 2 diabetes mellitus by reducing the adipose tissue. In addition, a previous study has proposed a novel index based on the relative proportion of adiponectin‐to‐resistin to diagnose of insulin resistance23. The present study also showed that the insulin sensitivity/resistance indexes (QUICKI and HOMA‐IR) have a strong correlation with the relative ratios of adiponectin/TNF‐α, adiponectin/IL‐1β, adiponectin/IL‐10, TNF‐α/IL‐10 and IL‐1β/IL‐10. Therefore, an index based on the levels of adipokines and adipose‐derive cytokines could be useful for diagnosis of insulin resistance. However, more studies are required to propose a new index and verify the diagnostic accuracy in clinical practice, and to establish a cut‐off value and reference range of insulin sensitivity for specific populations23.
In conclusion, the present study showed that the levels of adiponectin, TNF‐α, IL‐1β and IL‐10 are significantly modulated during the development of overweight and type 2 diabetes mellitus. The adiponectin levels were significantly correlated with the TNF‐α, IL‐1β and IL‐10 levels, and with clinical parameters of obesity and type 2 diabetes mellitus. Adipokines, together with pro‐inflammatory cytokines, could possibly modulate the pathogenesis of overweight and type 2 diabetes mellitus, and might serve as a prognostic marker and a therapeutic intervention for overweight‐related type 2 diabetes mellitus.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank all the study participants. This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106‐YS.02‐2014.36.
J Diabetes Investig 2017; 8: 295–305
References
- 1. Idf. Annual Report. 6th edn, 2013.
- 2. Guariguata L, Whiting DR, Hambleton I, et al Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137–149. [DOI] [PubMed] [Google Scholar]
- 3. Garber AJ, Handelsman Y, Einhorn D, et al Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from The American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract 2008; 14: 933–946. [DOI] [PubMed] [Google Scholar]
- 4. Monzillo LU, Hamdy O, Horton ES, et al Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 2003; 11: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 5. Pham NM, Eggleston K. Diabetes prevalence and risk factors among vietnamese adults: findings from community‐based screening programs. Diabetes Care 2015; 38: E77–E78. [DOI] [PubMed] [Google Scholar]
- 6. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11: 98–107. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Diagnosis and classification Of diabetes mellitus. Diabetes Care 2012; 35(Suppl 1): S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 9. Kahn SE. Clinical review 135: the importance of beta‐cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001; 86: 4047–4058. [DOI] [PubMed] [Google Scholar]
- 10. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 11. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol 2013; 3: 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guilherme A, Virbasius JV, Puri V, et al Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 14. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013; 9: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014; 15: 6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuzawa Y, Hibi K, Kimura K. Risk assessment for cardiovascular disease ‐ microvascular dysfunction. Circ J 2010; 74: 1296–1297. [DOI] [PubMed] [Google Scholar]
- 17. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92: 347–355. [DOI] [PubMed] [Google Scholar]
- 18. Lee JM, Kim SR, Yoo SJ, et al The relationship between adipokines, metabolic parameters and insulin resistance in patients with metabolic syndrome and type 2 diabetes. J Int Med Res 2009; 37: 1803–1812. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez M, Del Mar BM, Pons A, et al Inflammatory markers and metabolic syndrome among adolescents. Eur J Clin Nutr 2012; 66: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 20. Alikasifoglu A, Gonc N, Ozon ZA, et al The relationship between serum adiponectin, tumor necrosis factor‐alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. J Clin Res Pediatr Endocrinol 2009; 1: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idf Clinical Guidelines Task Force . Global Guideline For Type 2 Diabetes. Brussels: International Diabetes Federation, 2005. [Google Scholar]
- 22. World Health Organization . Preventing And Managing The Global Epidemic. Report of a Who Consultation on Obesity. Geneva, 1998. [PubMed] [Google Scholar]
- 23. Lau CH, Muniandy S. Novel Adiponectin‐Resistin (Ar) And Insulin Resistance (Irar) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study. Cardiovasc Diabetol 2011; 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spranger J, Kroke A, Mohlig M, et al Inflammatory Cytokines and the risk to develop type 2 diabetes: results of the prospective population‐based European Prospective Investigation into Cancer and Nutrition (Epic)‐Potsdam Study. Diabetes 2003; 52: 812–817. [DOI] [PubMed] [Google Scholar]
- 25. Kang YS. Obesity associated hypertension: new insights into mechanism. Electrolyte Blood Press 2013; 11: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knights AJ, Funnell AP, Pearson RC, et al Adipokines and insulin action: a sensitive issue. Adipocyte. 2014; 3: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 2013; 4: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haluzik M. Adiponectin and its potential in the treatment of obesity, diabetes and insulin resistance. Curr Opin Investig Drugs 2005; 6: 988–993. [PubMed] [Google Scholar]
- 29. Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord 2008; 6: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haluzik M, Parizkova J, Haluzik MM. Adiponectin and its role in the obesity‐induced insulin resistance and related complications. Physiol Res 2004; 53: 123–129. [PubMed] [Google Scholar]
- 31. Jung CH, Kim BY, Mok JO, et al Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus. J Diabetes Investig 2014; 5: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aleidi S, Issa A, Bustanji H, et al Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm J 2015; 23: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asayama K, Hayashibe H, Dobashi K, et al Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res 2003; 11: 1072–1079. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto S, Matsushita Y, Nakagawa T, et al Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diabetes 2014; 4: E130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai H, Lin N, Xing Z, et al Association between the level of circulating adiponectin and prediabetes: a meta‐analysis. J Diabetes Investig 2015; 6: 416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shu KH, Wu MJ, Chen CH, et al. Serum adipokine levels in renal transplant recipients. Transplant Proc 2014; 46: 381–384. [DOI] [PubMed] [Google Scholar]
- 37. Enli Y, Balci YI, Gonen C, et al. Adipocytokine concentrations in children with different types of beta‐thalassemia. Scand J Clin Lab Invest 2014; 74: 306–311. [DOI] [PubMed] [Google Scholar]
- 38. Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: new biomarkers and attractive therapeutic targets. World J Exp Med 2013; 3: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, Inflammation And Immunity. Nat Rev Immunol 2006; 6: 772–783. [DOI] [PubMed] [Google Scholar]
- 40. Wolf AM, Wolf D, Rumpold H, et al. Adiponectin induces the anti‐inflammatory cytokines Il‐10 and Il‐1ra in human leukocytes. Biochem Biophys Res Commun 2004; 323: 630–635. [DOI] [PubMed] [Google Scholar]
- 41. Acharya AB, Thakur S, Muddapur MV. Evaluation of serum interleukin‐10 levels as a predictor of glycemic alteration in chronic periodontitis and type 2 diabetes mellitus. J Indian Soc Periodontol 2015; 19: 388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van EE, Gussekloo J, De Craen AJ, et al. Low production capacity of interleukin‐10 associates with the metabolic syndrome and type 2 diabetes: the leiden 85‐plus study. Diabetes 2002; 51: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 43. Hua Y, Shen J, Song Y, et al. Interleukin‐10 ‐592c/A, ‐819c/T And ‐1082a/G polymorphisms with risk of type 2 diabetes mellitus: a huge review and meta‐analysis. PLoS ONE 2013; 8: E66568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banerjee M, Saxena M. Interleukin‐1 (Il‐1) family of cytokines: role in type 2 diabetes. Clin Chim Acta 2012; 413: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 45. Zhao G, Dharmadhikari G, Maedler K, et al. Possible role of interleukin‐1beta in type 2 diabetes onset and implications for anti‐inflammatory therapy strategies. PLoS Comput Biol 2014; 10: E1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 2013; 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bosch M, Lopez‐Bermejo A, Vendrell J, et al. Circulating Il‐18 concentration is associated with insulin sensitivity and glucose tolerance through increased fat‐free mass. Diabetologia 2005; 48: 1841–1843. [DOI] [PubMed] [Google Scholar]
- 48. Bruun JM, Stallknecht B, Helge JW, et al. Interleukin‐18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 2007; 157: 465–471. [DOI] [PubMed] [Google Scholar]
- 49. Straczkowski M, Kowalska I, Nikolajuk A, et al. Increased serum interleukin‐18 concentration is associated with Hypoadiponectinemia in obesity, independently of insulin resistance. Int J Obes (Lond) 2007; 31: 221–225. [DOI] [PubMed] [Google Scholar]
- 50. Bauche IB, El Mkadem SA, Pottier AM, et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology 2007; 148: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 51. Brichard SM, Delporte ML, Lambert M. Adipocytokines in anorexia nervosa: a review focusing on leptin and adiponectin. Horm Metab Res 2003; 35: 337–342. [DOI] [PubMed] [Google Scholar]
- 52. Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003; 46: 459–469. [DOI] [PubMed] [Google Scholar]
- 53. Jiang Y, Owei I, Wan J, et al. Adiponectin levels predict prediabetes risk: the pathobiology of prediabetes in A biracial cohort (Pop‐Abc) study. BMJ Open Diabetes Res Care 2016; 4: E000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang C, Momma H, Niu K., et al. High serum adiponectin levels predict incident falls among middle‐aged and older adults: a prospective Cohort Study. Age Ageing 2016; 45: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang ZV, Scherer PE. Adiponectin, The Past Two Decades. J Mol Cell Biol 2016; 8: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol 2016; 8: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]


