Abstract
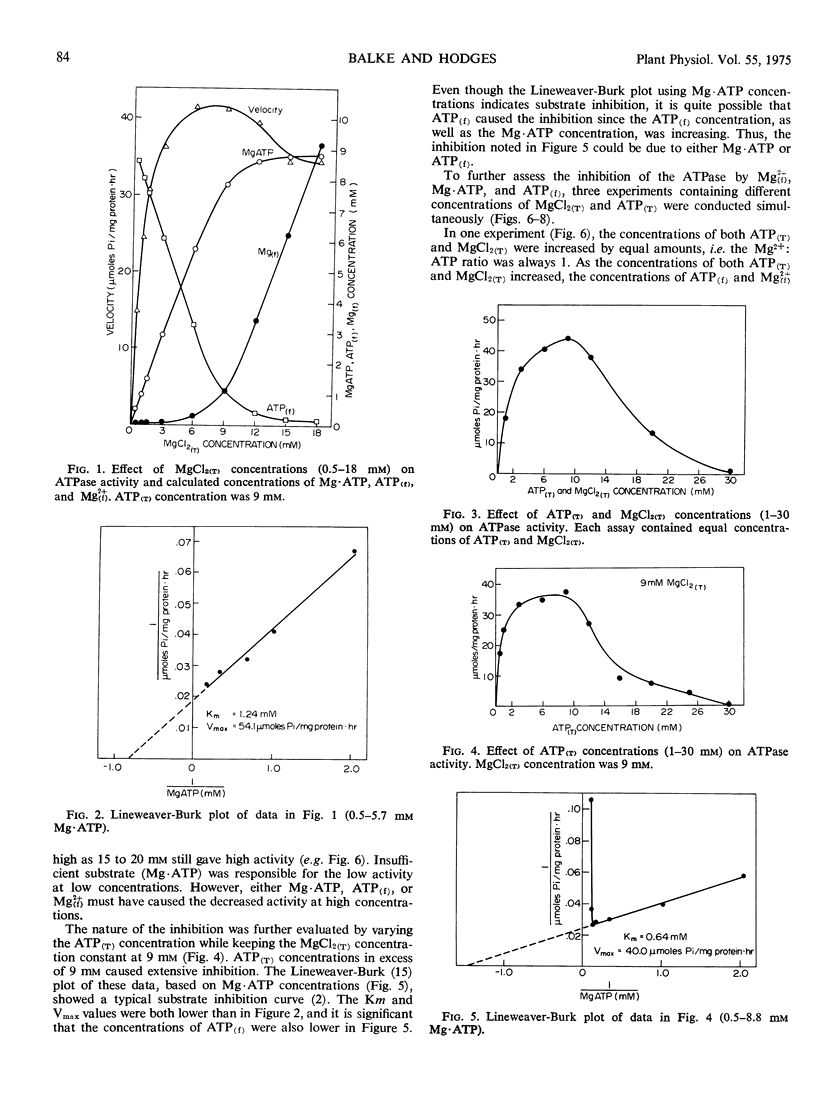
ATPase activity of plasma membrane vesicles isolated from oat (Avena sativa L. cv. Goodfield) roots was examined in the presence of various concentrations of MgCl2 and ATP. A Mg2+: ATP ratio of about 1 was required for maximal activity regardless of the concentrations used; the optimum concentration for both Mg2+ and ATP was 9 mm. Based on the ATPase activity at different concentrations of complexed Mg·ATP and free ATP, it is concluded that Mg·ATP is the true substrate of this enzyme.
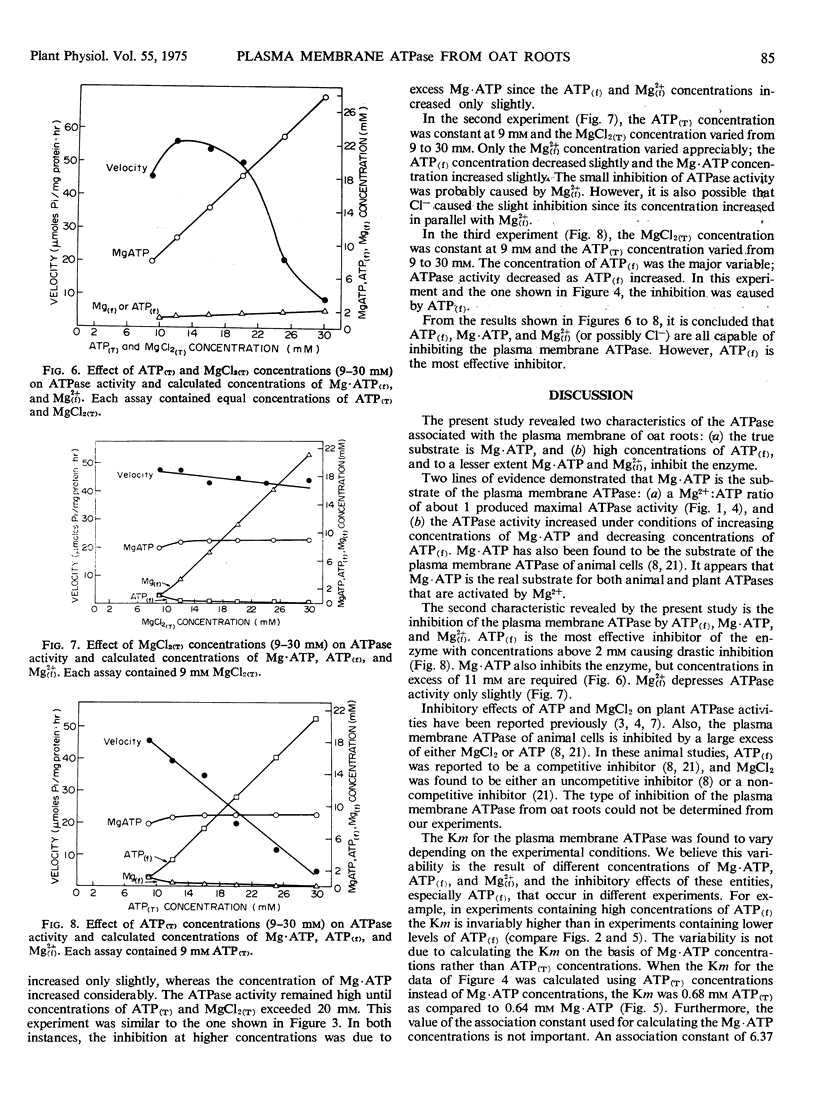
Under certain experimental conditions, high concentrations of MgCl2 and ATP inhibited the plasma membrane ATPase. On the basis of the relative amounts of free and complexed ATP and Mg2+, it was found that the different moieties caused different amounts of inhibition. Free ATP inhibited the ATPase at concentrations in excess of 2 mm. Mg·ATP concentrations above 11 mm inhibited the enzyme. Free Mg2+ caused only a slight inhibition of the ATPase.
The Km for Mg·ATP was found to vary from 0.64 to 1.24 mm depending on the experimental conditions. This variation is thought to be due to variable amounts of Mg·ATP, which serves as an inhibitor as well as the substrate, and free ATP, which also inhibits the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexum T., Samson F. E., Jr, Himes R. H. Kinetic studies of membrane (Na+-K+-Mg2+)-ATPase. Biochim Biophys Acta. 1970 Aug 15;212(2):322–331. doi: 10.1016/0005-2744(70)90213-5. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline M. H., Hexum T. D., Dahl J. L., Hokin L. E. Studies on the characterization of the sodium-potassium transport adenosinetriphosphatase. VII. Comparison of the properties of the membrane-bound and partially purified soluble and insoluble forms of the enzyme. Arch Biochem Biophys. 1971 Dec;147(2):781–787. doi: 10.1016/0003-9861(71)90439-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANNINGA L. B. The association constant of the complexes of adenosine triphosphate with magnesium, calcium, strontium, and barium ions. Biochim Biophys Acta. 1961 Dec 9;54:330–338. doi: 10.1016/0006-3002(61)90373-0. [DOI] [PubMed] [Google Scholar]
- Opit L. J., Charnock J. S. A molecular model for a sodium pump. Nature. 1965 Oct 30;208(5009):471–474. doi: 10.1038/208471a0. [DOI] [PubMed] [Google Scholar]
- Ratanabanangkoon K., Dixon J. F., Hokin L. E. Studies on the characterization of the sodium-potassium transport adenosinetriphosphatase. XI. Comparison of kinetic properties of the purified with the impure membrane-bound enzyme from Squalus acanthias. Arch Biochem Biophys. 1973 May;156(1):342–349. doi: 10.1016/0003-9861(73)90373-1. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Nucleotide and divalent cation interactions with the (Na+ plus K+)-dependent ATPase. Biochim Biophys Acta. 1974 Mar 21;341(1):232–247. doi: 10.1016/0005-2744(74)90084-9. [DOI] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]


