Abstract
The intrinsic ability of neurogenesis after stroke has been proven weak, which results in insufficient repair of injury in the nerve system. Recent studies suggest multiple microRNAs (miRNAs) are involved in the neuroremodeling process. Targeted miRNAs delivery for amplification of neurogenesis is promising in promoting the prognosis after ischemia. Here, we showed that modified exosomes, with rabies virus glycoprotein (RVG) fused to exosomal protein lysosome-associated membrane glycoprotein 2b (Lamp2b), could efficiently deliver miR-124 to the infarct site. Systemic administration of RVG-exosomes loaded with miR-124 promoted cortical neural progenitors to obtain neuronal identity and protect against ischemic injury by robust cortical neurogenesis. Our study suggests that RVG-exosomes can be utilized therapeutically for the targeted delivery of gene drugs to the brain, thus having great potential for clinical applications.
Keywords: targeted drug delivery, exosome, miRNA, ischemia, neurogenesis
Introduction
Stroke is one of the leading causes of death worldwide and results in disabilities in about 75% of its survivors.1, 2 It is of paramount importance to develop novel neuroprotective therapies to get enhanced recovery. Brain self-repair by neuronal replacement from endogenous neural stem cells and precursors is insufficient and results in irreversible loss of neurons. Amplification of this self-repair mechanism by neuroremodeling could be a promising strategy for stroke treatment. Tissue responds to injury and remodels after ischemia by altering their gene and protein expression. MicroRNAs (miRNAs), a family of non-coding RNAs of 20–25 nt, play pivotal roles during this remodeling process by regulating target genes at post-transcriptional level.3 Several studies have demonstrated significant alterations in the cerebral “miRNA-ome” following ischemia.4, 5 These reports suggest that miRNAs may act as innovative gene therapeutic candidates contributing to neurogenesis, angiogenesis, and neural plasticity.
Among numerous miRNAs, microRNA-124 (miR-124) is most abundantly expressed in the central nervous system (CNS).6 It has been reported that attenuation of endogenous miR-124 in neural progenitor cells in subventricular zone (SVZ) can abolish neuronal differentiation, whereas overexpression leads to acquisition of neuronal identity.7, 8 After ischemia, the expression of miR-124 was increased in ischemic penumbra. Exogenous miR-124, by using agomir or liposomated mimic, could reduce infarct area.9, 10 Recently, a study showed that viral vector expressing miR-124 yielded increased angioneurogenesis and significant neuroprotection against stroke.11 Studies above revealed the neuroprotective and neurorestorative potential of miR-124. However, blood brain barrier (BBB) has been proven to be the major obstacle for delivery of drugs to the CNS. It has been estimated that 98% of all small molecules do not cross the BBB.12 Recently, accumulating evidences showed that exosomes, which are lipid membrane vesicles of 30 to 100 nm in diameter, could cross BBB and carry cargoes such as proteins, lipids, and nucleotides, mediating the intertwined brain remodeling events after stroke.13, 14 In order to achieve neuron-specific targeting, rabies virus glycoprotein (RVG) has been engineered to exosomal surface via fused protein lysosome-associated membrane glycoprotein 2b (Lamp2b)-RVG.15 In this study, we wondered whether delivery of miR-124 utilizing RVG-modified exosomes could protect against cortical ischemia. Our results showed that RVG-exosome-mediated miR-124 delivery could efficiently ferry miRNA to the ischemic region and ameliorate brain injury by promoting neural progenitors to obtain neuronal phenotype at infarct site.
Results
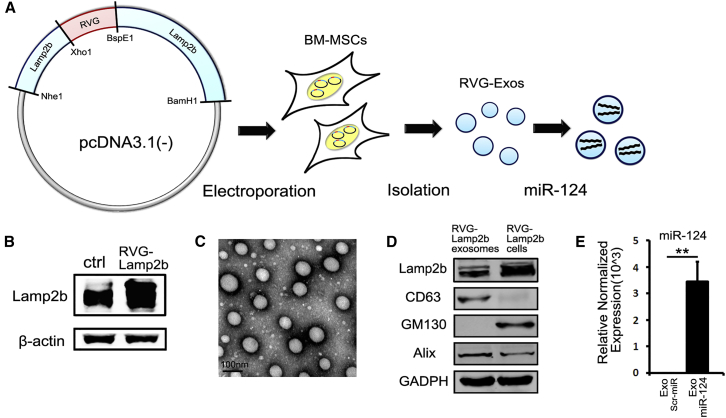
Characterization of RVG-Lamp2b-Modified Exosomes
To generate RVG-Exos, we fused the RVG peptide to Lamp2b and introduced the pcDNA3.1(−)-RVG-Lamp2b plasmid into BM-MSCs. Then we loaded the RVG-Exos with miR-124 mimics or scrambled miRNAs via electroporation (Figure 1A). To validate whether the RVG-Lamp2b plasmid was successfully electroporated into BM-MSCs, we assessed the protein level of Lamp2b (Figure 1B). Compared with BM-MSCs electroporated by pcDNA3.1(−) plasmid, the western blotting showed that the RVG-Lamp2b-modified cells expressed a higher level of Lamp2b. Then we purified exosomes from culture supernatants of BM-MSCs. As previously described,15 modification didn’t appear to affect the physical property of the modified exosomes observed by electron microscopy (EM) (Figure 1C). Western blot analysis of exosomal markers Lamp2b, CD63, Alix, and Golgi marker GM130 further confirmed the identity of the exosomes and inclusion of RVG-Lamp2b on the exosome (Figure 1D). Next, we examined the efficacy of loading exosomes with exogenous cargoes. The amount of encapsulated miR-124 was assayed by qRT-PCR. The results showed that the expression of miR-124 was significantly higher in exosomes loaded with miR-124 mimics (RVG-Exos-miR124) than that of exosomes loaded with scrambled miRNAs (RVG-Exo-Scr) (Figure 1E). Moreover, there were about 1% of the miRNAs encapsulated into the exosomes, meaning about 500 miRNAs per exosome. All of these data confirmed the efficient loading and stability of miRNA in the modified exosomes.
Figure 1.
Characterization of Exosomes
(A) The schematic diagram of plasmid recombination, BM-MSCs electroporation, exosome isolation, and cargo loading. (B) Western blotting of Lamp2b in electoporated BM-MSCs. Note the higher expression of Lamp2b in RVG-Lamp2b-modified cells compared with control (ctrl) ones. (C) Representative EM image of modified exosomes derived from BM-MSCs. The scale bar represents 100 nm. (D) Western blotting of Lamp2b, CD63, GM130, Alix in RVG-Lamp2b-modified exosomes, and the parental cells. (E) Expression level of miR-124 was quantified in exosomes electroporated with scrambled miRNAs (Scr-miR) or miR-124 by qRT-PCR analysis (n = 4). The data were normalized to U6 expression and expressed as mean ± SEM (**p < 0.01).
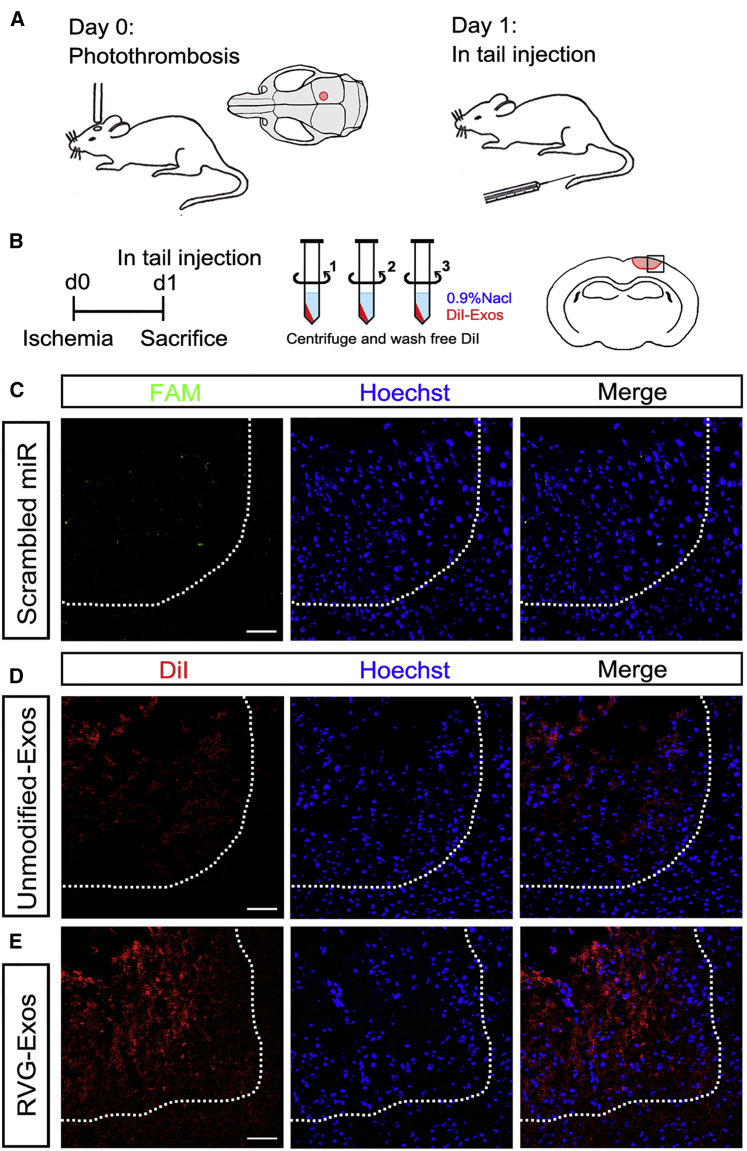
RVG-Lamp2b-Modified Exosomes Specially Target the Injured Region
To investigate the potential of RVG-Exos to specially deliver miR-124 to the injured region, we established a focal ischemia model. We performed photothrombosis in the somatosensory cortex (Figure 2A).16 Unmodified exosomes (unmodified-Exos) or RVG-Exos were labeled with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) dye. To wash away free DiI, exosomes were resuspended in 0.9% saline and centrifuged for three times before intravenous injection into C57BL/6 mice at 1 day post ischemia (dpi). Mice were sacrificed 2 hr after DiI labeling for observation of exosome distribution (Figure 2B). Serial coronal sections were made and images were taken by confocal microscopy. To test whether miRNAs could get to the ischemic region, we first injected fluorescein amidite (FAM)-labeled scrambled miRNAs intravenously at 1dpi. FAM could be scarcely detected 2 hr post injection (Figure 2C). This indicated that without encapsulation by exosomes, miRNAs could be hardly endocytosed by the cell. Then we injected DiI-labeled unmodified-Exos and RVG-Exos, respectively. In unmodified-Exos injected mice, few DiI could be observed at the core of the injured region (Figure 2D). However, DiI fluorescence was predominantly detectable in the ischemic region in RVG-Exos injected mice, with relatively weak fluorescent signal scattered in the normal brain (Figure 2E). These data showed that compared with unmodified-Exos, RVG-Exos could efficiently promote targeting delivery. Focal BBB disruption may also facilitate the cross of RVG-Exos at the infarct site, making RVG-Exos effective drug delivery tools for ischemia treatment. Notably, most of the ischemic area was DiI positive in the RVG-exosome delivery group, suggesting that besides neurons, astrocytes and endothelial cells might be also the recipient cells. Besides the brain localization of the injected RVG-exosomes, we also observed abundant exosome distribution in liver, spleen, and lung, which should be attributed to the reticuloendothelial system function (Figure S1). Moreover, we did not observe any significant change of food intake, body weight, and physical activity after exosomal injection, suggesting no obvious side effects of exosome injection.
Figure 2.
Distribution of RVG-Exos in Ischemic Region
(A) The schematic diagram of photothrombosis model and the location of local cortex ischemia. The mice were treated with exosomes at 1 dpi. (B) Strategy of carrying out ischemic model and DiI labeling. To wash away free DiI dye, DiI-labeled exosomes (DiI-Exos) were resuspended by 0.9% saline and centrifuged for three times. Mice were sacrificed 2 hr after injection and the brain coronal sections were harvested. The square indicates the selected region of the representative brain sections. (C–E) Representative immunofluorescence images of ischemic region from mice receiving free FAM-labeled scrambled miRNAs (C), DiI-labeld unmodified-Exos (D), and DiI-labeled RVG-Exos (E). The brain was harvested 2 hr after tail vein injection. The dashed line delineates the lesion area. Note the scarcely detected FAM in the scrambled miRNAs injected group, few DiI at the core of infarct site in the unmodified-Exos injected group, and predominant labeling of DiI in the ischemic region in the RVG-Exos injected group. The images presented were representative sections of the whole ischemic region. The scale bars represent 50 μm.
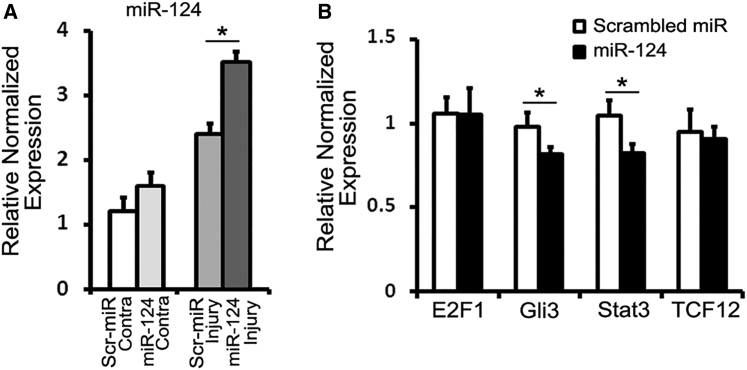
Effects of miR-124 on the Putative mRNA Target Expression
After loading modified exosomes with miR-124, we examined whether RVG-Exos could deliver miR-124 to the ischemic region. By qRT-PCR analysis, we compared the expression level of miR-124 in the contralateral and ipsilateral (to the injured side) cortex of RVG-Exos-miR124 and RVG-Exos-Scr treated mice (Figure 3A). In the contralateral cortex, the expression of miR-124 is relatively higher in RVG-Exos-miR124 treated mice than the control group, yet without statistical significance. In the injured cortex, the expression of miR-124 was significantly increased after RVG-Exos-miR124 injection. These results demonstrated that miR-124 could be efficiently delivered to the infarct site by RVG-Exos.
Figure 3.
Effects of miR-124 on Putative mRNA Target Expression
(A) qRT-PCR analysis of miR-124 in the contralateral (Contra) and ipsilateral cortex of RVG-Exos-miR124 or RVG-Exos-Scr treated mice (n = 4). The data were normalized to U6 and expressed as mean ± SEM (*p < 0.05). (B) Changes in expression of E2F1, Gli3, Stat3, and TCF12 in the ischemic region were assessed by qRT-PCR analysis (n = 4). The data were normalized to β-actin expression and expressed as mean ± SEM (*p < 0.05).
miRNAs target about 60% of mammalian genes and are involved in most biological and pathological processes.14 Based on the gene regulatory role of miRNAs, we suppose that ectopic miR-124 expression may have dramatic effect on the gene network after stroke. We produced the ischemic model and injected RVG-Exos-miR124 and RVG-Exos-Scr at 1 dpi. Then we harvested the ischemic tissue at 3 dpi and examined the mRNA expression of E2F1, Gli3, Stat3, and TCF12, which were previously predicted under the regulation of miR-124 and participated in neural differentiation17, 18, 19, 20, 21 (Figure 3B). The results of qRT-PCR showed that there was no significant difference of mRNA expression of E2F1 and TCF12, while significant decrease of Gli3 and Stat3 upon exosomal miR-124 injection, indicating the functional regulation of target genes by exosomal miR-124.
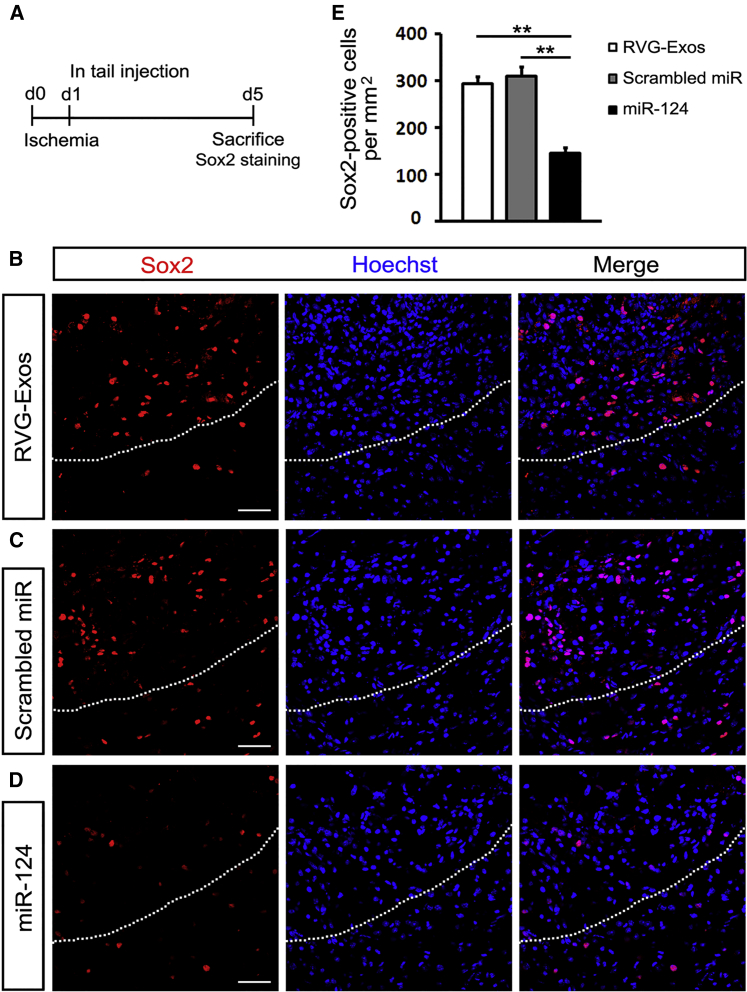
miR-124-Loaded Exosomes Ameliorate the Brain Injury by Promoting Neurogenesis
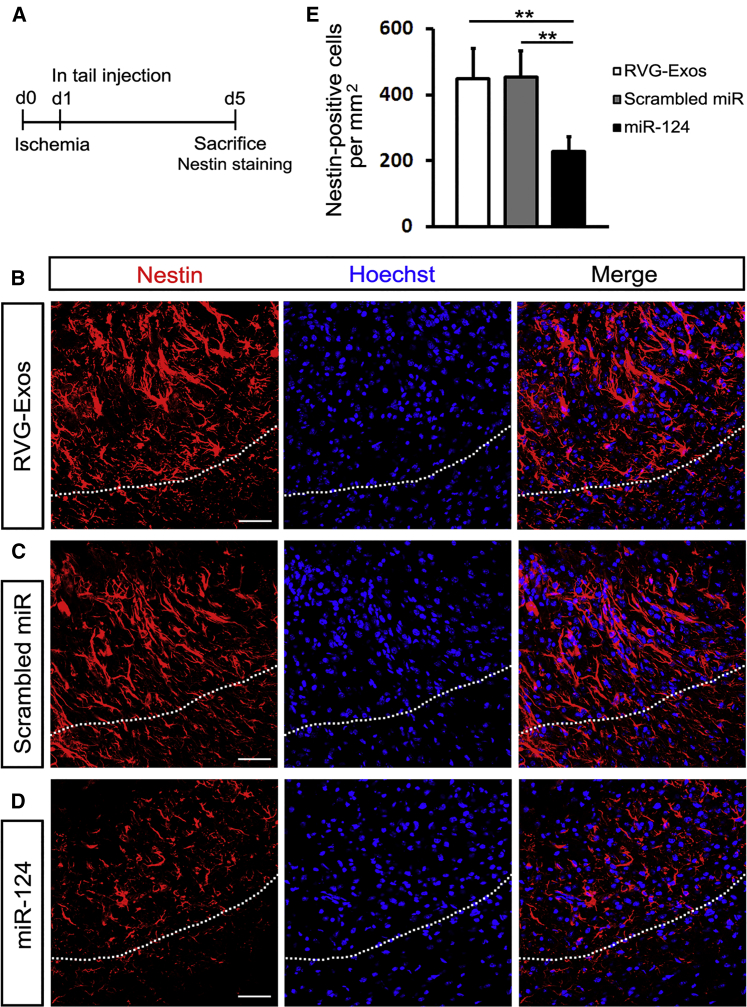
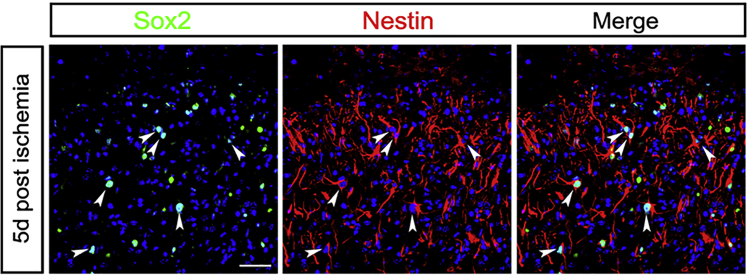
Since miR-124 is well known for its proneuronal role in both the developing and mature brain,22 we further investigated the effect of miR-124 on neural differentiation and neurogenesis post ischemia. We injected RVG-Exos with no miRNAs, RVG-Exos-Scr, and RVG-Exos-miR124 into ischemic mice at 1 dpi. We first examined the expression of neural progenitor marker Sox2 in the ischemic cortex at 5 dpi (Figure 4A). In the RVG-Exos and RVG-Exos-Scr group, Sox2 was induced rapidly around the lesion area at 5 dpi as previously reported (Figures 4B and 4C).23 However, cell quantification in the ischemic region showed that the number of Sox2-positive cells decreased significantly after RVG-Exos-miR124 injection (Figures 4D and 4E). The expression of another neural progenitor marker Nestin was also examined (Figures 5A and S2). Similarly, Nestin exhibited the same expression change at 5 dpi. In the injured cortex, cells richly upregulated Nestin in RVG-Exo and RVG-Exo-Scr treated mice (Figures 5B and 5C), while there was a notable reduction of Nestin-positive cells in RVG-Exos-miR124 treated mice, suggesting the fate change of neural progenitors (Figures 5D and 5E). Both Sox2 and Nestin are markers of the neural progenitor cells, and there are a robust percentage of the neural progenitor cells expressing both markers. Immunohistochemistry of Sox2 and Nestin in the ischemic cortex at 5 dpi revealed that nearly all the Sox2-positive neural progenitors around the injured area were Nestin positive (Figure 6), which suggests that exosomal miR-124 delivery might promote these progenitor cells toward neurogenesis.
Figure 4.
RVG-Exos-miR124 Reduces Sox2 Expression in Ischemic Region
(A) Strategy of carrying out ischemic model and Sox2 immunohistochemistry. (B–D) Immunohistochemistry of Sox2 in the ischemic cortex of (B) RVG-Exos-, (C) RVG-Exos-Scr-, and (D) RVG-Exos-miR124-treated mice at 5 dpi. (E) Cell quantification of Sox2-positive cells in the ischemic region via counting the ischemic area of every eighth slice of the whole ischemic region. Note the significantly decreased expression of Sox2 in RVG-Exos-miR124 treated mice compared with RVG-Exos and RVG-Exos-Scr injected mice (n = 5). The data were expressed as mean ± SD (**p < 0.01). The scale bars represent 50 μm.
Figure 5.
RVG-Exos-miR124 Reduces Nestin Expression in Ischemic Region
(A) Strategy of carrying out ischemic model and Nestin immunohistochemistry. (B–D) Immunohistochemistry of Nestin in the ischemic cortex of (B) RVG-Exos-, (C) RVG-Exos-Scr-, and (D) RVG-Exos-miR124-treated mice at 5 dpi. (E) Cell quantification of Nestin-positive cells in the ischemic region via counting the whole ischemic area of every eighth slice of the ischemic region. Note the significantly decreased expression of Nestin in RVG-Exos-miR124 treated mice compared with RVG-Exos and RVG-Exos-Scr injected mice (n = 5). The data were expressed as mean ± SD (**p < 0.01). The scale bars represent 50 μm.
Figure 6.
Immunohistochemistry of Sox2 and Nestin
Double staining of Sox2 and Nestin in the ischemic cortex at 5 dpi. Most of the neural progenitors were double-positive cells. The arrowheads point to double-stained representative cells. The scale bar represents 50 μm.
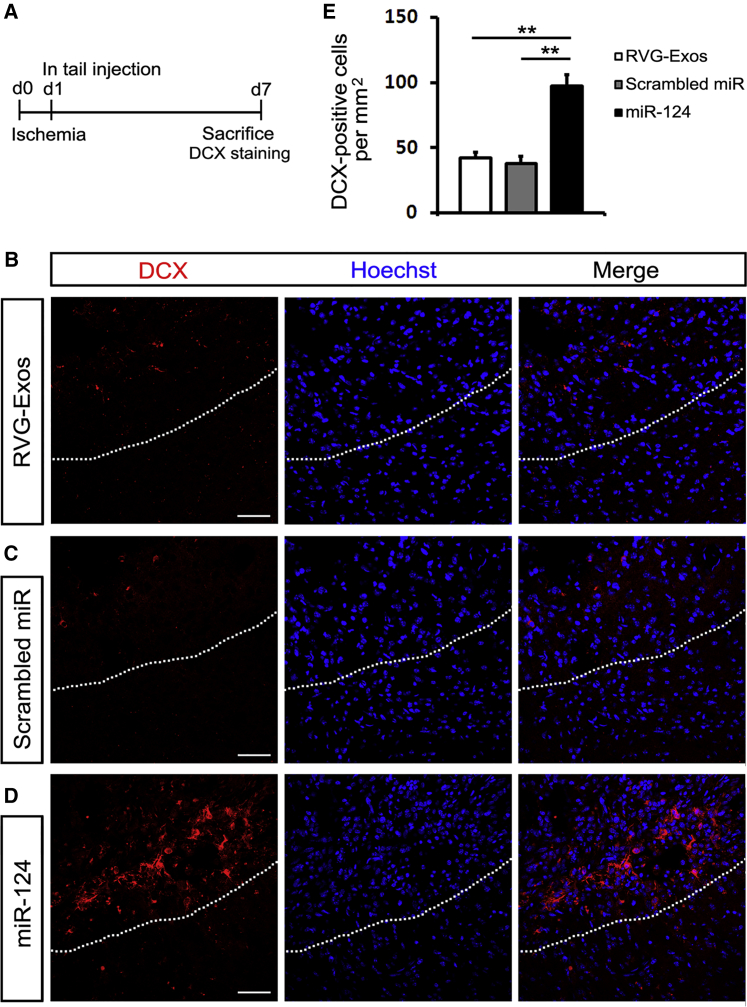
Considering the expression of Sox2 and Nestin decreased significantly after ischemia, we wondered whether the fate of these neural progenitors was changed into neuronal lineage under the effect of miR-124. Then we examined neurogenesis at 7 dpi (Figure 7A). The immature neuronal marker doublecortin (DCX) scattered at an infarct site in RVG-Exos and RVG-Exos-Scr treated mice (Figures 7B and 7C). But the number of DCX-positive cells in RVG-Exos-miR124 treated mice was approximately twice as much as that of control groups (Figures 7D and 7E). Our results showed that exosome-encapsulated miR-124 greatly promoted adult neurogenesis post ischemia via biasing neural progenitors into neuronal lineage.
Figure 7.
RVG-Exos-miR124 Promotes Robust Neurogenesis in Ischemic Region
(A) Strategy of carrying out ischemic model and DCX immunohistochemistry. (B–D) Immunohistochemistry of DCX in the ischemic cortex of (B) RVG-Exos-, (C) RVG-Exos-Scr-, and (D) RVG-Exos-miR124-treated mice at 7 dpi. (E) Cell quantification of DCX-positive cells in the ischemic region via counting the ischemic area of every eighth slice of the whole ischemic region. Note the robustly increased expression of DCX in RVG-Exos-miR124 treated mice compared with RVG-Exos and RVG-Exos-Scr injected mice (n = 5). The data were expressed as mean ± SD (**p < 0.01). The scale bars represent 50 μm.
Discussion
In the present study, we for the first time performed targeting delivery of miR-124 to the ischemic cortex using modified exosomes. The RVG-modified exosomes efficiently delivered miR-124 to the infarct region and promoted neurogenesis in the adult cortex after ischemia. Our results demonstrate that systemic administration of engineered exosomes could be a superior candidate for specifically transporting gene drugs for the treatment of stroke.
Neuroremodeling by neurogenesis after stroke has been proven existing, although weak, especially after adult cortical ischemia.24, 25 Therapeutically enhancing neurogenesis would greatly benefit recovery. As miRNAs regulate myriads of genes, a handful of studies target on miRNAs’ therapeutic potentials.26 miRNA-9, miRNA let-7b, miR-137, and miR-184 have been reported to regulate neural stem cell proliferation and differentiation at different stages.27, 28, 29, 30 MiR-124, the most brain-enriched miRNA, is SVZ neuronal fate determinant and mediates stroke-induced neurogenesis in adult SVZ and striatum.7, 11, 31 In our study, we found that ectopic expression of miR-124 in cortex promoted neural progenitors to differentiate into neuronal lineage and further yielded the amelioration against ischemia via cortical neurogenesis. We carefully limited the infarct region in the cortex and excluded all those corpus callosum injured cases. The DCX-positive cells formed clusters and distributed along the ischemic border. But we didn’t observe the migrating route of new born neurons from SVZ, the well acknowledged neural stem cell niche.32 It will be interesting to investigate the cellular origin of these cortical neuroblasts and explore the underlying mechanisms.
Since the first report that exosomes contain both mRNAs and miRNAs which can be delivered from parent cells to recipient cells,13 more and more studies revealed the roles of exosome-mediated intercellular genetic communication in CNS diseases. Glioblastoma-derived exosomes vehicle mRNAs/miRNAs to normal host cells to promote tumor growth.33 Schwann cell-derived miRNAs stimulated axonal growth and regeneration after nerve damage when taken up by adjacent neurons.34 Inflammation-induced endothelial cell-derived exosomes modulate the cellular status of pericytes by transferring miRNAs.35 Compared with other kinds of cells, cultured MSC is an efficient producer for exosomes, allowing the production on a larger scale.36 Emerging data show that exosomes released from MSCs make therapeutic benefits in stroke. MSC-derived exosomes are capable of transferring miR-133b to neurons and subsequently contribute to neurite outgrowth after stroke.37, 38 These indicate that MSC-derived exosomes not only cross the BBB, but also deliver functional cargo to modulate gene expression in the recipient cells. Due to the small size, exosomes can avoid phagocytosis by macrophages. They are naturally stable to escape endosomal-lysosomal degradation compared with polymeric nanoparticles and liposomes. Collectively, with lack of immunogenicity, these features make exosomes promising candidates for gene drug delivery.39 In our study, the qRT-PCR showed that the expression of miRNA-124 in the ischemic cortex was significantly increased at 3 dpi after exosome injection, indicating persistent and sufficient miR-124 concentration for treatment. Considering MSCs-derived exosomes package a repertoire of bioactive molecules and can promote the outcome of stroke without artificial loading,40 there may be other factors besides miR-124 contributing to the beneficial effects observed in our experiment.
To date, targeting drugs to brain cells is a troublesome challenge. In our study, DiI fluorescence ferried by RVG-Exos scattered in the normal brain, which was consistent with the distribution pattern reported by Alvarez-Erviti in 2011.15 However, compared with unmodified exosomes, the concentrated fluorescence in the ischemic region showed high targeting efficiency of RVG-Exos with BBB disruption. Except the RVG system, modifying exosomal surface proteins with targeting ligands or receptor-specific antibodies has also been used to achieve tailored delivery.41 Considering the therapeutic application of exosomes, the better alternative may need a homing device that is specific for a target which is induced or upregulated under pathological conditions.42 In our study presented here, most of the ischemic area was DiI positive in the RVG-exosome delivery group, suggesting that besides neurons, astrocytes and endothelial cells might be also the recipient cells. Future studies identifying the targeting peptide specific for neural progenitors and with high BBB penetrating capacity are still needed.
Conclusions
In summary, our results have shown that RVG-modified exosomes are efficient tools for gene drug delivery to the ischemic cortex. Systemic administration of RVG-exosomes loaded with miR-124 can promote cortical neural progenitors to obtain neuronal identity and attenuate ischemic injury by cortical neurogenesis. Considering the intrinsic weak ability of neurogenesis in the adult cortex after ischemia, our study suggests that treatment with RVG-Exos-miR124 during the acute phase of stroke can be a promising therapeutic strategy for neuroprotection and neuroremodeling via promoting neurogenesis.
Materials and Methods
Animals and Photothrombosis Model
Male C56BL/6 mice (8–10 weeks old, 22–25 g) were used. All animal experiments were carried out under protocols approved by the Animal Care and Use Committee of Fourth Military Medical University. Focal cortical ischemia was induced as previously described.16 Briefly, after the tail injection of Rosa Bengal (Sigma) at 25 mg/kg, a skull window was made without hurting brain tissue, ranging from 0.8 to 2.4 mm posterior to the Bregma and 0.5 to 2.1 mm right to the midline. The skull window was illuminated for 9.5 min by an optic fiber connected to a cold light source.
Lamp2b Modification
Lamp2b was cloned with cDNA from mice cortex and was inserted between Nhe1 and BamH1 into a pcDNA3.1(−) vector. Primers designed to encode RVG were used to introduce the targeting ligand between Xho1 and BspE1. BM-MSCs were electroporated with pcDNA3.1(−)-RVG-Lamp2b plasmid to produce RVG-positive exosomes (RVG-Exos).
Cell Culture
Bone marrow from adult male mice was mechanically harvested from femurs as previously described.37 Cells were washed by PBS and suspended in α-modified minimum essential medium (MEM, HyClone), supplemented with 20% fetal bovine serum (FBS, Gibco) and antibiotics. At 3 days later, cells tightly adhered to the plastic flasks were considered to be P0 BM-MSCs. BM-MSCs were used within the eighth passage for exosome collection.
Exosome Isolation and Characterization
Exosomes were purified from cell culture supernatant of BM-MSCs. Prior to culture medium collection, BM-MSCs were washed twice with PBS, and the medium was switched to serum-free medium. After incubation for 48 hr, the supernatant was collected and went through sequential ultracentrifugation at 2,000 × g for 30 min, 10,000 × g for 30 min, and 100,000 × g for 4 hr at 4°C. To avoid contamination by the FBS-derived exosomes, FBS was also spun before applied to BM-MSCs culture as previously described.43 The exosomes were washed once with PBS and resuspended for further characterization.
For fluorescence labeling of exosomes, a 4 mg/mL solution of DiI (Molecular Probes) was added to the PBS (1:200) and incubated following the manufacturer’s instructions. After the isolation by sequential ultracentrifugation as mentioned above, DiI-labeled exosomes (DiI-Exos) were resuspended in 0.9% saline and centrifuged at 10,000 × g for 30 min. This procedure was repeated for three times to wash free DiI away (Figure 2B). Then DiI-Exos were injected intravenously through tail vein into ischemic mice at 1 dpi.
For EM, purified exosomes from BM-MSCs were resuspended in PBS and imaged by transmission electron microscope as detailed before.44
miR-124 Loading
Exosomes at a total protein concentration of 12 μg (BCA Protein Assay Kit, Thermo Scientific) were electroporated with 12 μg miR-124 mimics (RVG-Exos-miR124) or scrambled miRNAs (GenePharma) (RVG-Exo-Scr) at 350 V and 150 μF in 0.4 cm electroporation cuvetes.15 To remove the unincorporated free miR-124, exosomes were washed with cold PBS twice by ultracentrifugation at 100,000 × g for 90 min. Efficiency of electroporation was validated by qRT-PCR for detection of miR-124 levels.
Western Blotting
For cell western blotting, control BM-MSCs or BM-MSCs electroporated with pcDNA3.1-RVG-Lamp2b plasmid were lysed by RIPA buffer. Blots were incubated with primary antibodies overnight at 4°C. The primary antibodies used were as follows: goat anti-Lamp2b (1:1,000, Abcam), mouse anti-β-actin (1:5,000, Sigma), mouse anti-CD63 (1:1,000, Abcam), rabbit anti-Alix (1:1,000, Abcam), rabbit anti-GM130 (1:1,000, Abcam), and mouse anti-GAPDH (1:5,000, Abcam). Corresponding HRP-conjugated anti-goat, anti-rabbit, or anti-mouse (1:10,000, Pierce) secondary antibodies were incubated for 1 hr at room temperature. Bands were visualized with an ECL kit (Pierce).
RNA Isolation and qRT-PCR
Total RNA was extracted from exosomes or ipsilateral/contralateral cortex using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions.
For analysis of mRNA levels, reverse-transcription was performed using PrimeScript First-Strand cDNA Synthesis Kit (Takara), and cDNAs were used for qRT-PCR using PrimeScript RT Master Mix (Takara).
For analysis of miRNA levels, total RNA was reverse-transcribed to cDNA using miRcute miRNA First-Strand cDNA Synthesis Kit (Tiangen Biotech), and qRT-PCR was carried out using miRcute miRNA qPCR Detection Kit (SYBR Green) (Tiangen Biotech).
All PCR reactions were run in triplicate and mRNA or miRNA expression, relative to β-actin or U6 snRNA, was calculated using the 2−ΔΔCt method.
Immunofluorescence
Serial coronal sections of 14 μm in thickness were prepared on a cryostat. After being incubated with 0.3% Triton X-100 and 3% bovine serum albumin (BSA) in PBS for 1 hr, the following primary antibodies were incubated overnight at room temperature: rabbit anti-Sox2 (1:200, Sangon Biotech), goat anti-Nestin (1:200, Santa Cruz Biotechnology), and rabbit anti-DCX (1:400, Abcam). Corresponding secondary antibodies as Alexa Fluor 488 and Alexa Fluor 594 (donkey anti rabbit or anti-goat IgG, 1:800, Invitrogen) were incubated for 3 hr at room temperature. Cellular nuclei were stained by Hoechst 33342 (1:100, Sigma).
For cell quantification, the ischemic region was defined by the outer lining of Nestin-positive cells as before.16 All the quantification was performed in this region (Figure S2). Cells were counted from every eighth slice of the ischemic region and five mice were included for comparison in each group. The cell counting was performed in a design-blind manner.
Statistical Analysis
Data are presented as mean ± SD or mean ± SEM. A two-tailed Student’s t test or ANOVA was used to analyze the relative expression of qRT-PCR and cell counting. p values less than 0.05 were considered as statistically significant.
Author Contributions
J.Y. and X.Z. conducted experiments and drafted the manuscript. X.C. contributed to the experiment design and statistical analysis. L.W. and G.Y. designed the experiments and revised the manuscript.
Conflicts of Interest
The authors claim no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China and Shaanxi Province to G.Y. (grant code: 31100979 and 2016JM8005, respectively) and L.W. (grant code: 81371540). The authors thank all the lab members of Yang lab for technical support and critical discussion of the manuscript.
Footnotes
Supplemental Information includes two figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.omtn.2017.04.010.
Contributor Information
Lei Wang, Email: hellowanglei068@163.com.
Guodong Yang, Email: yanggd@fmmu.edu.cn.
Supplemental Information
References
- 1.Lloyd-Jones D., Adams R., Carnethon M., De Simone G., Ferguson T.B., Flegal K., Ford E., Furie K., Go A., Greenlund K., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Lackland D.T., Roccella E.J., Deutsch A.F., Fornage M., George M.G., Howard G., Kissela B.M., Kittner S.J., Lichtman J.H., Lisabeth L.D., American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Quality of Care and Outcomes Research. Council on Functional Genomics and Translational Biology Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X.S., Chopp M., Zhang R.L., Zhang Z.G. MicroRNAs in cerebral ischemia-induced neurogenesis. J. Neuropathol. Exp. Neurol. 2013;72:718–722. doi: 10.1097/NEN.0b013e31829e4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharap A., Bowen K., Place R., Li L.C., Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J. Cereb. Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyaseelan K., Lim K.Y., Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 6.Mishima T., Mizuguchi Y., Kawahigashi Y., Takizawa T., Takizawa T. RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res. 2007;1131:37–43. doi: 10.1016/j.brainres.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Åkerblom M., Sachdeva R., Barde I., Verp S., Gentner B., Trono D., Jakobsson J. MicroRNA-124 is a subventricular zone neuronal fate determinant. J. Neurosci. 2012;32:8879–8889. doi: 10.1523/JNEUROSCI.0558-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L.C., Pastrana E., Tavazoie M., Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y., Gui H., Li Q., Luo Z.M., Zheng M.J., Duan J.L., Liu X. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci. Ther. 2013;19:813–819. doi: 10.1111/cns.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamzei Taj S., Kho W., Riou A., Wiedermann D., Hoehn M. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials. 2016;91:151–165. doi: 10.1016/j.biomaterials.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Doeppner T.R., Doehring M., Bretschneider E., Zechariah A., Kaltwasser B., Müller B., Koch J.C., Bähr M., Hermann D.M., Michel U. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol. 2013;126:251–265. doi: 10.1007/s00401-013-1142-5. [DOI] [PubMed] [Google Scholar]
- 12.Lakhal S., Wood M.J.A. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33:737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 13.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Xin H., Li Y., Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 16.Yang J., Zhang X., Wu Y., Zhao B., Liu X., Pan Y., Liu Y., Ding Y., Qiu M., Wang Y.Z., Zhao G. Wnt/β-catenin signaling mediates the seizure-facilitating effect of postischemic reactive astrocytes after pentylenetetrazole-kindling. Glia. 2016;64:1083–1091. doi: 10.1002/glia.22984. [DOI] [PubMed] [Google Scholar]
- 17.Santos M.C.T., Tegge A.N., Correa B.R., Mahesula S., Kohnke L.Q., Qiao M., Ferreira M.A., Kokovay E., Penalva L.O. miR-124, -128, and -137 orchestrate neural differentiation by acting on overlapping gene sets containing a highly connected transcription factor network. Stem Cells. 2016;34:220–232. doi: 10.1002/stem.2204. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Ge G., Uchida Y., Luu B., Ahn S. Gli3 is required for maintenance and fate specification of cortical progenitors. J. Neurosci. 2011;31:6440–6448. doi: 10.1523/JNEUROSCI.4892-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu F., Hata R., Ma Y.J., Tanaka J., Mitsuda N., Kumon Y., Hanakawa Y., Hashimoto K., Nakajima K., Sakanaka M. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J. Neurosci. Res. 2005;81:163–171. doi: 10.1002/jnr.20561. [DOI] [PubMed] [Google Scholar]
- 20.Uittenbogaard M., Chiaramello A. Expression of the bHLH transcription factor Tcf12 (ME1) gene is linked to the expansion of precursor cell populations during neurogenesis. Brain Res. Gene Expr. Patterns. 2002;1:115–121. doi: 10.1016/s1567-133x(01)00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper-Kuhn C.M., Vroemen M., Brown J., Ye H., Thompson M.A., Winkler J., Kuhn H.G. Impaired adult neurogenesis in mice lacking the transcription factor E2F1. Mol. Cell. Neurosci. 2002;21:312–323. doi: 10.1006/mcne.2002.1176. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Luo Z.M., Guo X.M., Su D.F., Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front. Cell. Neurosci. 2015;9:193. doi: 10.3389/fncel.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leker R.R., Soldner F., Velasco I., Gavin D.K., Androutsellis-Theotokis A., McKay R.D.G. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–161. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- 24.Benner E.J., Luciano D., Jo R., Abdi K., Paez-Gonzalez P., Sheng H., Warner D.S., Liu C., Eroglu C., Kuo C.T. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., Harms K.M., Ventura P.B., Lagace D.C., Eisch A.J., Cunningham L.A. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang Y.B., Stary C.M., Yang G.Y., Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr. Drug Targets. 2013;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C., Sun G., Li S., Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat. Struct. Mol. Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C., Sun G., Li S., Lang M.F., Yang S., Li W., Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc. Natl. Acad. Sci. USA. 2010;107:1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun G., Ye P., Murai K., Lang M.F., Li S., Zhang H., Li W., Fu C., Yin J., Wang A. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat. Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C., Teng Z.Q., Santistevan N.J., Szulwach K.E., Guo W., Jin P., Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X.S., Chopp M., Zhang R.L., Tao T., Wang X.L., Kassis H., Hozeska-Solgot A., Zhang L., Chen C., Zhang Z.G. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS ONE. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Buylla A., Lim D.A. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 33.Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Verrilli M.A., Picou F., Court F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S., Niida S., Azuma E., Yanagibashi T., Muramatsu M., Huang T.T., Sagara H., Higaki S., Ikutani M., Nagai Y. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci. Rep. 2015;5:8505. doi: 10.1038/srep08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo R.W.Y., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh B.J., Lim S.K. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z.G., Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., Zhang Z.G., Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin H., Li Y., Cui Y., Yang J.J., Zhang Z.G., Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huwyler J., Wu D., Pardridge W.M. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. USA. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., Batrakova E.V. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.