Abstract
Epidermal growth factor (EGF) is important for cancer cell proliferation, angiogenesis and metastasis in many types of cancer. However, the mechanisms involved in EGF-induced head and neck squamous cell carcinoma (HNSCC) metastasis remain largely unknown. In this study, we reveal that angiopoietin-like 4 (ANGPTL4) plays an important role in the regulation of EGF-induced cancer metastasis. We showed that EGF-induced ANGPTL4 expression promoted anoikis resistance and cancer cell migration and invasion in HNSCC. In addition, depletion of ANGPTL4 inhibited EGF-induced cancer cell invasion. Autocrine production of EGF-induced ANGPTL4 regulated the expression of matrix metalloproteinases (MMPs). The induction of MMP-1 gene expression by ANGPTL4-activated integrin β1 signalling occurred through the AP-1 binding site in the MMP-1 gene promoter. Furthermore, down-regulation of MMP-1 impeded EGF- and recombinant ANGPTL4-enhanced HNSCC cell migration and invasion. Depletion of ANGPTL4 significantly blocked EGF-primed extravasation and metastatic seeding of tumour cells and MMP-1 expression in lungs. However, no effect of ANGPTL4 on tumour growth was observed. These results suggest that EGF-induced expression and autocrine production of ANGPTL4 enhances HNSCC metastasis via the up-regulation of MMP-1 expression. Inhibition of ANGPTL4 expression may be a potential strategy for the treatment of EGFR-mediated HNSCC metastasis.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the most common type of cancer worldwide. Among men, HNSCC is the eighth leading type of cancer among the estimated new cancer cases in the United States.1 HNSCC represents a group of highly heterogeneous tumours. Over the last few decades, despite advances in treatment, the mortality rate of HNSCC has not significantly changed.2 Metastasis is the most important contributor to the mortality of cancer patients. The pathogenesis of cancer metastasis involves several processes, including the loss of cellular adhesion, increased cell invasion, survival in the circulation during extravasation and eventual colonization of distant organs.3
Tumour cells that survive in the circulation are characterized by anoikis resistance.4 Anoikis is a type of cell death that is induced upon cell detachment from the extracellular matrix, and it is a key mechanism in the maintenance of tissue homeostasis and development.5 Anoikis can occur through the activation of the death receptor and/or mitochondrial apoptosis pathway, resulting in caspase-3 activation. Defects in either of these pathways render malignant cells resistant to anoikis.4, 6 Up-regulation of anti-apoptotic proteins such as Bcl-2, Bcl-XL, Bax, Bim and BAD, and activation of integrins and the EGFR activated pro-survival PI3K-Akt pathway all contribute to anoikis resistance.6, 7 For example, the expression of cytokines confers anoikis resistance to tumour cells through the activation of survival pathways. Hepatocyte growth factor inhibits anoikis and mediates survival in HNSCC by activating the extracellular signal-regulated kinase (ERK)-dependent AP-1 signalling pathway.8 E-cadherin-mediated EGFR activation has also been demonstrated to protect HNSCC from anoikis and maintain cell survival.9 In addition, both hepatocyte growth factor and epidermal growth factor (EGF) play important roles in the progression and metastasis of HNSCC.10, 11, 12 Cytokines such as CXCL8 enhance the resistance of colorectal cancer cells to anoikis by increasing TOPK and activating AKT and ERK.13 IL-6 significantly augments STAT3-mediated anoikis resistance in pancreatic cancer cell lines. These results support the possibility that growth factors and cytokines enhance tumour metastasis by enhancing anoikis resistance of cancer cells. However, the mechanism involved in EGF-mediated regulation of anoikis resistance that leads to enhanced HNSCC metastasis remains unclear.
Angiopoietin-like 4 (ANGPTL4), a secreted protein consisting of N-terminal and C-terminal domains, is a member of the angiopoietin family, and it plays important roles in lipid and glucose metabolism.14 Interestingly, up-regulation of ANGPTL4 has been observed in various types of human cancers, including colorectal cancer, breast cancer, esophageal SCC and oral tongue SCC. The expression of ANGPTL4 in tumours is highly associated with metastasis. For example, constitutive activation of EGFR (EGFRvIII) induces ANGPTL4 expression through the ERK/c-Myc pathway and promotes tumour angiogenesis in malignant gliomas.15 ANGPTL4 induction by prostaglandin E2 (PGE2) under hypoxic conditions promotes colorectal cancer progression.16 ANGPTL4 promotes oral squamous cell carcinoma metastasis by stimulating cell invasion.17 ANGPTL4 induced by TGFβ via the Smad signalling pathway promotes breast cancer metastasis.18 Recent reports indicate that the effect of ANGPTL4 on tumour metastasis may be mediated by integrin activation. ANGPTL4 stimulates integrin-dependent survival signals through the activation of NADPH oxidase 1 (Nox1), mimics anchorage conditions and confers anoikis resistance to tumour cells by regulating oncogenic reactive oxygen species (ROS).19 Tumour-derived C-terminal ANGPTL4 (cANGPTL4) binds to integrin α5β1 and activates the downstream PAK/Rac signalling pathway, resulting in endothelial disruption in TGFβ-enhanced lung metastasis of breast cancer.18, 20 However, previous studies have shown that ANGPTL4 inhibits angiogenesis and prevents metastasis,21, 22 highlighting a controversial effect of ANGPTL4 on tumour metastasis. While the expression of ANGPTL4 is associated with angiogenesis and vascular permeability, the expression of ANGPTL4 or matrix metalloproteinases (MMPs) is correlated with cancer survival and invasion.23, 24 Interestingly, the co-expression of ANGPTL4 and MMPs has also been correlated with matrix remodelling in cartilage degradation and chondrogenic differentiation.25, 26 The ANGPTLs, such as ANGPTL1, repress lung cancer cell migration and invasion by regulating the integrin α1β1/FAK/ERK/SP1/miR-630/SLUG signalling axis.27 However, whether the expression of ANGPTL4 is associated with epithelial-to-mesenchymal transition is still unclear.
In this study, we reveal for the first time that autocrine production of EGF-induced ANGPTL4 in tumour cells not only enhances anoikis resistance but also promotes MMP-1 expression through activation of the integrin β1 signalling pathway, resulting in HNSCC metastasis. ANGPTL4 inhibition represents a new strategy for the treatment of EGFR-mediated HNSCC metastasis.
Results
EGF enhances ANGPTL4 expression in HNSCC
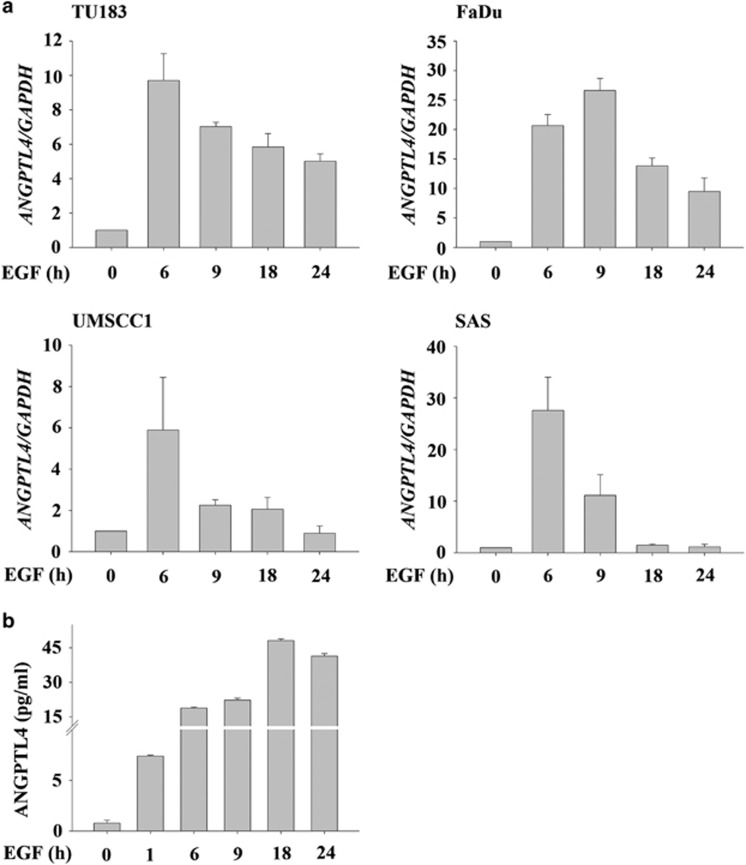
To investigate whether the expression of ANGPTL4 is associated with the activation of EGFR signalling in HNSCC, cells were treated with EGF for varying periods of time. As shown in Figure 1a, EGF significantly induced the expression of ANGPTL4 in a time-dependent manner in various HNSCC cell lines. To further confirm the induction of ANGPTL4 by EGF, the expression and secretion of ANGPTL4 were examined using an enzyme-linked immunosorbent assay (ELISA). An increase in ANGPTL4 was observed in the conditioned media harvested from EGF-treated cells (Figure 1b). These results suggest that EGF induces the expression and secretion of ANGPTL4 in HNSCC.
Figure 1.
EGF induces expression of ANGPTL4 in HNSCC cell lines. (a) HNSCC cell lines were treated with 50 ng/ml EGF for the indicated period of time. The expression of ANGPTL4 and GAPDH mRNA was analysed by real-time quantitative PCR. Relative levels of ANGPTL4 were normalized to GADPH. Values are indicated as the mean±s.e.m. (b) TU183 cells were treated with 50 ng/ml EGF for the indicated period of time. Secreted ANGPTL4 was harvested from conditioned medium and detected by ELISA. Values are indicated as the mean±s.e.m.
EGF-induced ANGPTL4 promotes HNSCC migration and invasion
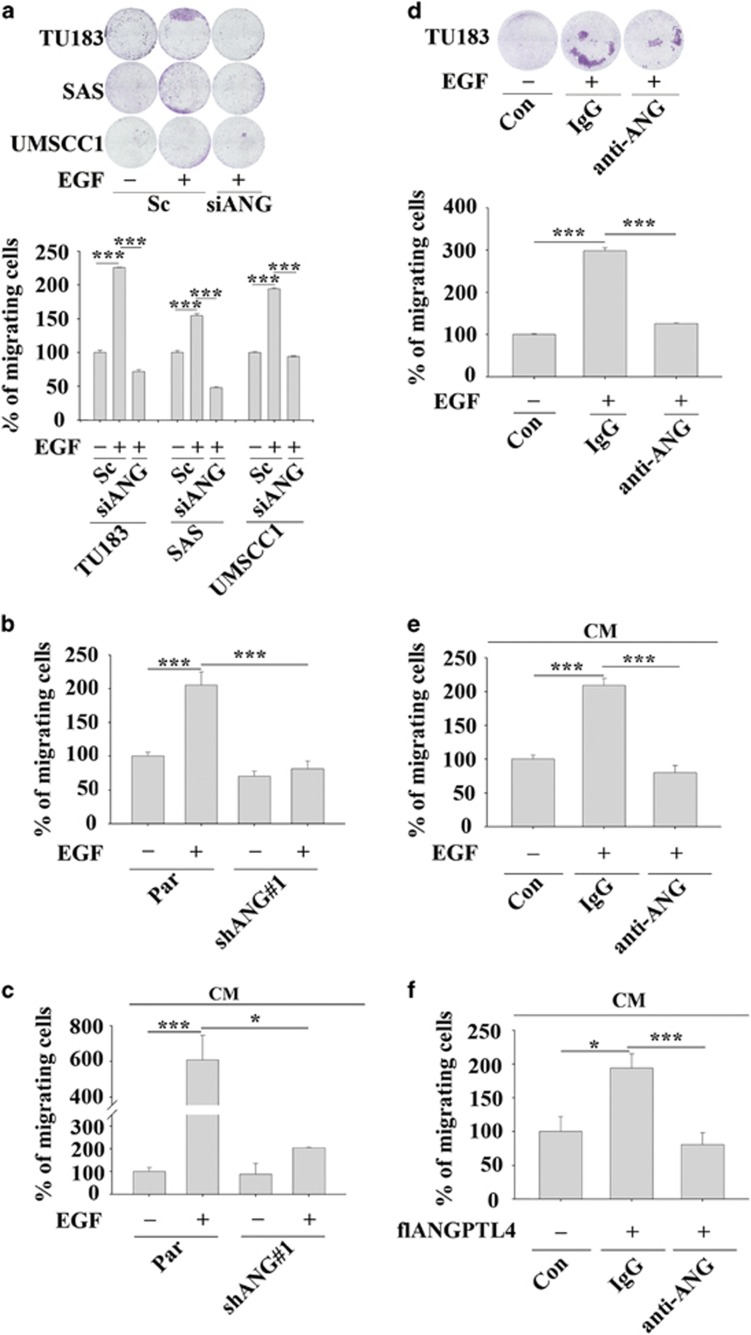
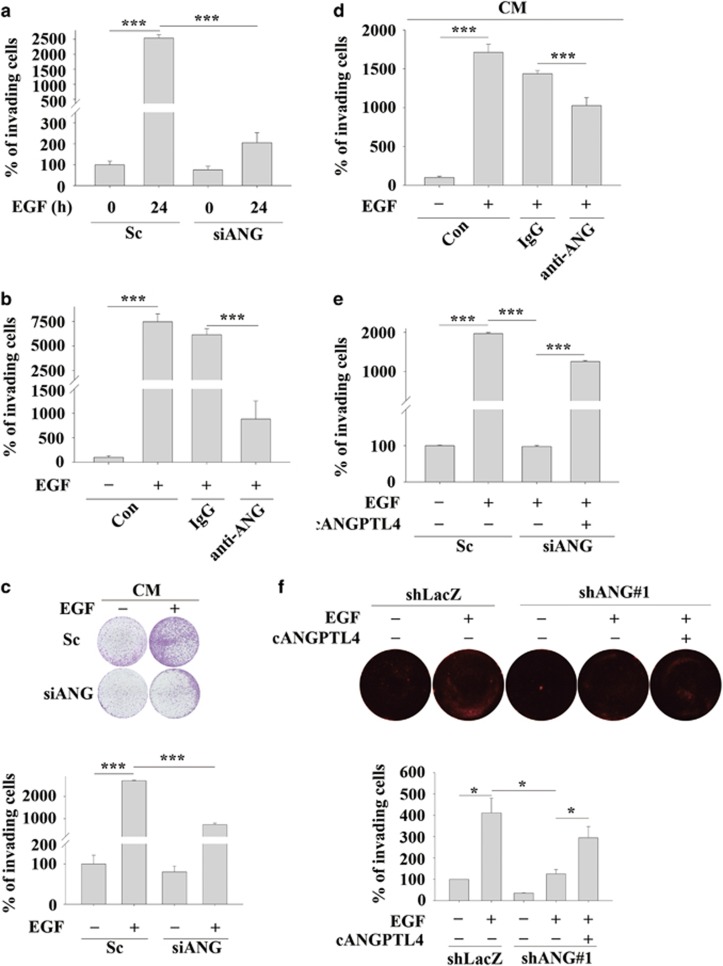
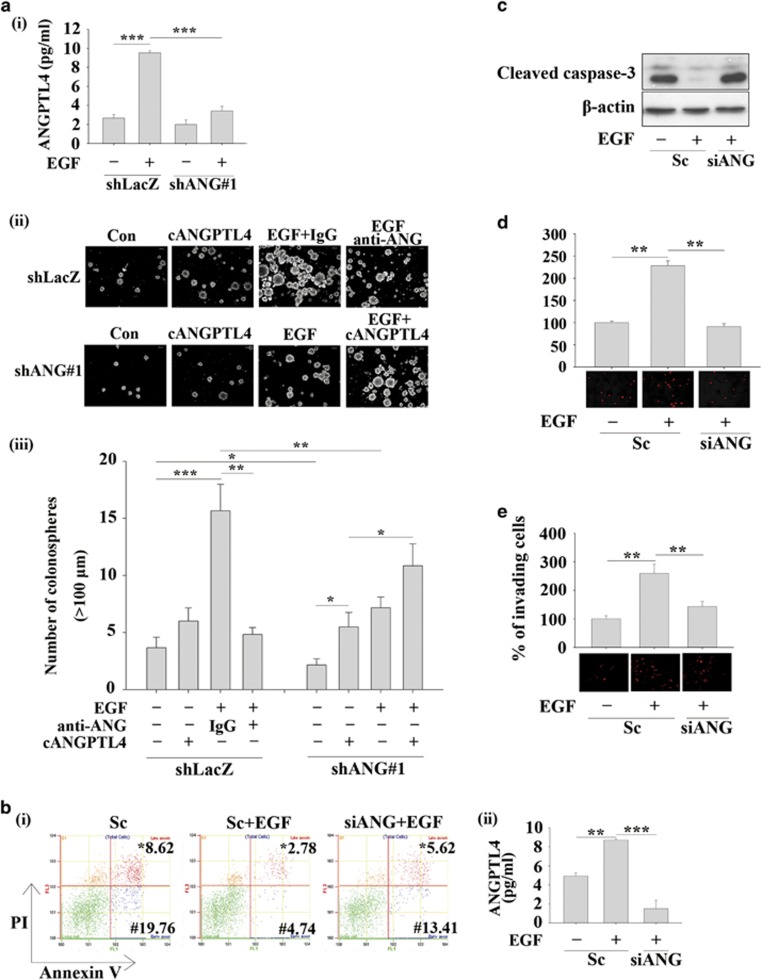
Recent studies have suggested that although ANGPTL4 mediates TGFβ-induced lung metastasis of breast cancer,18 it also inhibits the metastasis of melanoma cells.22 These conflicting results indicate that the role of ANGPTL4 in the regulation of cancer metastasis has yet to be clarified for different types of cancer. To better understand the biological significance of EGF-induced ANGPTL4 expression in tumour cells, siRNA targeting ANGPTL4 (siANGPTL4) and stable cell lines with ANGPTL4 depleted via shRNA (shANGPTL4) were used. The inhibition of ANGPTL4 expression by gene knockdown was confirmed in EGF-treated cells (Supplementary Figures 1a and b). The EGF-induced migration of HNSCC cells was reduced in ANGPTL4-knockdown cells (Figures 2a and b). To further confirm that the secretion of EGF-induced ANGPTL4 regulates cell migration, the conditioned media harvested from EGF-treated parental and shANGPTL4 cells were used to treat cancer cells. ANGPTL4 secreted from EGF-treated parental cells, but not shANGPTL4 cells dramatically induced cell migration in a time-dependent manner (Supplementary Figure 1c and Figure 2c). The involvement of ANGPTL4 in the regulation of EGF-induced migration was also examined by disrupting the function of ANGPTL4 with anti-ANGPTL4 antibodies. The antibody-induced neutralization of ANGPTL4 significantly blocked EGF- and conditioned media-induced cell migration (Figures 2d and e). In addition, the conditioned media harvested from cells overexpressing ANGPTL4 (Supplementary Figure S1d) also induced tumour cell migration (Figure 2f). However, the cell migration induced by the overexpression of ANGPTL4 was also inhibited by the neutralization of ANGPTL4 in the culture media (Figure 2f). These results suggest that EGF-induced ANGPTL4 regulated HNSCC migration. The contribution of EGF-induced ANGPTL4 to tumour invasion was further investigated. As shown in Figures 3a and b, depletion and neutralization of ANGPTL4 inhibited EGF-induced HNSCC cell invasion. Conditioned media harvested from EGF-treated cells induced tumour invasion that was also blocked by siANGPTL4 and neutralization of ANGPTL4 (Figures 3c and d). Inhibition of EGF-induced invasion by siANGPTL4 was reversed when the cells were treated with recombinant ANGPTL4 (Figure 3e). The effect of ANGPTL4 on invasion was further verified using the transendothelial invasion assay. Briefly, endothelial cells were grown until they formed a monolayer on the bottom of a thick layer of extracellular matrix proteins to mimic intravasation. As shown in Figure 3f, EGF-induced invasion was significantly inhibited in shANGPTL4 cells, and the recombinant ANGPTL4 significantly rescued the invasion in ANGPTL4-depleted cells. To further clarify whether ANGPTL4-enhanced migration and invasion was correlated with the activation of EGF signalling, the recombinant ANGPTL4 protein was used alone in serum-free cell culture. As shown in Supplementary Figure S2, ANGPTL4 significantly promoted tumour migration and invasion. In summary, these results suggest that EGF-induced expression and secretion of ANGPTL4 promoted HNSCC migration and invasion.
Figure 2.
EGF-induced ANGPTL4 enhances HNSCC migration. The migratory properties of HNSCC cells were analysed using a transwell assay as described in ‘Materials and methods'. (a) TU183 cells were transfected with or without 50 nM ANGPTL4 siRNA oligonucleotides (siANG) by lipofection. After treatment with 50 ng/ml EGF for 24 h, migrating cells were fixed, stained with crystal violet and examined using a microscope (upper panel). Original magnification, × 20. Cells were stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader (lower panel). Values are the mean±s.e.m. (b) Parental (Par) and shANGPTL4 (shANG) TU183 cells were treated with 50 ng/ml EGF for 24 h. Migrating cells were fixed and stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (c) TU183 cells were treated with conditioned medium (CM) collected from EGF-treated parental (Par) and shANGPTL4 (shANG) TU183 cells. Migrating cells were fixed and stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (d) TU183 cells were treated with 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG), 10 μg/ml immunoglobulin G (IgG) and 50 ng/ml EGF for 24 h. Migrating cells were fixed, stained with crystal violet and examined using a microscope (upper panel). Original magnification, × 20. The crystal violet solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader (lower panel). Values are the mean±s.e.m. (e) CM was collected from TU183 cells treated with 50 ng/ml EGF. TU183 cells were then treated with 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG), 10 μg/ml immunoglobulin G (IgG) and CM for 24 h. Migrating cells were fixed and stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (f) CM was collected from flANGPTL4-overexpressing TU183 cells. TU183 cells were then treated with 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG), 10 μg/ml immunoglobulin G (IgG) and CM for 24 h. Migrating cells were fixed and stained by crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. *P<0.05; ***P<0.005.
Figure 3.
EGF-induced ANGPTL4 enhances HNSCC invasion. The invasive properties of the cells were examined using an invasion assay as described in ‘Materials and methods'. (a) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection. Following treatment with 50 ng/ml EGF for 24 h, the invading cells were fixed and stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (b) TU183 cells were treated with 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG), 10 μg/ml IgG and 50 ng/ml EGF for 48 h. The invading cells were fixed and stained by crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (c and d) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection, and CM was collected from EGF-treated or non-treated cells. TU183 cells were then treated with 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG), 10 μg/ml immunoglobulin G (IgG) and CM for 48 h. The invading cells were fixed, stained with crystal violet and examined using a microscope (c, upper panel). Original magnification, × 20. The crystal violet solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader (c, lower panel and d). Values are the mean±s.e.m. (e) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection, and then treated with 50 ng/ml EGF and 250 ng/ml recombinant C-terminal ANGPTL4 (cANGPTL4) for 48 h. The invading cells were fixed and stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (f) The transendothelial invasion of cancer cells was performed as described in ‘Materials and methods'. Endothelial cells were grown to form a monolayer on the bottom of a thick layer of extracellular matrix proteins to mimic intravasation in transendothelial invasion assays. shLacZ and shANG TU183 cells were treated with or without 50 ng/ml EGF and cANGPTL4 for 48 h. The total number of invading cells was quantified by Image J and images were photographed. The percentage of invading cells was determined in three independent experiments. Values are the mean±s.e.m. **P<0.01; ***P<0.005.
EGF-induced ANGPTL4 promotes tumour-endothelial cell interactions and anoikis resistance
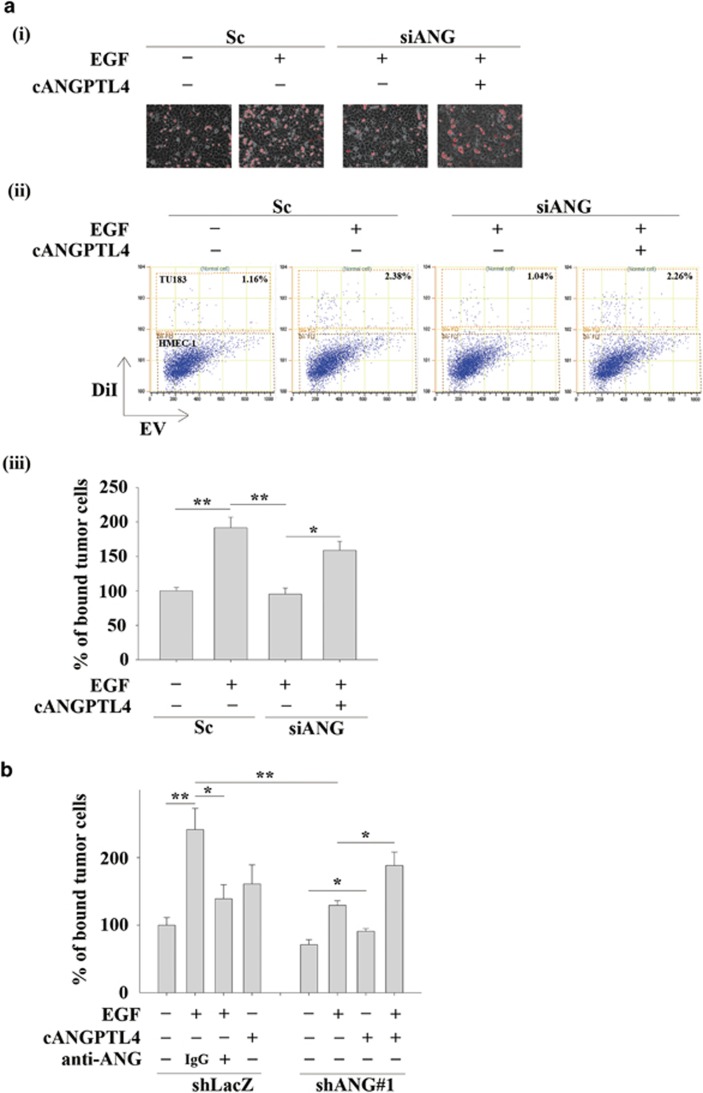
During tumour metastasis, the infiltration of tumour cells into distant locations initially relies on their attachment to blood vessels. Therefore, the ability of EGF-induced ANGPTL4 to promote the interaction between tumour cells and endothelial cells was examined. The enhancement of binding between tumour and endothelial cells was observed in EGF-treated parental but not ANGPTL4-depleted cells (Figure 4a). Although recombinant ANGPTL4 alone produced minor effect on the interaction (Figure 4b), treatment with EGF and ANGPTL4 reversed the inhibition of the tumour-endothelial cell interaction in the ANGPTL4-knockdown cells (Figure 4). In addition, the interaction was also blocked by the neutralization of ANGPTL4 with anti-ANGPTL4 antibodies (Figure 4b). These results indicated that autocrine production of EGF-induced ANGPTL4 stimulated the binding of tumour cells to endothelial cells, which may enhance the ability of tumour cells to penetrate blood vessels. In addition, to investigate whether EGF-induced ANGPTL4 conferred anoikis resistance to regulate the distal dissemination of metastatic tumour cells, the anchorage-independent survival of tumour cells was examined. As shown in Figure 5a, the secretion of EGF-induced ANGPTL4 in suspension culture was inhibited in shANGPTL4 cells, resulting in the inhibition of EGF-induced anoikis resistance. The reduction in anoikis resistance was reversed by treating shANGPTL4 cells with recombinant cANGPTL4 protein, even cANGPTL4 alone, which produced a minor effect on parental cells (Figure 5a). In addition, EGF-induced anoikis resistance was reduced by the neutralization of ANGPTL4 (Figure 5a). The depletion of ANGPTL4 reduced EGF-enhanced cell survival in suspension culture (Figures 5b and c). To further examine whether EGF-induced ANGPTL4 regulated the interaction between anoikis resistant cells and endothelial cells, siRNA was used to deplete ANGPTL4 in suspension cells. The knockdown of ANGPTL4 significantly repressed anoikis-resistant tumour-endothelial cell interactions (Figure 5d). Notably, the ability of EGF-promoted anoikis-resistant cells to undergo transendothelial invasion was also reduced by the depletion of ANGPTL4 (Figure 5e). These results revealed that EGF-induced ANGPTL4 promoted anoikis resistance and interactions with endothelial cells, which may enhance metastasis.
Figure 4.
ANGPTL4 regulates EGF-enhanced tumour-endothelial cell interactions. (a) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection, and the cells were treated with or without 50 ng/ml EGF and 250 ng/ml cANGPTL4 for 3 h. The cells were then labelled with DiI and cultured with endothelial cells for 30 min. Cell attachment was examined using a microscope (i). The bound tumour cells (adherent cells) were analysed using a flow cytometer. TU183 cells were DiI-positive, and HMEC-1 cells were DiI-negative (ii). The percentage of bound tumour cells was quantified in three independent experiments by flow cytometry (iii). Values are the mean±s.e.m. (b) shLacZ and shANGPTL4 (shANG) TU183 cells were treated with or without 50 ng/ml EGF, 250 ng/ml cANGPTL4, 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG) and 10 μg/ml immunoglobulin G (IgG) for 3 h. The cells were then labelled with DiI and cultured with endothelial cells for 30 min. The percentage of bound tumour cells was quantified in three independent experiments using a flow cytometer. Values are the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.005.
Figure 5.
Expression of ANGPTL4 is essential for EGF-enhanced anoikis resistance. The anoikis resistance assay was applied as described in ‘Materials and methods'. (a) Secreted ANGPTL4 from TU183 cells treated with 50 ng/ml EGF for 48 h was detected by ELISA (i). shLacZ and shANGPTL4 (shANG) TU183 cells were treated with or without 10 μg/ml anti-ANGPTL4 antibodies (anti-ANG), 10 μg/ml IgG, 50 ng/ml EGF and 250 ng/ml cANGPTL4 for 48 h. Images of colonospheres were examined using a microscope (ii). The arrow indicates a colonosphere size>100 μm. Original magnification, × 200. The number of colonospheres (>100 μm) was counted (iii). (b) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection. Cells were treated with 50 ng/ml EGF and grown in suspension for 48 h. Apoptotic cells were stained with Annexin V-FITC/PI and analysed by flow cytometry (i). * late apoptosis; # early apoptosis. Secreted ANGPTL4 was detected by ELISA (ii). (c) Activation of caspase-3 was examined in EGF-treated and ANGPTL4-depleted cells and by western blotting. (d and e) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection and then grown in suspension for 48 h. The cells were then labelled with DiI and cultured with endothelial cells for 30 min. The percentage of bound tumour cells was quantified in three independent experiments by flow cytometry (upper panel). The attachment of cells was examined using a microscope (lower panel) (d). Values are the mean±s.e.m. The transendothelial invasion assay was performed (e). The total number of invading cells was quantified by Image J and images were photographed. The percentage of invading cells was determined in three independent experiments (upper panel). The images of invading cells were examined using a microscope (lower panel). Values are the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.005.
Expression of c-Jun is essential for ANGPTL4-induced MMP-1 expression
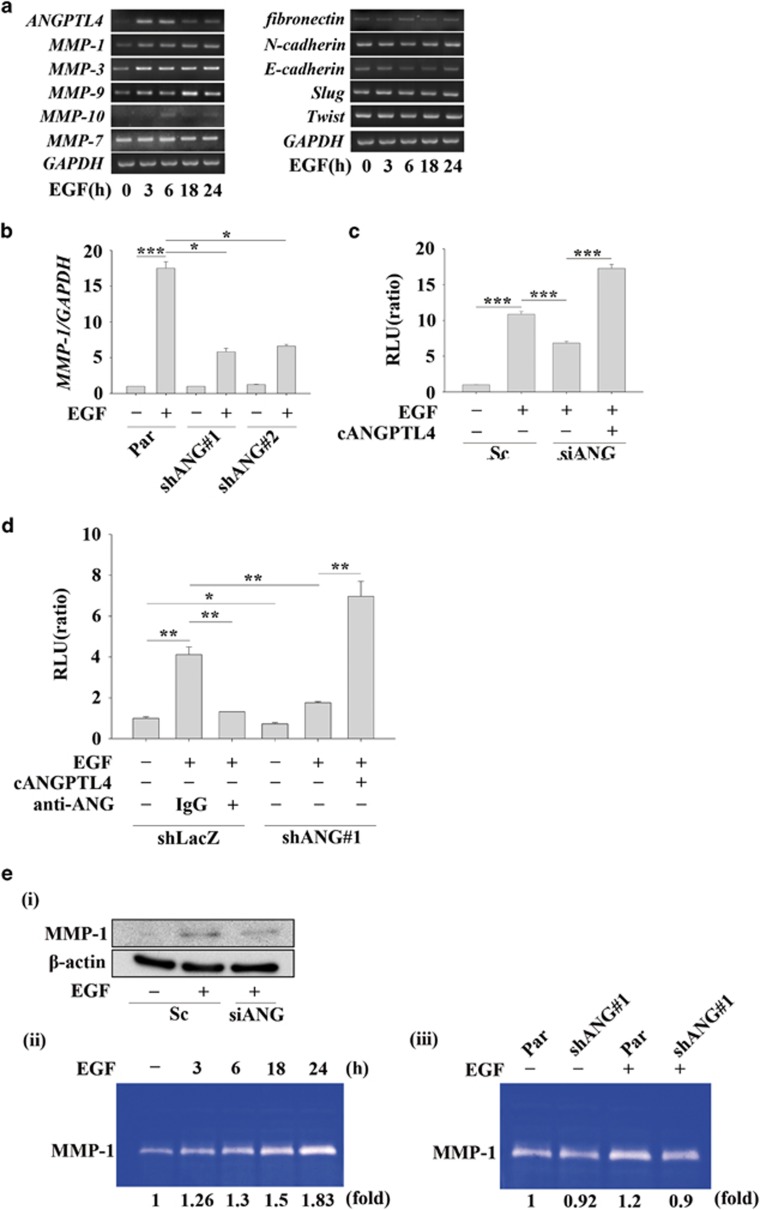
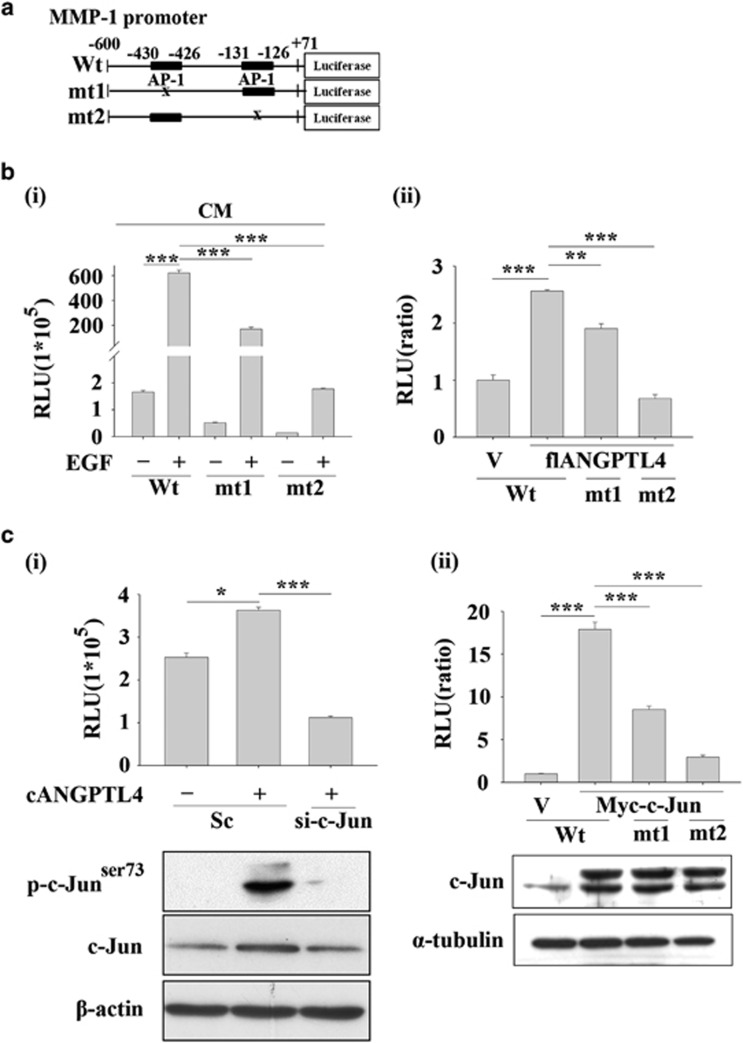
Based on the observation that ANGPTL4 expression was essential for EGF-enhanced cancer cell migration and invasion, we next investigated the mechanisms involved in ANGPTL4-regulated cell metastasis. Although no changes in fibronectin, E-cadherin, N-cadherin, slug or twist were observed in EGF-treated cells, the expression levels of MMPs, including MMP-1, MMP-3, MMP-9 and MMP-10, were increased (Figure 6a). Previous studies have shown that the expression of MMPs is dependent on ANGPTL4 levels and correlates with cancer metastasis.23, 24 To further confirm whether ANGPTL4 was required for EGF-induced MMP expression, the effects of ANGPTL4 knockdown on gene expression were examined. Indeed, depletion of ANGPTL4 inhibited EGF-induced MMP expression (Figure 6b and Supplementary Figures S3a and b). EGF-enhanced MMP-1 promoter activity was also repressed either following knockdown of ANGPTL4 (Figure 6c) or neutralization of the protein using anti-ANGPTL4 antibodies (Figure 6d). Inhibition of MMP-1 promoter activity was reversed following treatment with EGF and recombinant cANGPTL4 in ANGPTL4-depleted cells (Figures 6c and d). In addition, EGF-induced MMP-1 protein expression and enzymatic activity were also reduced in ANGPTL4-depleted cells (Figure 6e). These results suggested that the expression of ANGPTL4 was essential for EGF-induced MMP-1 expression. Although we confirmed that MMP expression was regulated by ANGPTL4, the mechanism involved in ANGPTL4-regulated expression of MMPs such as MMP-1 remains unclear. Previous studies have shown that the activated c-Jun/Fra-1 complex binds to the AP-1-binding site of the MMP-1 promoter to enhance MMP-1 gene expression.28 To further clarify whether AP-1-binding sites mediate ANGPTL4-regulated MMP-1 promoter activity, a promoter containing mutated AP-1-binding sites was constructed (Figure 7a). As shown in Figure 7b, both conditioned media harvested from EGF-treated and ANGPTL4 overexpressing cells significantly induced MMP-1 promoter activity. However, the induction was dramatically inhibited with the mutated constructs (mt1 and mt2). These results indicated that the AP-1-binding sites located in the −430 to −426 bp region and −131 to −126 bp region were essential for EGF- and ANGPTL4-induced MMP-1 expression. Next, we examined whether c-Jun was essential for ANGPTL4-induced MMP-1 expression. As shown in Supplementary Figure S3c and Figure 7c, EGF and ANGPTL4 induced the activation of c-Jun; however, the depletion of c-Jun dramatically inhibited ANGPTL4-induced MMP-1 promoter activity. In addition, overexpression of c-Jun also enhanced promoter activity, but the induction was repressed in constructs with mutated AP-1 site (Figure 7c). These results suggest that ANGPTL4-induced MMP-1 expression was regulated by the c-Jun signalling pathway.
Figure 6.
EGF-induced ANGPTL4 regulates the expression of MMP-1. (a) TU183 cells were treated with 50 ng/ml EGF for the indicated period of time. The expression levels of ANGPTL4, MMP-1, MMP-3, MMP-7, MMP-9, MMP-10, fibronectin, E-cadherin, slug, twist and GAPDH mRNA were analysed by RT-PCR and examined by 2% agarose gel electrophoresis. (b) Parental (Par) and shANGPTL4 (shANG) TU183 cells were treated with 50 ng/ml EGF for 9 h. The expression of MMP-1 mRNA was analysed by real-time quantitative PCR. The relative levels of MMP-1 were normalized to GADPH. Values are the mean±s.e.m. (c) TU183 cells were co-transfected with 0.5 μg MMP-1 promoter and 20 ng renilla luciferase construct, 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection. The cells were treated with or without 50 ng/ml EGF and 250 ng/ml cANGPTL4 for 18 h. The firefly and renilla luciferase activities were then determined and normalized. Values are the mean±s.e.m. (d) shLacZ and shANGPTL4 (shANG) TU183 cells were co-transfected with 0.5 μg MMP-1 promoter and 20 ng renilla luciferase construct. Cells were pre-treated with 10 μg/ml of anti-ANGPTL4 antibodies (anti-ANG) and 5 μg/ml IgG for 1 h, and then with 50 ng/ml EGF for 18 h. The firefly and renilla luciferase activities were then determined and normalized. Values are the mean±s.e.m. (e) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG) and scramble siRNA (Sc) by lipofection, and the cells were treated with or without 50 ng/ml EGF for 18 h. The expression levels of β-actin and MMP-1 were analysed by western blotting (i). TU183 cells were treated with 50 ng/ml EGF for the indicated period of time (ii). Parental (Par) and shANGPTL4 (shANG) TU183 cells were treated with 50 ng/ml EGF for 24 h (iii). The enzymatic activity of MMP-1 in CM was analysed using the casein-zymography assay. *P<0.05; **P<0.01.
Figure 7.
ANGPTL4-induced MMP-1 is dependent on c-Jun expression. (a) The MMP-1 promoter containing wild-type (Wt) and AP-1 binding site mutants (mt1 and mt2) was constructed. (b) TU183 cells were co-transfected with 0.5 μg MMP-1 promoter and 20 ng renilla luciferase construct, and then treated with CM collected from EGF-treated cells (i). TU183 cells were co-transfected with 0.5 μg MMP-1 promoter and 20 ng renilla luciferase construct and 1 μg flANGPTL4-expressing vector (ii). The firefly and renilla luciferase activities were then determined and normalized. Values are the mean±s.e.m. (c) TU183 cells were co-transfected with 0.5 μg MMP-1 promoter, 20 ng renilla luciferase construct, 50 nM c-Jun siRNA oligonucleotides, scramble siRNA (Sc) (i) and myc-c-Jun-expressing vector (ii) by lipofection and then with or without 250 ng/ml cANGPTL4 for 18 h. The firefly and renilla luciferase activities were then determined and normalized. Values are the mean±s.e.m. The expression levels of c-Jun, β-actin, α-tubulin and phosphorylated c-Junser73 were analysed by western blotting. ***P<0.005.
Integrin β1 is essential for EGF- and ANGPTL4-induced MMP-1 expression and tumour-endothelial cell interactions
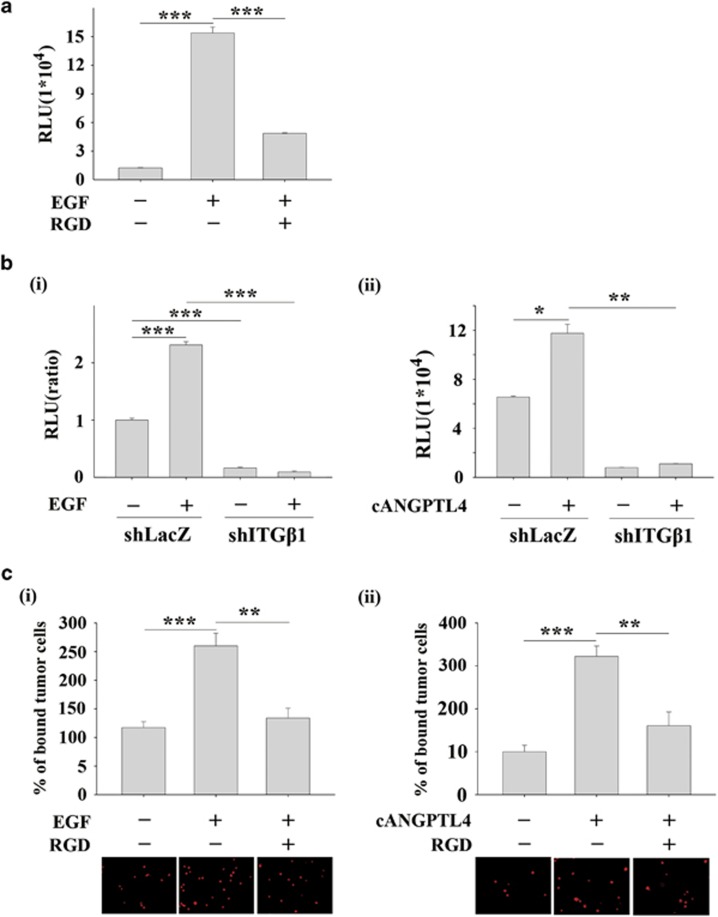
The binding of ANGPTL4 to integrin α5β1 has been reported to be essential for the activation of Rac signalling and to enhance the permeability of blood vessels.29 Therefore, we further verified the requirement of integrin β1 for EGF- and ANGPTL4-induced MMP-1 expression. As shown in Figures 8a and b, either the integrin antagonist (Arg-Gly-Asp) peptides or knockdown of integrin β1 inhibited EGF- and ANGPTL4-induced MMP-1 promoter activity. The inhibition of integrin also blocked EGF- and ANGPTL4-induced tumour-endothelial cell interactions (Figure 8c). These results suggest that activation of the integrin β1 signalling pathway was at least partially essential for EGF- and ANGPTL4-induced MMP-1 expression and tumour-endothelial cell interactions.
Figure 8.
The activation of integrin β1 signalling is essential for EGF- and ANGPTL4-induced MMP-1 expression and tumour-endothelial cell interactions. (a and b) TU183 cells were transfected with 0.5 μg MMP-1 promoter and 20 ng renilla luciferase construct and then treated with 50 ng/ml EGF and 1 mM (Arg-Gly-Asp) peptides for 18 h (a). shLacZ and integrin β1-depleted (shITGβ1) cells were co-transfected with 0.5 μg MMP-1 promoter and 20 ng renilla luciferase construct and then treated with 50 ng/ml EGF (i) and cANGPTL4 (250 ng/ml) (ii) for 18 h (b). The firefly and renilla luciferase activities were then determined and normalized. Values are the mean±s.e.m. (c) TU183 cells were treated with 50 ng/ml EGF (i), 250 ng/ml cANGPTL4 (ii) and 1 mM (Arg-Gly-Asp) peptides for 3 h. Cells were then labelled with DiI and cultured with endothelial cells for 30 min. The attachment of cells was examined using a microscope (lower panel). The percentage of bound tumour cells was quantified in three independent experiments by flow cytometry (upper panel). Values are the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.005.
The EGF/ANGPTL4/MMP-1 axis promotes HNSCC metastasis
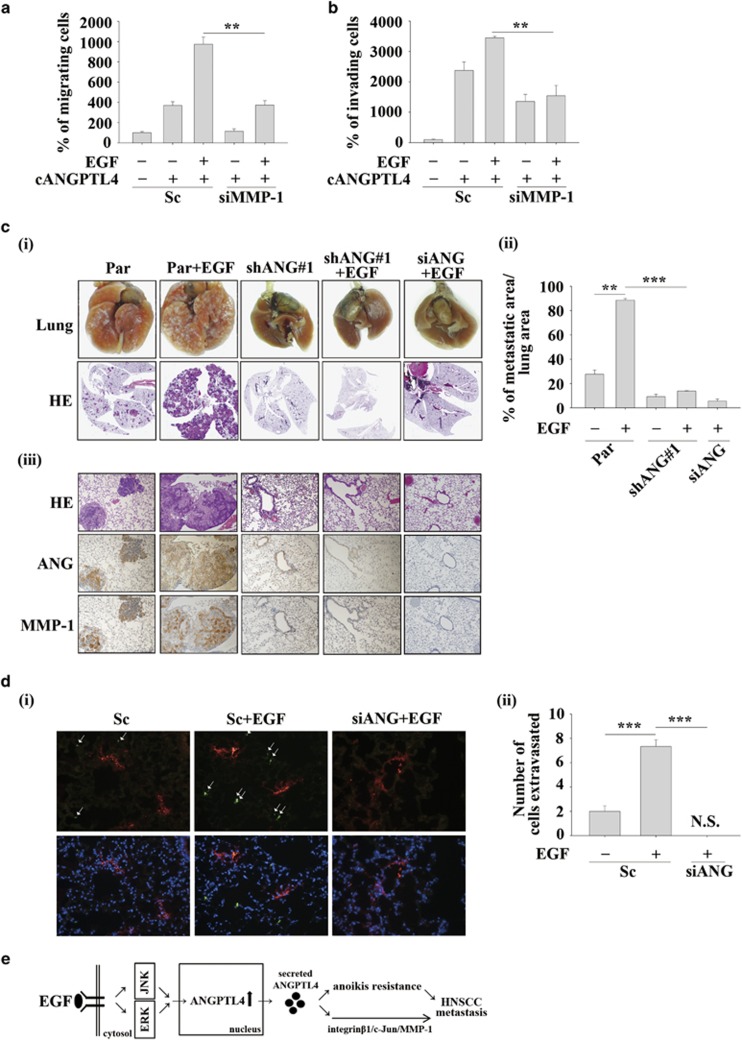
To further confirm the role of the EGF/ANGPTL4/MMP-1 axis in tumour metastasis, the effect of MMP-1 knockdown on EGF- and ANGPTL4-induced cell migration and invasion was studied. The results showed that ANGPTL4-induced migration and invasion were blocked in MMP-1 knockdown cells (Figures 9a and b). The synergistic effect of EGF and ANGPTL4 on migration and invasion was also reduced in MMP-1 knockdown cells (Figures 9a and b). Next, we examined whether ANGPTL4 was essential for EGF-enhanced metastasis in vivo by injecting EGF-pretreated parental and shANGPTL4 cells into the tail veins of mice. As shown in Figure 9c, increased metastatic nodules in lung tissues were found in mice injected with EGF-primed parental cells. Consistent with the observations in the transendothelial invasion assay, depletion of ANGPTL4 inhibited the metastatic seeding of EGF-primed tumour cells in the lungs. In addition, the expression of MMP-1 protein in lung tissues was observed in EGF-primed parental but not shANGPTL4 cells (Figure 9c). To further clarify whether the reduction of metastatic nodules of ANGPTL4-depleted cells was associated with the inhibition of tumour growth, tumour cell proliferation was examined in parental and shANGPTL4 cells. Cell proliferation and division rates remained unchanged between parental and shANGPTL4 cells (Supplementary Figures S4a and b). Furthermore, the growth of shANGPLT4 and parental cells in the subcutaneous space of mice did not differ (Supplementary Figure 4c). Again, transient knockdown of ANGPTL4 by siRNA was confirmed. The results suggested that the effect of siRNA oligonucleotides on ANGPTL4 inhibition was presented within 48 h in EGF-treated cells (Supplementary Figure 4d). However, ANGPTL4 siRNA also significantly inhibited EGF-primed pulmonary metastasis (Figure 9c). In addition, the extravasation assay30, 31, 32 revealed that EGF-promoted tumour cell penetration of blood vessels was significantly inhibited in ANGPTL4-depleted cells (Figure 9d). These results revealed that ANGPTL4-regulated pulmonary metastasis occurred via activation of tumour extravasation but not tumour growth. Taken together, induction of the ANGPTL4/MMP-1 axis was essential for EGF-induced HNSCC metastasis. However, depletion of ANGPTL4 was not associated with tumour growth.
Figure 9.
The EGF/ANGPTL4/MMP-1 axis promotes HNSCC metastasis. (a and b) TU183 cells were transfected with 50 nM MMP-1 siRNA oligonucleotides (siMMP-1) and scramble siRNA (Sc) by lipofection and then treated with 250 ng/ml cANGPTL4 and 50 ng/ml EGF for 24 or 48 h. To determine the percentages of migrating (a) and invading (b) cells, the cells were fixed and stained with crystal violet, solubilized with acetic acid and absorbance (OD, 595 nm) was measured in a microplate reader. Values are the mean±s.e.m. (c) Parental (Par) TU183 cells were transfected with 50 nM ANGPTL4 siRNA oligonucleotides (siANG). Par and shANGPTL4 (shANG) TU183 cells (5x105) were then treated with 50 ng/ml EGF for 3 h and then injected into the tail vein of severe combined immunodeficiency mice. Colonies in the lungs were examined and photographed at 1.5 months. Images of lung and H&E staining (i); the tumour area was analysed using ImageJ (ii); and ANGPTL4 and MMP-1 expression were examined by IHC (iii). n=5 for each groups. (d) Parental (Par) and shANGPTL4 (shANG) TU183 cells were treated with 50 ng/ml EGF for 3 h and then stained with DiO for 30 min. Cells were (5x105 cells/100 μl) injected into the tail vein of severe combined immunodeficiency mice for 24 h and then sacrificed for examining the metastatic tumour cells surrounding the lung tissue as described in ‘Materials and methods'. The penetration of tumour cells was examined using a microscope (i). Original magnification, × 20; arrows indicate the DiO-labelled tumour cells (green); DiI-labelled blood vessels (red); DAPI-labelled nucleus (blue). Tumour cells were quantified by analysing at least four sections and six fields to determine the number of tumour cells that underwent extravasation (ii). n=5 for each groups. Values are the mean±s.e.m. (e) The putative EGF/ANGPTL4/integrin β1/MMP1 pathway regulates HNSCC metastasis. **P<0.01. N.S., non signal (Non DiO-labelled signal was observed).
Discussion
Although the role of ANGPTL4 in the regulation of lipid metabolism is clear, the function of ANGPTL4 in the regulation of metastasis remains controversial. For example, ANGPTL4 prevents Lewis lung carcinoma metastasis through the inhibition of vascular permeability and tumour cell motility and invasiveness.22 However, the induction of ANGPTL4 via the Smad signalling pathway by TGFβ promotes breast tumour metastasis.18 Therefore, to further clarify the function of ANGPTL4 in HNSCC metastasis, we examined the effect of ANGPTL4 on EGF-induced HNSCC metastasis. For the first time, we revealed that tumour-derived ANGPTL4 promoted HNSCC migration and invasion. In an in vivo assay, penetration of blood vessels for metastatic seeding of the lungs by EGF-primed tumour cells was inhibited by the depletion of ANGPTL4. Previous studies have also indicated that tumour-derived ANGPTL4 promotes cell survival and tumour growth and increases the permeability of lung capillaries, thus enhancing lung metastasis of breast tumours.18, 33 These results suggest that tumour-derived ANGPTL4 is essential for cancer metastasis.
According to our previous report34 and this study, we found that EGF-induced gene expression including PTX3 and ANGPTL4 promoted HNSCC metastasis through the induction of MMPs. The depletion of ANGPTL4 inhibited EGF-induced MMP-1, MMP-3, MMP-9 and MMP-10. However, among EGF-induced MMPs, only MMP-9 was regulated by PTX3. In addition, the induction of MMP-1 occurred earlier than MMP-9 in EGF-treated HNSCC cells. These results support the possibility that the expression of PTX3 may also be regulated by EGF-induced ANGPTL4. Indeed, our results (unpublished data) revealed that knockdown of ANGPTL4 inhibited EGF-induced PTX3 in HNSCC, suggesting that PTX3 was one of the ANGPTL4-regulated targets. Therefore, activation of the ANGPTL4/PTX3/MMPs signalling axis was at least one of pathways involved in the regulation of EGF-induced HNSCC metastasis. In addition, ANGPTL4 and PTX3, as a lipoprotein lipase inhibitor and an inflammatory molecule, respectively, are useful biomarkers for cardiovascular disease.35 In a summary, these results provide clinical implications for PTX3 and ANGPTL4 not only in cardiovascular disease but also in HNSCC tumour metastasis.
Upon entering the blood circulation, tumour cells must acquire resistance to anoikis, anchor to and finally interact with endothelial cells to successfully metastasize to distant sites. We found that EGF dramatically enhanced HNSCC anoikis resistance by inducing autocrine production of ANGPTL4. Previous studies have demonstrated that tumour-derived ANGPTL4 stimulates the production of ROS, resulting in activation of Src, PI3K/PKBα and ERK survival pathways to confer anoikis resistance.19 Interestingly, we found that the expression of ANGPTL4 and ROS levels were increased in anoikis resistant cells. However, depletion of ANGPTL4 dramatically reduced ROS levels in these cells (our unpublished data). These results support the possibility that the elevation of ROS levels protects against anoikis, at least in part, via EGF-induced secretion of ANGPTL4. Previous studies have demonstrated that a deficiency in ANGPTL4 activates caspase-2, -3, -8 and -9 activities and induces apoptosis upon anoikis in tumour cells.19 In our investigations, we clarified that ANGPTL4 participated in EGF-elicited inhibition of caspase-3 during the anchorage-independent growth of cancer cells. In addition, we found that ANGPTL4 enhanced not only tumour-endothelial cells' interactions but also invasion of anoikis-resistant cells. Taken together, these results further indicate that antioxidants may abrogate the impact of EGF-induced ANGPTL4 on circulating cancer cell survival, adhesion and invasion. Previous studies have demonstrated that ANGPTL4 disrupts endothelial junctions by activating endothelial cell integrin α5/β1 signalling to increase endothelial leakiness and promote breast cancer metastasis.29 Consistent with these results, we found that tumour cell integrin β1 was required for EGF-induced MMP-1 expression and the interaction between tumour and endothelial cells. These results indicate that ANGPTL4 enhanced tumour metastasis not only by mediating vessel permeability but also by stimulating the adhesion of tumour cells to blood vessels. However, the mechanism involved in ANGPTL4-mediated stimulation of tumour-endothelial cell interaction remains to be clarified. We also cannot rule out the possibility that the activation of integrin α5/β1 signalling in endothelial cells may also contribute to their interaction with tumour cells in response to EGF-induced ANGPTL4.
ANGPTL4 directly affects communication between cells and the extracellular matrix by altering the availability of intact matrix proteins during keratinocyte wound healing.36 Previous studies have shown that the co-expression of ANGPTL4 and MMPs correlated with cancer invasion and metastasis.23, 24 Although ANGPTL4 has been reported to enhance the expression of MMP-1 and MMP-3,25, 26 the mechanism involved in regulating MMP-1 expression remains unclear. In this study, we clarified that ANGPTL4-induced MMP-1 expression was mediated by the integrin β1/c-Jun signalling pathway. The AP-1 binding sites located in the MMP-1 promoter region were essential for the activation of the promoter by EGF-induced ANGPTL4. These results indicate that a down-stream component of the integrin β1 signalling pathway, such as c-Jun, is required for the up-regulation of MMP-1 gene expression, resulting in the promotion of tumour invasion. The enhancement of ANGPTL4 expression has been reported in many tissues. For example, ANGPTL4 is highly expressed in human liver, adipose tissue, blood plasma, placenta, small intestine and heart.37, 38 ANGPTL4 mRNA has been found to be up-regulated in tumours including clear cell renal carcinomas, oral tongue squamous cell carcinomas and human gastric cancers.39, 40 The up-regulation of ANGPTL4 could be mediated by several endogenous cytokines, such as PGE2 and TGFβ. In addition to these cytokines, we further clarified that EGF also enhanced ANGPTL4 expression and secretion, resulting in HNSCC metastasis. In contrast to the function of cytokine-induced ANGPTL4 in promoting tumorigenesis, overexpression of ANGPTL4 in cells has been reported to down-regulate tumour metastasis. For example, ANGPTL4 prevents the metastasis of Lewis lung carcinoma 3LL cells by inhibiting tumour cell intravasation from the primary tumour to lymphatic or blood vessels.22 The inhibition of tumour metastasis by ANGPTL4 was mediated by the suppression of vascular tube formation in the tumour microenvironment.21 Although the effect of ANGPTL4 on tumour metastasis is still controversial, these results suggest that the differential functions of ANGPTL4 may be dependent on its induction by endogenous cytokines such as EGF and TGFβ, or the overexpression of ANGPTL4.
In conclusion, we demonstrated the biological functions of ANGPTL4 in EGF-induced HNSCC metastasis. As shown in Figure 9e, EGF induced ANGPTL4 expression by activating the JNK and ERK pathways (our unpublished data). Autocrine production of ANGPTL4 further activated the integrin β1/c-Jun signalling pathway to enhance MMP-1 expression. Our results show that the induction of ANGPTL4 not only enhances anoikis resistance and tumour-endothelial cell interactions, but also up-regulates MMP-1 expression to promote the metastatic seeding of tumour cells in the lungs. Measurement of ANGPTL4 expression levels may provide prognostic information regarding HNSCC metastasis, which suggests that ANGPTL4 could be a novel therapeutic target for EGFR-overexpressing HNSCC.
Materials and methods
Cell culture
The FaDu head and neck cancer cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The human microvascular endothelial cell line (HMEC-1) was kindly provided by Dr Trai-Ming Yeh (Department of Medical Laboratory Science and Biotechnology, College of Medicine, National Cheng Kung University, Tainan, Taiwan). The head and neck cancer cell lines TU183, UMSCC1 and SAS were kindly provided by Dr Kwang-Yu Chang (National Health Research Institutes, NHRI, Taiwan).41 These head and neck cancer cell lines were maintained at 37 °C with 5% CO2 in 10-cm plastic dishes containing 7 ml of Dulbecco's Modified Eagle's Medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Invitrogen), 100 μg/ml streptomycin (Invitrogen) and 100 units/ml penicillin (Invitrogen). The FaDu cell line was maintained at 37 °C with 5% CO2 in 10-cm plastic dishes containing 10 ml of Eagle's Minimum Essential Medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin and 100 units/ml penicillin. The HMEC-1 cells were maintained in MCDB131 culture medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, 100 units/ml penicillin and 15 μg/ml endothelial cell growth supplement (Millipore, Bedford, MA, USA).
Conditioned medium and antibodies
Full details are available in Supplementary Materials and Methods.
Reverse transcription-PCR and real-time quantitative RT-PCR
Full details are available in Supplementary Materials and Methods.
Enzyme-linked immunosorbent assay
Full details are available in Supplementary Materials and Methods.
Knockdown experiments
Full details are available in Supplementary Materials and Methods.
Transfection with siRNA oligonucleotides
Full details are available in Supplementary Materials and Methods.
Migration and invasion assay
Full details are available in Supplementary Materials and Methods.
Transendothelial invasion assay
Full details are available in Supplementary Materials and Methods.
Cell adhesion assay
Briefly, tumour cells were treated with 50 ng/ml of EGF or 250 ng/ml of cANGPTRL4 for 3 h and then labelled for 30 min at 37 °C with DiI (Invitrogen Life Technologies, Carlsbad, CA, USA) and washed twice with phosphate-buffered saline. The tumour cells (1 × 105 cells /ml) were resuspended and added to a monolayer of HMEC-1 cells. After incubation for 30 min at 37 °C, the wells were gently washed twice with phosphate-buffered saline to remove non-adherent cells. The cells were photographed and the number of adherent tumour cells and HMEC-1 cells was quantified by Cell Lab Quanta SC flow cytometry (Beckman Coulter, Fullerton, CA, USA) equipped with an argon laser. The DiI emission was measured at 575 nm (FL2) and the data was analysed by FlowJo software (Treestar, Inc., San Carlos, CA, USA). The percentage of bound tumour cells was determined by the ratio of the bound tumour cells (adherent cells) that were DiI-positive to the HMEC-1 cells that were DiI-negative (5 × 103 cells per group).
Anoikis assay
Cells were seeded and grown on polyHEMA (Sigma)-coated plates in 6-cm plastic dishes containing 3 ml of Dulbecco's Modified Eagle's Medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin-streptomycin-glutamine. Next, they were treated with or without EGF, cANGPTL4 and ANGPTL4 antibody for 48 h. Samples were analysed in a Cell Lab Quanta SC flow cytometry (Beckman Coulter) equipped with an argon laser and the data was analysed by FlowJo software (Treestar). Ten thousand events were collected for each sample. For the analysis of cell death, cells were harvested and then washed with PBS. 1 × 105 cells were resuspended in 100 μl binding buffer containing Annexin V-FITC (1:100) (BD Pharmingen, San Diego, CA, USA) and propidium iodide (1:2000, BD Pharmingen) for 15 min at room temperature and then determined by flow cytometer.
Luciferase assays and plasmid construction
Full details are available in Supplementary Materials and Methods.
Tumour metastasis assay in an animal model
Tumour metastasis was determined by tail vein intravenous injection of cancer cells into 6- to 8-week-old male severe combined immunodeficiency mice. There is no randomization method and blinding group for allocation of animals. Briefly, 5 × 105 tumour cells were resuspended in 100 μl with Hank's Balanced Salt Solution and then injected into the tail vein of mice. To evaluate lung metastasis, mice were sacrificed up to 1.5 months after injection. H&E staining was performed by the Human Biobank, Research Center of Clinical Medicine, NCKU Hospital. For the lung extravasation assay, 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) labelled cells were injected into the tail vein of mice and then sacrificed up to 24 h after injection. The blood vessels were directly labelled by cardiac perfusion using a specially formulated aqueous solution containing 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI), which incorporates into endothelial cell membranes upon contact.31 Lung tissues of mice were cryosectioned (8 μm) for fluorescence imaging to visualize the vasculature (red) and tumour cells (green).30 Quantification was performed by analysing at least four sections and six fields to determine the number of tumour cells that underwent extravasation. All mice were obtained from the National Cheng Kung University (NCKU) Laboratory Animal Center (Tainan, Taiwan). All animal experiments in this study were approved (Approved No. NCKU-IACUC-102-078) by the Laboratory Animal Committee of NCKU.
Statistical analysis
Data are expressed as the means±s.e.m. The statistical analysis was performed using SigmaPlot version 10.0 for Microsoft Windows, and an independent-samples t-test was used for comparisons between two groups. Centre values indicate as mean. A P-value less than 0.05 was considered significant and denoted by *. P-values less than 0.01 and 0.001 are denoted by ** and ***, respectively.
Acknowledgments
This work was supported by the Ministry of Science and Technology of Taiwan [Grant NSC 102–2628-B-006– 011-MY3; MOST 105-2320-B-006-022-MY3] and National Cheng Kung University [the Headquarters of University Advancement]. We thank for the kind gift of MMP-1 gene promoter-PGL3 from Dr Ju-Ming Wang.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
The authors declare no conflict of interest.
Supplementary Material
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics. 2013 . CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer 2005; 114: 806–816. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil' hypothesis revisited. Nat Rev Cancer 2003; 3: 453–458. [DOI] [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett 2008; 272: 177–185. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994; 124: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 2013; 1833: 3481–3498. [DOI] [PubMed] [Google Scholar]
- Humtsoe JO, Kramer RH. Differential epidermal growth factor receptor signaling regulates anchorage-independent growth by modulation of the PI3K/AKT pathway. Oncogene 2010; 29: 1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Chen S, You Z, Yang F, Carey TE, Saims D et al. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFkappa B. J Biol Chem 2002; 277: 25203–25208. [DOI] [PubMed] [Google Scholar]
- Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol 2004; 165: 1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JC, Timpson P, Kalna G, Machesky LM. Mtss1 regulates epidermal growth factor signaling in head and neck squamous carcinoma cells. Oncogene 2012; 31: 1781–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng GQ et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem 2011; 112: 2508–2517. [DOI] [PubMed] [Google Scholar]
- Masuelli L, Budillon A, Marzocchella L, Mrozek MA, Vitolo D, Di Gennaro E et al. Caveolin-1 overexpression is associated with simultaneous abnormal expression of the E-cadherin/alpha-beta catenins complex and multiple ErbB receptors and with lymph nodes metastasis in head and neck squamous cell carcinomas. J Cell Physiol 2012; 227: 3344–3353. [DOI] [PubMed] [Google Scholar]
- Xiao YC, Yang ZB, Cheng XS, Fang XB, Shen T, Xia CF et al. CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis. Cancer Lett 2015; 361: 22–32. [DOI] [PubMed] [Google Scholar]
- Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med 2005; 11: 473–479. [DOI] [PubMed] [Google Scholar]
- Katanasaka Y, Kodera Y, Kitamura Y, Morimoto T, Tamura T, Koizumi F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer 2013; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park YY, Kim SW, Lee JS, Wang D, DuBois RN. ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res 2011; 71: 7010–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Irie T, Yamamoto G, Yasuhara R, Isobe T, Hokazono C et al. ANGPTL4 regulates the metastatic potential of oral squamous cell carcinoma. J Oral Pathol Med 2015; 44: 126–133. [DOI] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 2008; 133: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS, Nwariaku FE. Escaping anoikis through ROS: ANGPTL4 controls integrin signaling through Nox1. Cancer Cell 2011; 19: 297–299. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD et al. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi-Takada E, Hattori N, Tsukamoto T, Miyamoto K, Ando T, Ito S et al. ANGPTL4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene 2014; 33: 2273–2278. [DOI] [PubMed] [Google Scholar]
- Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA 2006; 103: 18721–18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akishima-Fukasawa Y, Ishikawa Y, Akasaka Y, Uzuki M, Inomata N, Yokoo T et al. Histopathological predictors of regional lymph node metastasis at the invasive front in early colorectal cancer. Histopathology 2011; 59: 470–481. [DOI] [PubMed] [Google Scholar]
- Sun Y, Long J, Zhou Y. Angiopoietin-like 4 promotes melanoma cell invasion and survival through aldolase A. Oncol Lett 2014; 8: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Yudo K, Nakamura H, Chiba J, Okamoto K, Suematsu N et al. Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J Orthop Res 2009; 27: 50–57. [DOI] [PubMed] [Google Scholar]
- Mathieu M, Iampietro M, Chuchana P, Guerit D, Djouad F, Noel D et al. Involvement of angiopoietin-like 4 in matrix remodeling during chondrogenic differentiation of mesenchymal stem cells. J Biol Chem 2014; 289: 8402–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TC, Tan CT, Chang YW, Hong CC, Lee WJ, Chen MW et al. Angiopoietin-like protein 1 suppresses SLUG to inhibit cancer cell motility. J Clin Invest 2013; 123: 1082–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Ishikawa C, Rokkaku T, Janknecht R, Mori N. Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim Biophys Acta 2011; 1813: 1543–1553. [DOI] [PubMed] [Google Scholar]
- Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 2011; 118: 3990–4002. [DOI] [PubMed] [Google Scholar]
- Tichet M, Prod'Homme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun 2015; 6: 6993. [DOI] [PubMed] [Google Scholar]
- Li Y, Song Y, Zhao L, Gaidosh G, Laties AM, Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc 2008; 3: 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 2000; 6: 100–102. [DOI] [PubMed] [Google Scholar]
- Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 2011; 19: 401–415. [DOI] [PubMed] [Google Scholar]
- Chang WC, Wu SL, Huang WC, Hsu JY, Chan SH, Wang JM et al. PTX3 gene activation in EGF-induced head and neck cancer cell metastasis. Oncotarget 2015; 6: 7741–7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa Y, Nagai T, Anzai A. Pentraxins: CRP and PTX3 and cardiovascular disease. Inflamm Allergy Drug Targets 2011; 10: 229–235. [DOI] [PubMed] [Google Scholar]
- Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem 2010; 285: 32999–33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Muller M et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem 2006; 281: 934–944. [DOI] [PubMed] [Google Scholar]
- Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK et al. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol 2009; 29: 969–974. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hirakawa H, Shibata K, Nazneen A, Abe K, Nagayasu T et al. Expression of angiopoietin-like 4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol Rep 2011; 25: 929–935. [DOI] [PubMed] [Google Scholar]
- Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol 2003; 162: 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KY, Tsai SY, Wu CM, Yen CJ, Chuang BF, Chang JY. Novel phosphoinositide 3-kinase/mTOR dual inhibitor, NVP-BGT226, displays potent growth-inhibitory activity against human head and neck cancer cells in vitro and in vivo. Clin Cancer Res 2011; 17: 7116–7126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.