Abstract
Accumulated evidence suggests that polyphenolic antioxidants present in herbs play important roles in prevention of AD; the molecular mechanisms behind neuroprotective actions rely on the phenols through different effects on the amyloid-aggregation pathway. Fagopyrum dibotrys is a traditional herbal medicine which contains high quantity phenols. In present study, we investigate the beneficial pharmacological actions of Fagopyrum dibotrys extract in the APP/PS1 transgenic mouse mode; meanwhile, effects of the FDE on the fibrillation and cytotoxicity of Aβ peptide were evaluated in vitro. After 9-month treatment, FDE exhibited multifunctional properties on Aβ-related pathologies, which cleaned Aβ deposits in the brain and decreased Aβ burden in the plasma, inhibited microhaemorrhage, and reduced reactive microglia in APP/PS1 transgenic mice and also promoted Aβ fibrils disaggregation and inhibited neurotoxicity induced by Aβ in SH-SY5Y cells. These results highlighted that FDE is an AD type pathology modulator with therapeutic potential against AD.
1. Introduction
Alzheimer disease (AD) is progressive and neurodegenerative disorder disease among the elderly, characterized by progressive loss of memory and cognition [1]. Histopathological hallmarks of AD include deposition of β-amyloid plaques and formation of neurofibrillary tangles, intracellular neurofibrillary tangles, reactive microgliosis, and astrogliosis [2]. Although several compounds undergoing research appeared to be protective and therapeutic effects on AD model, none of these drugs was able to stop or reverse the course in patients with AD.
Fagopyrum dibotrys (D. Don.) Hara is an erect perennial herb with clusters of small pinkish or white flowers and edible triangular seeds, growing mainly in China, India, Vietnam, Thailand, and Nepal [3, 4]. In China, its rhizome was regarded as folk medicine for the treatment of lung diseases, inflammation, dysentery, traumatic injuries and rheumatism [5]. The Fagopyrum dibotrys extract (FDE) was characterized by the abundance of proanthocyanidins, hecogenin, ferulic acid, catechin, gallic acid, epicatechin, and so on. Some polyphenol compounds such as ferulic acid and epicatechin have been reported to exhibit neuroprotective properties including therapeutic action in AD [6, 7] that may be attributed to these polyphenol metabolites which are able to cross the BBB and to penetrate and accumulate in the brain at pharmacologically relevant sub-μM to μM concentration [8], tightly controlling the influx in the brain of metabolites and nutrients as well as of drugs [9]. The total polyphenols content in Fagopyrum dibotrys is about 37.7% according to the test report provided by R&D department of Qujin Gelikang Science and Technological Co. Ltd. But up till now no studies have been carried out to show the effect of total polyphenols content in Fagopyrum dibotrys on the modulation of Aβ-related pathologies. Here, we report that FDE can influence the Aβ aggregation and Aβ-related pathology process.
2. Materials and Methods
2.1. Preparation of Fagopyrum dibotrys Extract
Fresh plant materials were collected from GAP planting base of Fagopyrum dibotrys in Qujing city, Yunnan province of China, which were identified by expert of Kunming Institute of Botany, Chinese Academy of Sciences. The whole Fagopyrum dibotrys plant was washed and cut into small pieces and dried in shade. 1000 g fresh plant dried in shade yielded approximately 200 g powder. The dried powder 100 g was milled and extracted with 60% EtOH at 90°C (extract 3 times with 1 h each time) and the crude extract was eluted with 60% ethanol and then absorbed and purified by polyamide column chromatography. Ethanol eluted extract was concentrated by a rotary evaporator at 40°C and freeze dried and a standard extract of Fagopyrum dibotrys (36 g) was obtained.
2.2. HPLC Analysis of Polyphenols in FDE
The standard extract of Fagopyrum dibotrys was analyzed by high performance liquid column (HPLC/UV). In brief, 25 mg FDE was dissolved in ethanol-0.1% formic acid (10 : 90) to final concentration 0.5 mg/ml. An aliquot of the sample solution 10 μL was automatically injected into the column for analysis of polyphenol components. The separation was performed on ODS-C18 column (100 × 4.6 mm, 3 μm) with gradient elution by the mobile phase A, methanol-0.1% formic acid (10 : 90), and mobile phase B, methanol-acetonitrile-0.1% formic acid (50 : 30 : 20); the gradient elution processes is 0 min (0% B), 15 min (8% B), 45 min (30% B), 60 min (35% B), and 75 min (100% B); the flow rate was 1.0 mL/min; the column temperature was 40°C; and the detection wave length was set at 280 nm. This method was accurate in identification and determination of compounds in Fagopyrum extracts and most of chromatogram peaks were identified by HPLC-MS/MS.
2.3. Aβ Disaggregation Assay
2.3.1. Aβ Preparation
Synthetic Aβ42 was purchased from American Peptide and prepared following the protocols described previously [10]. In brief, the Aβ42 peptide was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, Sigma) at 1 mg/ml and was aliquoted in Eppendorf tubes. The HFIP was allowed to evaporate in the fume hood, and the resulting clear peptide film was dried under vacuum overnight.
2.3.2. Thioflavin T Fluorescence Assay
To test the inhibitory effect of FDE on Aβ aggregation, 1 μM Aβ42 monomer was first incubated with a serial gradient of FDE in DMEM at 37°C for 10 d to form Aβ fibril; the resultants were incubated with 5 μM Th T solution. Fluorescence intensity was monitored at an excitation wavelength of 450 nm and an emission wavelength of 482 nm by a spectrometer (Turner Biosystems).
2.3.3. Electron Microscopy (EM) Assay
In brief, copper grids were preplaced on the bottom of wells in a 24-well plate where 1 μM Aβ42 monomer was incubated with or without FDE at a concentration gradient for 7 days at 37°C. Following incubation, the mixtures were stained with 2% (wt/vol) aqueous phosphotungstic acid for 30 min. The images were collected using a Hitachi7650 TEM equipped with Mega view 3 Digital Camera.
2.3.4. SH-SY5Y Cell Culture and Viability Assay
Human neuroblastoma cell line SH-SY5Y was cultured in RPMI-1640 supplemented with 10% FBS at 37°C. Cells at 60–70% confluence were treated with 1 μM Aβ with or without FDE (2.5 mg/mL, 5 mg/mL, or 10 mg/mL) for 24 hours, followed by incubation with MTT (0.5 mg/ml) for 4 h and 10% SDS solution for another 15 min at 37°C. The optical density at 560 nm was evaluated by a microplate reader (Turner Biosystems).
2.3.5. Transgenic Mouse Model
APP/PS1 transgenic mice were provided by the South model animal institute, Shanghai. These mice were constructed on a C57BL/6 background and bear a chimeric mouse/human (Mo/Hu) APP695 with mutations linked to familial AD (KM 593/594 NL) and human PS1 carrying the exon-9-deleted variant associated with familial AD (PS1dE9) in one locus under control of a brain- and neuron-specific murine Thy-1 promoter element [11]. Genotypes of the off spring were determined by PCR analysis of tail DNA. Mice were maintained on ad libitum food and water with a 12 h light/dark cycle.
2.3.6. Diet Treatment
The transgenic mice were randomly divided into 3 groups, each group comprising 12 mice, male : female = 1 : 1. The FDE (Tg) group were treated with 0.65% FDE mixed food; the dosage (0.103 mg/kg/d) used in the current study was based on clinical data. Control (Tg) and another age and sex matched wild-type mice group were fed with standard commercial food (Beijing Keao Xieli Feed Company, Beijing, China). From 3 months old, all the mice were fed the above diets for another 9 months. Food consumption and animal body weight were detected every 3 months during treatment period.
In this study, all experiments were performed in accordance with the European Communities Council Directive (2010/63/UE) and NIH for the Care and Use of Laboratory Animals. The study had been approved by the Committee for Animal Experiments and Ethics at the Kunming Medical University.
3. AD Type Pathology and Bioanalysis
3.1. Tissue Sampling
After 9 months of treatment, the mice were anesthetized with 10% chloral hydrate and the blood was collected from the eyeball for biochemical detection. Next, the brain was perfused with 50 ml chilled standard saline solution and was removed and bisected in the mid-sagittal plane. The left brain hemisphere was fixed in 4% paraformaldehyde (pH 7.4) for 24 h and incubated for another 48 h in 30% sucrose for subsequent cryoprotection. Coronal sections of the brain were cut at 35 μm thickness d with a cryosectioning microtome and stored at 4°C in PBS containing 40% glycol until use. The right brain was snap frozen in liquid nitrogen and stored at −80°C for future biochemical analysis.
3.2. Pathological Staining and Image Analysis
A series of five equally spaced tissue sections (~1.3 mm apart) were randomly selected and stained using free-floating immunohistochemistry (Biotin-conjugated mouse anti-Aβ antibody 6E10, Serotec) and Congo red for total Aβ, activated microglia (rat monoclonal anti-CD45 Chemicon, USA), and astrocyte (rabbit polyclonal antiglial fibrillary acidic protein, Dako, Denmark), respectively. Sections were incubated overnight with primary antibodies at 4°C, further developed with biotinylated secondary antibodies, and visualized with diaminobenzidine (Slide Kit Chemical international, Inc. Millipore).
For the compact Aβ plaque staining, a series of sections was mounted and stained with Congo red. In brief, the sections were treated with working sodium chloride solution (containing sodium chloride saturated in 80% alcohol and 0.01% sodium hydroxide) at room temperature for 30 min, then placed directly into working Congo red solution (containing saturated Congo red in working sodium chloride solution) for 1 h, and dehydrated rapidly in absolute alcohol.
The region of neocortex and hippocampus manually was selected for quantification of total Aβ plaques and microgliosis. The images were acquired in the same session and collected at 4x magnification using constant bulb temperature and exposure (Olympus DP73), yielding the area fraction of the total positive staining against the area of tissue analyzed; the average number of deposits was calculated per each brain area; all image analyses were developed in a blind manner.
3.3. Quantification of Aβ Peptide Levels by ELISA
ELISA analysis of the brain Aβ was processed as described previously [12]. Briefly, frozen brain was homogenized and sonicated in TBS containing protease inhibitors. Homogenates were centrifuged at 100,000 ×g for 1 h at 4°C, and the resultant supernatant was collected, representing the TBS-soluble fraction (Aβ-TBS). The resultant pellet was suspended and sonicated in water containing 2% SDS and protease inhibitors. The SDS solubilized homogenates were centrifuged at 100,000 ×g for 1 h at 4°C, and the resultant supernatant was collected, representing the SDS-soluble fraction (Aβ-SDS). The resultant pellet was then extracted in 70% formic acid (FA) and centrifuged, and the resultant supernatant was collected, representing the SDS-insoluble fraction (Aβ-FA). Before ELISA assay, formic acid extracts were neutralized by 1 : 20 dilution into 1 M Tris phosphate buffer, pH 11, and then diluted in sample buffer. Concentrations of Aβ40 and of Aβ42 in brain extract and serum were quantitatively measured by ELISA following the manufacturer's instructions (ELISA kits, Millipore).
3.4. Quantification of IL-1β, TNF-α, and IFN-γ in the Mouse Plasma by ELISA
IL-1β, TNF-α, and IFN-γ in the plasma of mice were measured using ELISA kits as per manufacturer's instructions (ELISA kits, eBioscience).
3.5. Assessment of Toxicity of FDE
Total bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were analyzed by Analytical Biochemical Laboratory of First Affiliated Hospital of Kunming Medical University.
4. Statistical Analysis
Results were generally presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using one-way ANOVA followed by post hoc Student's test where appropriate; p < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS for Windows version 20.0 (SPSS Inc.).
5. Results
5.1. HPLC Analysis of Polyphenol Compounds in FDE
The presence of polyphenols in the extracts was confirmed by identifying characteristic spectral features and comparison with standard compounds (>95% purity by HPLC, Shanghai Tauto Biotech Co., Ltd.). The use of reversed phase high performance liquid chromatography allowed the identification of 7 phenolic constituents; there were gallic acid, protocatechin acid, catechin, dimer gallic acid ester and epicatechin, trimer gallic acid ester, and epicatechin gallate (Figure 1).
Figure 1.
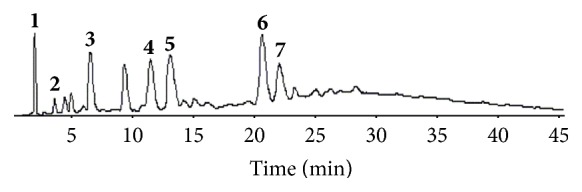
The HPLC/UV chromatograms of Fagopyrum dibotrys extract. Peaks: 1. gallic acid; 2. protocatechuic acid; 3. catechin; 4. dimer gallic acid ester; 5 epicatechin; 6. trimer gallic acid ester; 7. epicatechin gallate.
5.2. Effects of the FDE on the Fibrillation and Cytotoxicity of Aβ Peptide
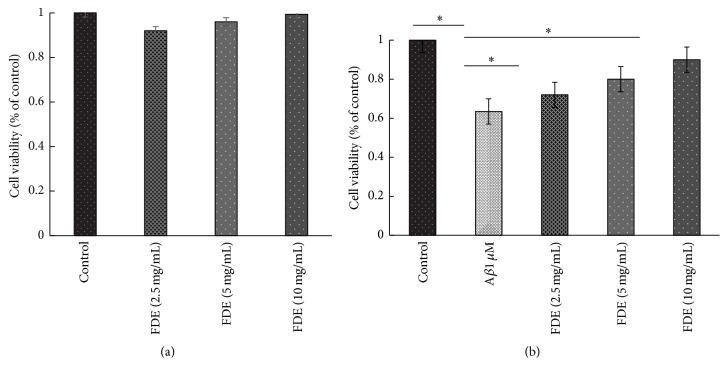
To investigate the effects of FDE on the fibrillation and cytotoxicity of Aβ protein, the inhibitory effects of FDE on Aβ1–42 fibril formation were determined by using thioflavin T fluorescence (Th-T) and Electron Microscopy (EM); the protective effects against cytotoxicity induced by Aβ1–42 in SH-SY5Y cells were evaluated by MTT assay. The data from Th-T fluorescence assay showed that FDE had a dose-dependent effect on disaggregation of preformed Aβ1–42 fibril (ANOVA, F = 10.762, p < 0.001, Figure 2(c)), it also reduced the neurotoxicity of Aβ1–42 on the cultured SH-SY5Y cells (Figures 3(a) and 3(b), p < 0.05).
Figure 2.
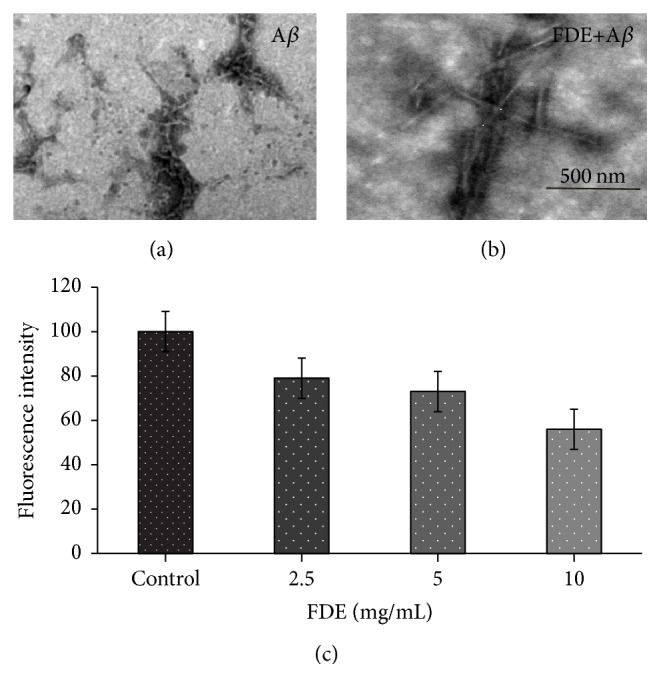
FDE disaggregate of Aβ fibrils. (a) Preformed Aβ 1 μM was incubated alone at 37°C 5 d. (b) Aβ with FDE at 37°C for an additional 3 d. Scale bar, 500 nm. (c) Th.T assays for effect of FDE on disaggregation of preformed Aβ fibrils (n = 3, p < 0.05).
Figure 3.
FDE attenuates the toxic effect of Aβ42 in SH-SY5Y cells. MTT assay of cell viability of SH-SY5Y cells treated with (a) various doses of FDE for 24 h (∗p > 0.05) and (b) 1 μM Aβ42 with various doses of FDE (∗p < 0.05).
The morphologies of the assemblies present following Aβ incubations with or without FDE were examined using Electron Microscopy (EM). When Aβ42 was incubated under fibril-forming conditions, different morphology of amyloid fibrils such as nonbranching fibrils, twisted long fibrils, and some small aggregates mixed with fibrils could be observed (Figure 2(a)). Conversely, when preformed Aβ fibrils were further incubated with FDE for an additional 3 days (Figure 2(b)), there were hardly any long fibrils on squares of grids.
These data indicate that FDE can effectively inhibit Aβ1–42 fibril formation and significantly lower the neurotoxicity on the cultured SH-SY5Y cells, suggesting potential effect of the FDE on Aβ clearance.
5.3. FDE Is Well Tolerated in APP/PS1 Transgenic Mice
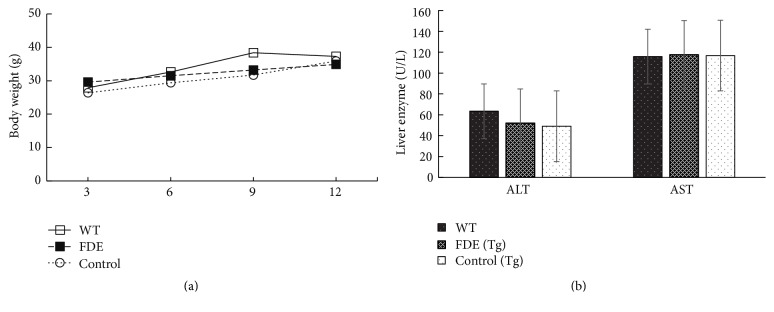
During treatment period, we did not observe any animal death occurring and any differences in animal viability general activities among the groups. It is important to note that the long-term daily consumption FDE diet for 9 months in APP/PS1 transgenic mice did not significantly influence animal body weight and liver function (Figures 4(a) and 4(b), p > 0.05, the serum level was too low to be detected). The FDE was well tolerated in AD mice.
Figure 4.
FDE is well tolerated in APP/PS1 transgenic mice. (a) Body weight was monitored at 3, 6, 9, and 12 months of age. (b) Serum levels of ALT and AST (p > 0.05). Points and bar graphs represent group mean (±SEM).
5.4. FDE Attenuates Aβ-Related Pathologies
In order to know Aβ deposition in the brain after FDE treatment, the AD brain sections were stained with anti-Aβ antibody 6E10 and Gongo red; a histological dye is commonly used to detect fibrillar Aβ plaques [13]. Aβ plaques were observed primarily in the neocortical and hippocampal areas of the brain, as observed in Figures 5(a), 5(b), 5(d), and 5(e).
Figure 5.
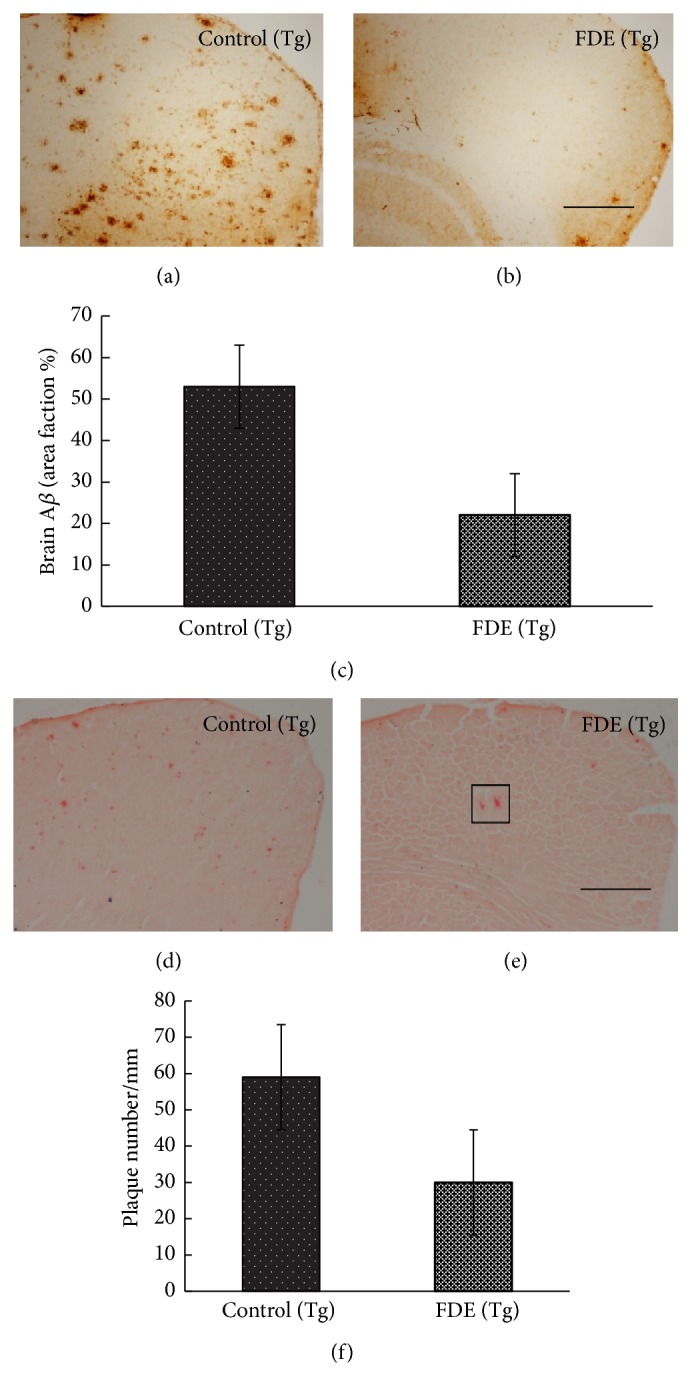
A plaque burden in the brain of mice. (a-b) IHC-positive Aβ plaques in neocortex of 9-month-old mice. (c) Comparison of IHC-positive Aβ plaque density in neocortex of 9-month-old mice. (d-e) Congo red-positive Aβ plaques in neocortex of 9-month-old mice. (f) Comparison of Congo red-positive Aβ plaque density in neocortex of 9-month-old animals. Denote p < 0.05 versus control group.
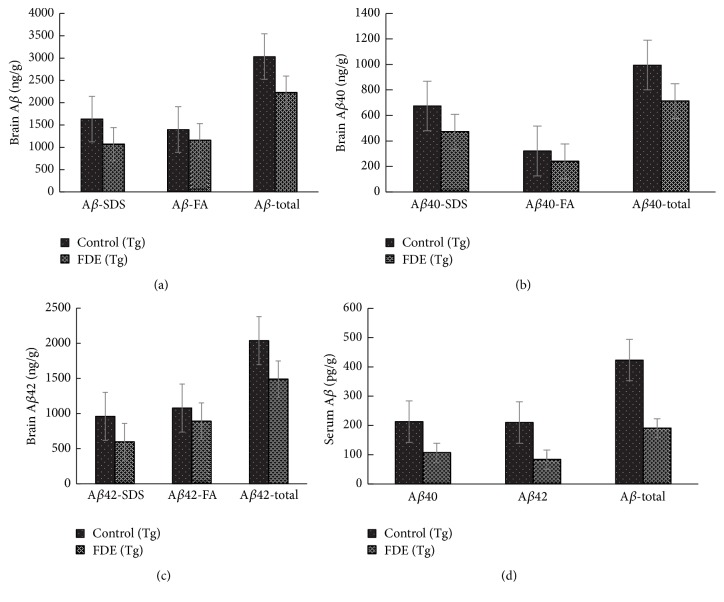
The total number of Aβ plaques stained by immunohistochemistry and Congo red in the brain was, respectively, reduced by 57% and 49% (Figures 5(c) and 5(f)); FDE treatment group had a significantly lower Aβ plaques numbers than control group fed with normal diet (ANOVA, F = 5.420, p < 0.05, and ANOVA, F = 6.480, p < 0.05). We further examined the brain and plasma Aβ levels by ELISA tests; the levels of total Aβ40 and Aβ42 in SDS and FA fractions of brain homogenates of FDE treatment group were significantly declined compared to control group (Tg) (ANOVA, F = 71.044, p < 0.05; ANOVA, F = 15.481, p < 0.001; ANOVA, F = 8.425, p < 0.05); the levels of Aβ40 and Aβ42 in formic acid (FA) also showed significant decline (Figures 6(b) and 6(c), p < 0.05). In addition, the total Aβ level in plasma of FDE treatment group obviously reduced by 55% (Figure 6(d)), which correlated with the change tendency of total brain Aβ levels (Pearson r = 0.883, p < 0.05).
Figure 6.
Effects of FDE consumption on Aβ levels in the brain and serum of APP/PS1 transgenic mice. (a) Comparison of total Aβ, Aβ in SDS fraction (Aβ-SDS), and Aβ in formic acid fraction (Aβ-FA) among groups. (b) Comparison of total Aβ40, Aβ40-SDS, and Aβ 40-FA. (c) Comparison of total and Aβ42-FA. (d) Comparison of total, Aβ42, Aβ42-SDS Aβ 40, and Aβ 42 in serum. Denote p < 0.05 or p < 0.01 versus APP/PS1 transgenic mice fed with control diet. Scale bar, 1 mm.
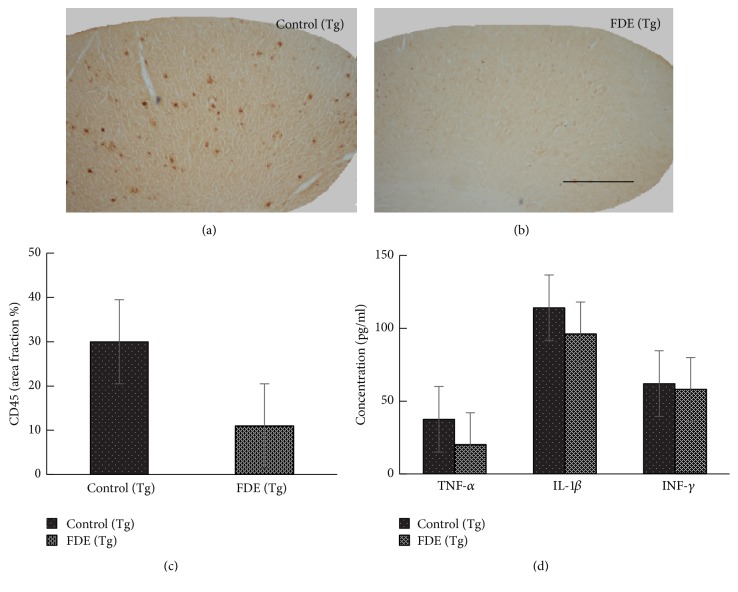
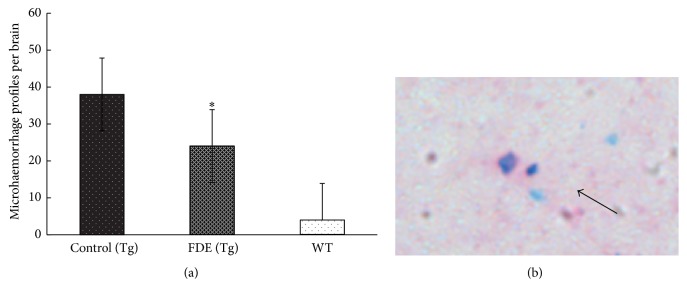
Activated microglia are associated with the progression of Alzheimer's disease (AD); therefore microglia are key targets for therapeutic intervention [14]. Here, we examined the activated microglia with rat monoclonal anti-CD45 in the neocortical and hippocampal areas of the brain; the levels of activated microglia in FDE treatment group were obviously decreased compared to the transgenic mice fed with normal diet (11 ± 3 versus 30 ± 4.5, p < 0.001, Figure 7(c)). Moreover, we measured the levels of IL-1β, TNF-α, and IFN-γ in the plasma; ELISA assay showed that the level of proinflammatory cytokine TNF-α in serum in the FDE treatment group was lower than APP/PS1 control group (36.7 ± 4.3 versus 20 ± 2.7, p < 0.05, Figure 7(d)), but no significantly lower levels of IL-β and IFN-γ were observed in FDE treatment group. In Addition, characteristic blue haemosiderin-positive profiles on the stained brain slices were observed. The microhaemorrhage rates in the FDE group were detected at a rate of 24.5 ± 3.6 per hemibrain (p < 0.05) which was lower than Control group fed with normal diet (38.0 ± 6 per hemi-brain, p < 0.05, Figure 8).
Figure 7.
FDE attenuates inflammation in APP/PS1 transgenic mice. (a–c) Representative images of staining in APP/PS1. No obvious microgliosis was observed in the brain of FDE treatment transgenic mice. (c) Comparison of CD45 area fraction in neocortex among groups. (d) Quantification of IL-1β, IL-6, TNF-α, and IFN-γ in plasma. Denote p < 0.05 or p < 0.01 versus control (Tg) mice, as determined by one-way ANOVA. Scale bar, 1 mm.
Figure 8.
Effects of FDE treatment on microhaemorrhage profiles in the brain. (a) Comparison of microhaemorrhage profiles; denote ∗p < 0.05 versus wild-type littermate fed with control diet. (b) An example of microhaemorrhage profile (solid arrow) observed in neocortex.
6. Discussion
The present results showed the neuroprotective ability of FDE on AD-like symptoms in transgenic mice. Besides, the antiamyloidogenic effects of FDE in vitro were detected in this study. To our knowledge, this is the first time to report that polyphenol extract of Fagopyrum dibotrys has preventive effects on an animal model of AD pathology.
Our data demonstrate that FDE cleared Aβ deposits that appeared in the brain and decreased Aβ from the plasma, reduced microhaemorrhage, decreased reactive microglia in brain, and reduced the TNF-α level in serum. But, we did not observe FDE reverse deficits in motor function and spatial working memory in the Morris water maze test (result is not shown).
Other putative agents tested for enhancement of cognition in animal models can improve memory. For example, blueberry appears to have a pronounced effect on short term memory and has been shown to improve long-term reference memory following 7 weeks of supplementation [15]; the flavonoid-rich plant extract of Ginkgo biloba has also been shown to induce positive effects on memory, learning, and concentration [16]. The differences in effects on memory between these results and those in the present manuscript may be attributed to different strains of mice tested or different chemical structures of the compounds tested. Hence, the beneficial effects and mechanisms of polyphenols on memory deficits caused by Aβ need further research [17].
Amyloid β (Aβ) is toxic to neurons and is associated with multiple pathogenic cascades. Inhibition of Aβ aggregation or disaggregation of Aβ fibrils are important strategies for prevention and treatment of AD. In this study, we found that FDE significantly attenuated Aβ42 fibril-induced toxicity on SH-SY5Y cells, inhibited Aβ1–42 fibril formation in all tested concentrations, consistent with the effects of other polyphenols contained in grape seed extract, wine, bilberry, and blackcurrant, which have been shown to destabilize the preformed Aβ fibrils in vitro, and exhibited antiamyloidogenic activity [18].
New research suggests that pathological accumulation of Aβ is a key factor that drives neuroinflammatory responses in Alzheimer's disease; these Aβ aggregates bind to cell-surface receptors on microglia, inducing an inflammatory activation that results in the secretion of proinflammatory cytokines [19], including TNF-α, interleukin 1β, and IFN-γ. Overexpression of inflammatory cytokines has been shown to increase tau pathology [20]. Our data showed the plasma level of TNF-α and reactive microglia are decreased after FDE treatment, indicating that the FDE can modulate neuroinflammation and alleviate harmful effects formed under pathological conditions. Cerebral amyloid angiopathy (CAA) has been recognized as one of the morphologic hallmarks of Alzheimer disease (AD), resulting from deposition of β-amyloid in the media and adventitia of small arteries and capillaries of the leptomeninges and cerebral cortex, which is associated with cerebral microbleeds and white matter hyperintensities [21]. The present study found that FDE is beneficial in ameliorating cerebral microbleeds, possibly a result of clearance of the 12 amyloid-β peptide from the brain. This finding may suggest FDE's potential as preventive agent for cerebral amyloid angiopathy (CAA).
In conclusion, the present study showed that the oral administration of FDE prevented the development of AD pathology in an animal model by targeting multiple pathways. It provides a novel suggestion that FDE might show promise in the prevention or treatment of AD.
Acknowledgments
This work was supported by grants from National Natural Science Foundation (81441136) and “Innovation Team Development program of the Ministry of Education of China,” the State Key Laboratory of Stem Cell and Regenerative Medicine of Yunnan Province.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Chen Liang and Jian-Ping Yuan contributed equally to this work.
References
- 1.Querfurth H. W., LaFerla F. M. Alzheimer's disease. The New England Journal of Medicine. 2010;362(4):329–344. doi: 10.1056/nejmra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Heneka M. T., O'Banion M. K. Inflammatory processes in Alzheimer's disease. Journal of Neuroimmunology. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Li A. R. Flora of China. Vol. 25. Beijing, China: Science Press and the Missouri Botanical Garden Press; 2003. Fagopyrum. [Google Scholar]
- 4.Manandhar N. P., Manandhar S. Plants and People of Nepal. London, UK: Timber Press; 2002. [Google Scholar]
- 5.Editorial Board of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine. Zhong Huaen Cao. Vol. 2. Shanghai, China: Shanghai Science and Technology Press; 1999. [Google Scholar]
- 6.Mori T., Koyama N., Guillot-Sestier M.-V., Tan J., Town T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE. 2013;8(2):16. doi: 10.1371/journal.pone.0055774.e55774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng Y.-Q., Wang Y.-J., Zhou X.-F. Effects of (-)epicatechin on the pathology of APP/PS1 transgenic mice. Frontiers in Neurology. 2014;5, article 69 doi: 10.3389/fneur.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasinetti G. M., Wang J., Ho L., Zhao W., Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer's disease prevention and treatment. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2014;1852(6):1202–1208. doi: 10.1016/j.bbadis.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey K. B., Rizvi S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlgren K. N., Manelli A. M., Blaine Stine W., Jr., Baker L. K., Krafft G. A., Ladu M. J. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. Journal of Biological Chemistry. 2002;277(35):32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 11.Jankowsky J. L., Slunt H. H., Ratovitski T., Jenkins N. A., Copeland N. G., Borchelt D. R. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular Engineering. 2001;17(6):157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.-J., Wang X., Lu J.-J., et al. p75NTR regulates Aβ deposition by increasing Aβ production but inhibiting Aβ aggregation with its extracellular domain. Journal of Neuroscience. 2011;31(6):2292–2304. doi: 10.1523/JNEUROSCI.2733-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frid P., Anisimov S. V., Popovic N. Congo red and protein aggregation in neurodegenerative diseases. Brain Research Reviews. 2007;53(1):135–160. doi: 10.1016/j.brainresrev.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.McGeer P. L., McGeer E. G. Targeting microglia for the treatment of Alzheimer's disease. Expert Opinion on Therapeutic Targets. 2015;19(4):497–506. doi: 10.1517/14728222.2014.988707. [DOI] [PubMed] [Google Scholar]
- 15.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J. P. E. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes and Nutrition. 2008;3(3-4):115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ude C., Schubert-Zsilavecz M., Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clinical Pharmacokinetics. 2013;52(9):727–749. doi: 10.1007/s40262-013-0074-5. [DOI] [PubMed] [Google Scholar]
- 17.Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Medicine and Cellular Longevity. 2012;2012:16. doi: 10.1155/2012/914273.914273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi T., Ono K., Murase A., Yamada M. Phenolic compounds prevent Alzheimer's pathology through different effects on the amyloid-β aggregation pathway. American Journal of Pathology. 2009;175(6):2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heneka M. T., Carson M. J., El Khoury J., et al. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/s1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S., Wu M. D., Shaftel S. S., et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an alzheimer's mouse model. Journal of Neuroscience. 2013;33(11):5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viswanathan A., Greenberg S. M. Cerebral amyloid angiopathy in the elderly. Annals of Neurology. 2011;70(6):871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]