Abstract
Objectives:
This study assessed the efficacy and safety of plecanatide, a guanylate cyclase-C (GC-C) agonist and the first uroguanylin analog approved for the treatment of chronic idiopathic constipation (CIC).
Methods:
This phase III, multicenter, double-blind, placebo-controlled study randomized 1,394 patients with CIC. Patients received either plecanatide (3 or 6 mg) or placebo, orally, once daily, for 12 weeks. The primary efficacy endpoint was the percentage of patients who were durable overall complete spontaneous bowel movement (CSBM) responders over the 12-week treatment period. Patients were instructed to record their daily bowel movements, stool consistency scores, and abdominal symptoms in an electronic diary. Treatment-emergent adverse events (AEs) were collected.
Results:
Each dose of plecanatide resulted in a significantly greater percentage of durable overall CSBM responders (21.0%, 3 mg; 19.5%, 6 mg) as compared with placebo (10.2% P<0.001 for both). Plecanatide (3 and 6 mg) also significantly increased mean weekly CSBM frequency from baseline (increase of 2.5 and 2.2/week, respectively) vs. placebo (1.2/week; P<0.001 for both) and mean weekly spontaneous bowel movement frequency (increase of 3.2 and 3.1/week, respectively) vs. placebo (1.3/week; P<0.001, for both) over the 12-week treatment period. Both plecanatide doses significantly improved all secondary and additional efficacy endpoints. The most common AE, diarrhea, occurred in 1.3% (placebo), 5.9% (3 mg) and 5.7% (6 mg) of patients.
Conclusions:
Plecanatide significantly improved constipation and its related symptoms with a low rate of adverse events. These results suggest that plecanatide will be a useful treatment option in the management of CIC. ClinicalTrials.gov: NCT01982240.
Introduction
Chronic idiopathic constipation (CIC) is one of the most common functional gastrointestinal (GI) disorders, with prevalence rates ranging from 2 to 27%, and an overall average prevalence of 14.8% (1, 2). For several years, CIC had been defined by a single symptom, the frequency of bowel movements (BMs). Over time, however, that definition has expanded to include the additional symptoms of straining, lumpy or hard stools, the sensation of incomplete BMs, and abdominal symptoms such as bloating and abdominal discomfort. Individual symptoms can be severe, adversely affecting patients' quality of life and elevating health care costs (3). Patients with CIC often report dissatisfaction with traditional treatment options, such as dietary fiber with supplemental bulking agents, exercise, bowel habit training, and over-the-counter laxatives (4). Current guidelines suggest that constipated patients should be initially treated with over-the-counter laxatives for episodic constipation (5, 6). More recently, a number of prescription pharmacotherapies have been approved in the United States for the treatment of patients with CIC, including linaclotide (Linzess, Ironwood Pharmaceuticals, Inc., Cambridge, MA, USA) and lubiprostone (Amitiza, Takeda Pharmaceutical Company, Osaka, Japan) (7, 8). However, no single or combined treatment has been shown to work in all patients and thus there remains a need for new treatment options (9).
Plecanatide is a 16-amino acid peptide analog of uroguanylin. Uroguanylin is an endogenous agonist that binds and activates guanylate cyclase-C (GC-C) receptors expressed in the epithelial lining of the GI mucosa in a pH-sensitive manner (10, 11). The sole difference between the two peptides is the replacement of one pH-sensing residue with another, Asp with Glu, at the third position near the N-terminus (12, 13). Therefore, plecanatide, like uroguanylin, binds to and activates GC-C receptors in a pH-sensitive manner and has demonstrated eight times the binding potency of uroguanylin in preclinical models (14). GC-C receptor activation stimulates cyclic guanosine monophosphate production, which increases cystic fibrosis transmembrane conductance regulator activity (15), leading to chloride and bicarbonate secretion into the intestinal lumen. In addition, activation of GC-C signaling decreases the activity of the sodium-hydrogen exchanger, leading to decreased sodium absorption (16). The resulting ionic gradient allows for fluid secretion that serves to hydrate the stool and facilitate BMs (17). GC-C activation also decreases other CIC-related symptoms by decreasing visceral hypersensitivity to relieve abdominal discomfort and to accelerate stool transit through the intestine, facilitating BMs (18, 19, 20, 21, 22, 23).
Previous studies in healthy volunteers and in patients with CIC have demonstrated plecanatide to be safe and effective in relieving the symptoms of CIC (12, 24). The objective of the present study was to assess, on a larger scale, the efficacy and safety of two plecanatide doses when administered to patients with CIC for 12 weeks.
Methods
Study design
This study (NCT01982240) was randomized, 12 weeks in duration, double-blinded and placebo-controlled. Patients with CIC (N=1,394) were randomized at one of 164 clinical centers (153 in the United States and 11 in Canada) between 3 December 2013 and 23 April 2015 (last patient last visit). Informed consent was obtained from each patient before admission into the study and initiation of any study-related procedures, according to the regulatory and legal requirements of the participating country (US or Canada). The terms of the consent and when it was obtained were also documented. Each site-specific investigator and coordinator recruited and enrolled participants. The study was conducted in accordance with the International Conference on Harmonisation E6 Consolidated Guidance for Good Clinical Practice, the United States Code of Federal Regulations Title 21, Parts 50 and 56, and the ethical principles of the Declaration of Helsinki (as amended in 1996). No important changes to methods were made after the trial was commenced. All authors had access to the study data and had reviewed and approved the final manuscript.
Following informed consent, patients entered a screening period. The last 2 weeks of the screening period consisted of a pre-treatment assessment period to confirm eligibility and establish each patient's baseline for efficacy outcome measurements. Patients were instructed to use an electronic diary to maintain a record of Daily BMs (number, time, consistency, completeness of evacuation, and rescue medication use) and Daily Symptoms. Recordings were to be made for each day, with no allowance for returning to complete data for previous days. To maintain eligibility for participation in the trial, patients were required to complete 6 of the 7 required daily diary entries (among other criteria) in each of the two pre-treatment assessment weeks.
Patients who maintained eligibility at the end of the 2-week pre-treatment assessment were randomized on day 1 of the 12-week treatment period in a 1:1:1 ratio (stratified by gender) to one of the following three treatment groups: plecanatide 3 mg, plecanatide 6 mg, or placebo. Randomization was dynamic by site, with each site initially assigned two randomization blocks (one for each gender) of 9 per strata. At weeks 4, 8, and 12 of the treatment period and 2 weeks following the last dose of medication (week 14), patients returned to the clinic to undergo efficacy and safety assessments. Patients continued to complete daily diary entries throughout the treatment and post-treatment periods.
Patient population
Eligible for inclusion were male and female (not pregnant or lactating) patients with CIC aged 18–80 years who had body mass index of 18–40 kg/m2 and were willing to participate in the 2-week pre-treatment assessment, 12 weeks of treatment, and a 2-week post-treatment period. Patients had to meet the Rome III functional constipation criteria, modified for this study, for at least 3 months before the screening visit and had to demonstrate symptom onset for at least 6 months before the diagnosis, which included a history of fewer than three BMs per week, no use of manual maneuvers (such as digital evacuations or support of pelvic floor) to facilitate defecations, and at least two of the following: straining during at least 25% of defecations, lumpy or hard stool for at least 25% of defecations, sensation of incomplete evacuation for at least 25% of defecations, and sensation of anorectal blockage/obstruction for at least 25% of defecations (25). Patients were excluded if they met the Rome III criteria for irritable bowel syndrome or if they reported loose stool more than rarely without the use of laxatives. Other key exclusion criteria were diseases or conditions associated with constipation, diseases or conditions that could affect GI motility or defecation, medical history of cancer, or other uncontrolled medical conditions. Patients were to maintain a stable diet for at least 30 days prior to screening, use contraception where applicable, and were not to have participated in a previous plecanatide clinical trial. Patients were allowed to continue the use of fiber if they were using a high fiber diet or fiber supplements for the 30 days before screening and could enroll provided that they agreed to remain on that diet or supplement for the duration of the study. Following the completion of the 2-week pre-treatment assessment, patients had to meet the following criteria before randomization: less than 3 complete spontaneous bowel movements (CSBMs) per week, scores on the Bristol Stool Form Scale (BSFS) of 6 or 7 in less than 25% of spontaneous bowel movements (SBMs), and one of the following: BSFS of 1 or 2 in at least 25% of defecations, a straining value reported on at least 25% of days on which a BM was recorded, or at least 25% of BMs resulting in a sense of incomplete evacuation. A full listing of inclusion and exclusion criteria is listed in Supplementary Appendix A online.
Treatments
Patients were randomly assigned to one of the three treatment groups (plecanatide 3 mg, plecanatide 6 mg, or placebo) utilizing a web-based randomization and trial supply management (RTSM) system. All treatments were given orally, once daily from day 1 through 12 weeks of the treatment period. Patients received their assigned study drug on the day of randomization (day 1 of week 1), took their first dose at the clinic site, and were instructed to take their study medication on a daily basis with or without food. At the day 1, week 4, and week 8 visits, the site performed drug-dispensing activities by logging into the RTSM system to get a study drug kit allocation for each patient. No interruptions in daily therapy were permitted. Compliance was assessed by pill count, with patients who had taken at least 80% of assigned doses to be considered compliant. All study drugs were supplied in identical blister packs, and tablets were similar in color, smell, taste, and appearance, thereby assuring double-blind conditions for all investigators and patients. Patients agreed to maintain a stable diet and fluid intake over the course of the study. Patients were provided bisacodyl 5 mg tablets as rescue medication and were instructed to take 1 or 2 tablets only if they had not had a BM for 3 or more days. During the pre-treatment assessment period, patients were not to exceed 2 days of rescue medication use in each week. BMs occurring within 24 h of rescue medication use were not counted towards the SBM or CSBM frequency endpoint. In the event of an emergency, the investigator and sub-investigators at the clinical site had the ability to break the treatment code using the RTSM system. No break of the treatment code occurred in this study.
Assessments and endpoints
Patients were required to report all BMs in the BM Diary in real time or on a daily basis, indicating the time of the BM, with no ability to report data from the previous day. The primary efficacy endpoint was the percentage of patients who were durable overall CSBM responders during the 12-week treatment period. A CSBM weekly responder was defined as a patient who had ≥3 CSBMs for a given week and an increase from baseline of ≥1 CSBM for that same week. An overall CSBM responder was a patient who was a weekly CSBM responder for at least 9 of the 12 treatment weeks, and a durable overall CSBM responder was also a weekly responder in at least 3 of the last 4 weeks. An SBM was defined as a BM that occurs in the absence of laxative use (for example, rescue medication; as entered in the diary) within 24 h of the BM, and a CSBM was defined as an SBM with the sense of complete evacuation. Secondary and additional endpoints reported from the BM Diary included frequency of CSBMs and SBMs within 24 h after the first dose of study medication and stool consistency from the BSFS score for each BM. No changes in trial outcomes were made.
The Daily Symptom Diary was completed for additional endpoints. This electronic diary was completed each day in the evening and captured straining, abdominal bloating, and abdominal discomfort on a Likert scale of 0–4 (0=none, 4=very severe). The Patient Assessment of Constipation–Symptoms (PAC-SYM) and Patient Assessment of Constipation–Quality of Life (PAC-QoL) questionnaires, as well as the Patient Global Assessment questionnaire, were also completed at the clinic at weeks 4, 8, 12 (end of treatment), and 14 (end of study).
Safety evaluations included physical examinations, electrocardiograph recordings, vital sign measurements, and standard laboratory tests. Adverse events (AEs) were captured, assessed for severity, and classified for relatedness to study medication.
Statistical analysis
The intention-to-treat population was used for the efficacy analyses. The safety population (defined as all randomized patients who received at least one dose of the study drug) was used for safety analyses.
The primary efficacy endpoint was the percentage of patients who were durable overall CSBM responders. The Cochran–Mantel–Haenszel test, stratified by gender, was used to test a hierarchical comparison between plecanatide 6 mg and placebo and between plecanatide 3 mg and placebo. For this analysis, patients who had fewer than four complete diary days were considered non-responders. The Holm-based tree-gatekeeping procedure was used for adjustment of P values to control the family-wise type I error rate at 5% (two-sided) by taking into account multiple doses and multiple primary endpoints.
For secondary efficacy endpoints, treatment comparisons of changes from baseline were analyzed using a linear mixed-effects model with fixed effects for gender (stratification variable), treatment, week, the interaction of treatment and week, and the corresponding baseline value and random intercept for patients.
Treatment comparisons of changes from baseline for each patient-reported daily symptom were made using a linear mixed-effects model under the assumption of normally distributed residuals with treatment group, week, interaction of treatment and week, gender, and the corresponding baseline value as fixed effects, and random intercept for patient. Comparisons of patients' symptoms, measured using PAC-SYM and PAC-QoL questionnaires, were made of changes from baseline for the total score between each plecanatide treatment and placebo using an analysis of covariance (ANCOVA) linear mixed-effects model with fixed effects for gender (stratification variable), treatment, week, the interaction of treatment and week, and the corresponding baseline value, and a random intercept for patient.
The sample size for this study was based on results of a previously completed large, multicenter, 12-week dose-ranging study of plecanatide in patients with CIC and on consideration of overall safety exposure requirements (26). The power calculation for the primary endpoint assumed that the durable overall responder rates for plecanatide 3 and 6 mg were equal. Using these assumptions and based on a χ2 continuity-corrected test with the intention of providing ~90% power at 5% significance level, enrollment of at least 450 patients per treatment arm was required. Assuming a 50% screen failure and discontinuation rate, 2,864 patients were screened. No interim analyses were planned or conducted in this study.
Results
Patient disposition, compliance, and baseline demographics
Of the 2,864 patients screened (Figure 1), 1,346 comprised the intention-to-treat population (plecanatide 3 mg, n=453; plecanatide 6 mg, n=441; placebo, n=452). The safety population included the 1,389 patients who received at least one dose of study drug. There were 1,153 patients (82.7%) who completed treatment (plecanatide 3 mg, n=390; plecanatide 6 mg, n=375; placebo, n=388), and 1,140 patients (81.8%) completed the study (through week 14). The majority of patients in the intention-to-treat population were compliant with study drug, with compliance defined as 80% of assigned doses and calculated from returned pill counts. Medication compliance was comparable across all groups (plecanatide 3 mg, 96.5% plecanatide 6 mg, 96.6% and placebo, 98.0%). Demographic characteristics of the study population were comparable and balanced across the three treatment groups (Table 1). The proportion of females (80.8%) and those identifying as black/African American (25.6%) were comparable to those found in the general CIC patient population (2, 4).
Figure 1.
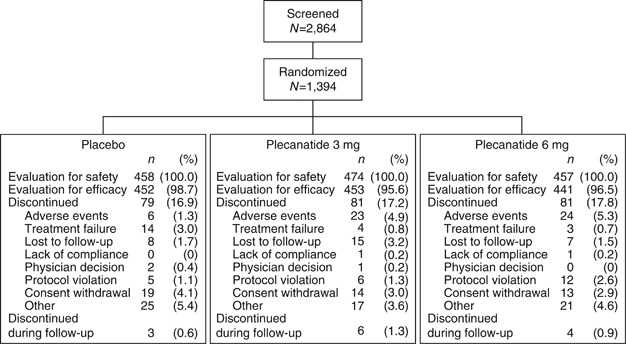
Disposition of the study population.
Table 1. Summary of demographics and baseline patient characteristicsa.
| Placebo (N=452) | Plecanatide 3 mg (N=453) | Plecanatide 6 mg (N=441) | |
|---|---|---|---|
| Age (years) | 46.4 | 45.0 | 45.1 |
| Range | (18–78) | (18–79) | (18–79) |
| Gender (% female) | 79.0 | 81.2 | 82.1 |
| Race (% of population) | |||
| White | 71.5 | 66.7 | 68.5 |
| Black | 23.9 | 28.5 | 24.5 |
| Other | 4.6 | 4.8 | 7.0 |
| Body mass index | 28.1±5.3 | 28.1±5.3 | 28.2±5.3 |
| CSBMs/week | 0.4±0.6 | 0.3±0.5 | 0.3±0.5 |
| SBMs/week | 2.2±2.0 | 2.0±1.8 | 1.8±1.8 |
| Stool consistencyb | 2.6±1.1 | 2.5±1.1 | 2.6±1.2 |
| Straining scorec | 2.3±0.8 | 2.3±0.8 | 2.3±0.9 |
| Abdominal discomfort scored | 1.8±0.9 | 1.8±0.8 | 1.8±0.9 |
| Abdominal bloating scoree | 1.9±0.9 | 1.9±0.9 | 1.9±1.0 |
CSBM, complete spontaneous bowel movement; ITT, intention-to-treat; SBM, spontaneous bowel movement.
Values are mean±s.d. in the ITT population unless otherwise stated.
Stool consistency was assessed with the use of the 7-point Bristol Stool Form Scale, where 1 indicates separate, hard lumps, like nuts (hard to pass); 2 sausage-shaped but lumpy; 3 like a sausage but with cracks on the surface; 4 like a sausage or snake, smooth and soft; 5 soft blobs with clear-cut edges (passed easily); 6 fluffy pieces with ragged edges or a mushy stool; and 7 watery, no solid pieces (entirely liquid).
Assessed using the Daily Symptom Diary; patients who indicated having a bowel movement for that day were asked: For today, when you had a bowel movement, rate your straining at its worst on a scale of 0 to 4. Straining was rated on a 5-point Likert scale where 0=none, 1=mild, 2=moderate, 3=severe, and 4=very severe.
Assessed using the Daily Symptom Diary; patients were asked: For today, rate your abdominal discomfort at its worst on a scale of 0 to 4. Abdominal discomfort was rated on a 5-point Likert scale where 0=none, 1=mild, 2=moderate, 3=severe, and 4=very severe.
Assessed using the Daily Symptom Diary; patients were asked: For today, rate your abdominal bloating at its worst on a scale of 0 to 4. Abdominal bloating was rated on a 5-point Likert scale where 0=none, 1=mild, 2=moderate, 3=severe, and 4=very severe.
Efficacy measures
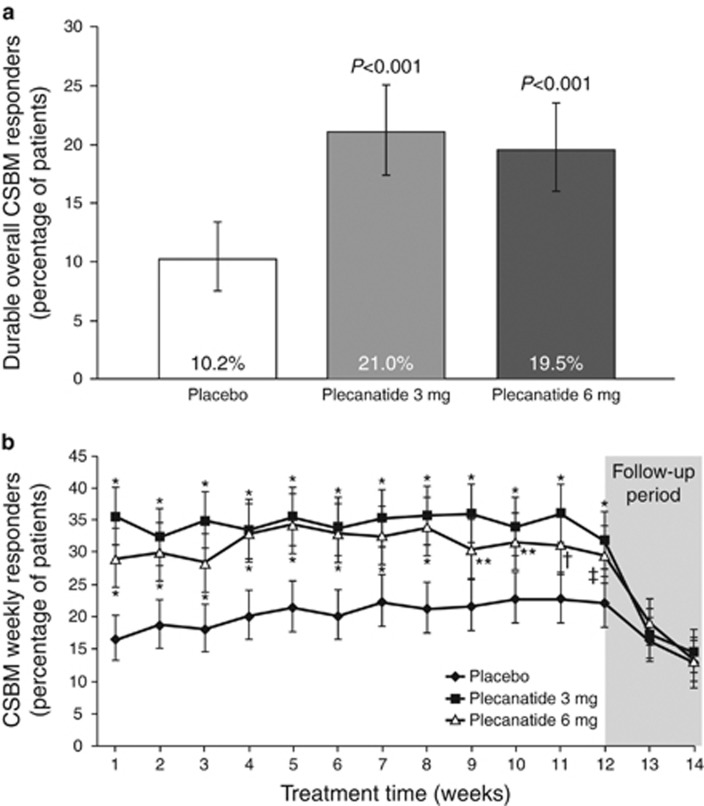
The primary efficacy measure was achieved with both plecanatide doses (Figure 2a). Both plecanatide 3 and 6 mg resulted in a significantly greater percentage of patients who were durable overall CSBM responders compared with those in the placebo group (plecanatide 3 mg, 21.0% plecanatide 6 mg, 19.5% placebo, 10.2% P<0.001 for each drug dose vs. placebo). The percentage of weekly CSBM responders in both plecanatide groups was greater than with placebo within the first week of treatment (plecanatide 3 mg, 35.8% plecanatide 6 mg, 29.3% placebo, 16.6% P<0.001 for each drug dose vs. placebo), and this difference was maintained for the duration of the 12-week treatment period (Figure 2b). Following cessation of plecanatide administration (follow-up period), the proportions of CSBM weekly responders in both plecanatide dose groups decreased and were comparable with placebo.
Figure 2.
(a) Percentage of patients in each treatment group assessed as a durable overall complete spontaneous bowel movement (CSBM) responder in the intention-to-treat (ITT) population, the primary efficacy endpoint. Durable overall CSBM responders were defined as patients who fulfilled both ≥3 CSBMs per week and an increase of ≥1 CSBM from baseline, in the same week, for ≥9 of the 12 treatment weeks, including ≥3 of the last 4 weeks of treatment. Error bars represent 95% confidence intervals. (b) Weekly evolution of the percentage of CSBM responders in the ITT population. Values are LS means; bars represent 95% confidence intervals. *P=0.001, **P=0.003, †P=0.005, ‡P=0.011 vs. placebo.
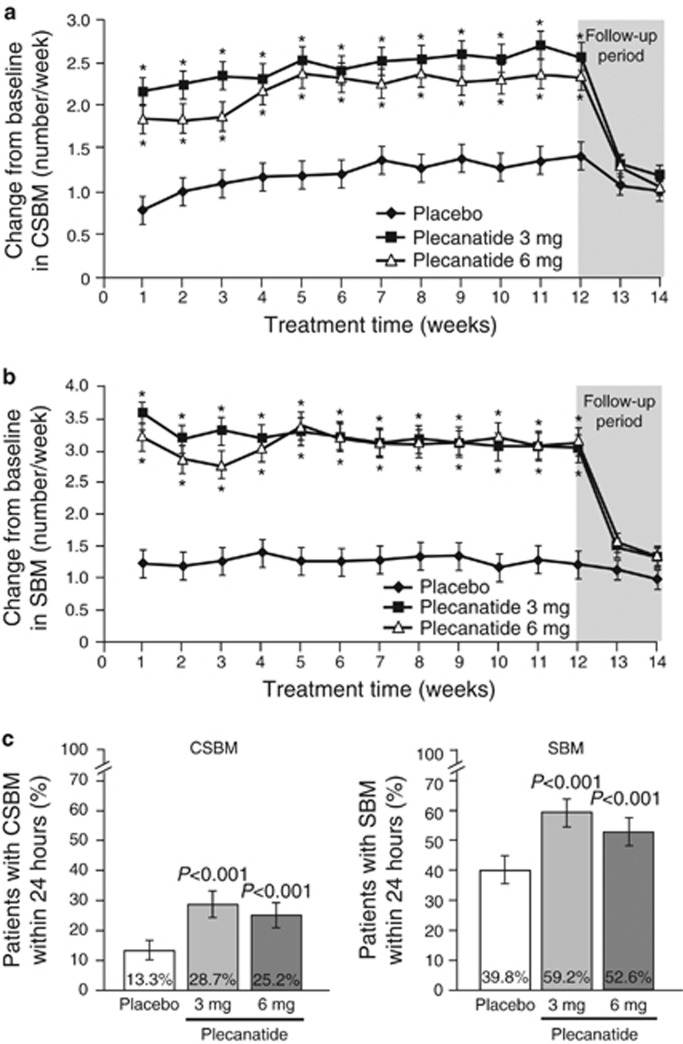
Both plecanatide doses significantly increased the weekly CSBM and SBM frequencies from baseline (Figure 3a,b). Increases from baseline were evident within the first week of treatment and were statistically different from the increase resulting from placebo treatment. These statistically significant increases were maintained throughout the duration of treatment. Over the 12-week treatment period, there were clinically and statistically significant least squares (LS) mean changes from baseline in weekly CSBM frequencies with 3- and 6-mg doses of plecanatide (2.5 and 2.2/week, respectively) as compared with placebo (1.2/week; P<0.001 for each dose). By week 14, 2 weeks following the cessation of treatment, the values for plecanatide treatment returned toward those of placebo treatment and did not go lower than baseline levels. Statistically significant changes in SBMs/week, similar to the patterns of change in CSBMs/week, also resulted with both doses of plecanatide. The LS mean increase in weekly SBM frequency over the 12-week treatment period was 3.2 and 3.1 for plecanatide 3 and 6 mg, respectively, and 1.3/week for placebo (P<0.001 for each dose compared with placebo). The onset of plecanatide activity was rapid and occurred within the first week of treatment. Both doses of plecanatide statistically increased the percentage of patients experiencing CSBMs and SBMs within 24 h compared with placebo (Figure 3c).
Figure 3.
(a) Changes from baseline in weekly complete spontaneous bowel movement (CSBM) frequency. Values are least squares (LS) mean; bars represent s.e. *P<0.001 vs. placebo. (b) Changes from baseline in mean weekly spontaneous bowel movement (SBM) frequency. Values are LS mean; bars represent s.e. *P<0.001 vs. placebo. (c) Percentage of patients experiencing a CSBM or SBM within 24 h after the first dose of study medication. *P<0.001 vs. placebo.
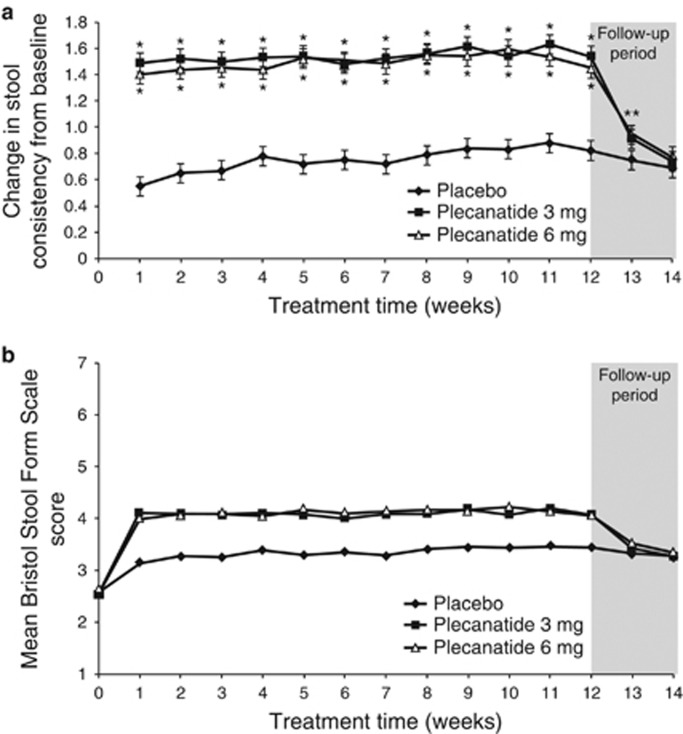
Plecanatide significantly improved stool consistency and symptom-related secondary endpoints over the 12-week treatment period compared with placebo (Figure 4a,b and Table 2). Stool consistency improved from baseline with both plecanatide doses by 1.5 points on the BSFS scale over the 12-week treatment period as compared with 0.8 points for placebo (P<0.001 for each dose compared with placebo). These improvements resulted in BSFS stool scores of 4.1 (mean) over 12 weeks for both the plecanatide 3- and 6-mg doses.
Figure 4.
(a) Changes from baseline in weekly stool consistency. Stool consistency was measured by the Bristol Stool Form Scale (BSFS). Values are least squares (LS) mean; bars represent s.e. *P<0.001 vs. placebo. (b) Weekly BSFS scores during the study. Values are LS mean.
Table 2. Changes from baseline in stool consistency and symptoms associated with treatment over the 12-week treatment perioda.
| Placebo (N=452) | Plecanatide 3 mg (N=453) | P valueb | Plecanatide 6 mg (N=441) | P valueb | |
|---|---|---|---|---|---|
| Stool consistency | |||||
| Baseline | 2.6 (1.1) | 2.5 (1.1) | – | 2.6 (1.2) | – |
| Change | 0.8 (0.1) | 1.5 (0.1) | <0.001 | 1.5 (0.1) | <0.001 |
| Daily symptoms scores | |||||
| Strainingc | |||||
| Baseline | 2.3 (0.8) | 2.3 (0.8) | – | 2.3 (0.9) | – |
| Change | −0.6 (0.0) | −0.9 (0.0) | <0.001 | −0.9 (0.0) | <0.001 |
| Abdominal bloatingc | |||||
| Baseline | 1.9 (0.9) | 1.9 (0.9) | – | 1.9 (1.0) | – |
| Change | −0.4 (0.0) | −0.5 (0.0) | 0.002 | −0.4 (0.0) | 0.045 |
| Abdominal discomfortc | |||||
| Baseline | 1.8 (0.9) | 1.8 (0.9) | – | 1.8 (0.9) | – |
| Change | −0.4 (0.0) | −0.5 (0.0) | <0.001 | −0.5 (0.0) | 0.014 |
| Patient global assessmentsd | |||||
| Constipation severitye | |||||
| Baseline | 3.5 (0.9) | 3.6 (0.9) | – | 3.5 (1.0) | – |
| Change | −1.0 (0.1) | −1.4 (0.1) | <0.001 | −1.4 (0.1) | <0.001 |
| Treatment satisfaction scoref | 2.8 (0.1) | 3.6 (0.1) | <0.001 | 3.5 (0.1) | <0.001 |
| Treatment continuation scoref | 3.4 (0.1) | 3.8 (0.1) | <0.001 | 3.8 (0.1) | <0.001 |
LS, least squares.
Baseline values are mean (s.d.). Change values are LS mean (s.e.).
P value compared with placebo group.
The severity of straining, bloating, and discomfort during bowel movements was assessed on a 5-point Likert scale where 0=none, 1=mild, 2=moderate, 3=severe, and 4=very severe.
Assessments at week 12.
Assessments of constipation severity: patients were asked to rate their constipation severity on a 1–5 scale, with a higher score indicating more severe constipation.
Assessments of treatment satisfaction and continuation: patients were asked to rate how satisfied they were with treatment on a 1–5 scale, with a higher score indicating higher satisfaction. The same scoring system was used for assessing treatment continuation. Values are mean (s.e.).
Statistical improvements in mean weekly Daily Symptom Diary scores were observed with plecanatide treatment compared with placebo (Table 2). Improvements in straining score were observed early in treatment and were maintained throughout the study. The LS mean changes in weekly straining scores over the 12-week treatment period were −0.9 for plecanatide 3 mg and −0.9 for plecanatide 6 mg, while the placebo change was −0.6 (P<0.001 for each dose compared with placebo). The symptoms of abdominal bloating and abdominal discomfort were also significantly improved over the 12-week treatment period with plecanatide compared with placebo. The LS mean changes in abdominal bloating were −0.4 for placebo, −0.5 for plecanatide 3 mg (P=0.002), and −0.4 for plecanatide 6 mg (P=0.045), and the LS mean changes in abdominal discomfort were −0.4 for placebo, −0.5 for plecanatide 3 mg (P<0.001), and −0.5 for plecanatide 6 mg (P=0.014).
Several patient assessment tools demonstrated that plecanatide treatment, at both doses, significantly improved patient symptoms and health-related quality of life. Evaluation of Patient Global Assessments showed that constipation severity was significantly reduced with both plecanatide doses compared with placebo (Table 2). Furthermore, a significantly greater percentage of patients treated with plecanatide 3 mg (71.5%) and plecanatide 6 mg (68.7%) responded that they experienced reductions in their assessment of constipation severity when compared with placebo (56.9%). Patient satisfaction with treatment and their intention to continue treatment was statistically greater with the plecanatide treatment groups than with the placebo group (Table 2). Scores between the two doses were comparable. The LS mean improvement in PAC-SYM Total Score was −0.7 for placebo and −0.9 for both plecanatide doses, respectively (P<0.001 for each dose compared with placebo; negative values represent improvement). Similarly, PAC-QoL Total Score improved by −0.7 for placebo and −1.0 for both plecanatide doses, respectively (P<0.001 for each dose compared with placebo; negative values represent improvement).
Safety
Approximately one-third of patients experienced at least one AE during the course of the 12-week treatment period (plecanatide 3 mg, 35.4% plecanatide 6 mg, 33.0% placebo, 32.8% Table 3). The majority of AEs were mild to moderate in severity. A total of 15 patients (1.1%) experienced a serious AE (SAE) across the treatment groups, with comparable rates between treatments (Table 3); two of the reported SAEs (both in the plecanatide 3 mg group) were pregnancies (sites were instructed to capture all pregnancies as SAEs). Of the 13 actual SAEs, 4 occurred with plecanatide 3 mg, 5 with plecanatide 6 mg, and 4 with placebo group. SAEs in the system organ class of infections and infestations were reported by the highest percentage of patients: 2 (0.4%) in the plecanatide 6-mg group and 3 (0.7%) in the placebo group (none reported with plecanatide 3 mg). Of the 13 SAEs reported overall, only one event, diverticulitis (placebo group), was considered to be possibly related to study drug. The rate of discontinuing study medication due to an AE was 5.1% with plecanatide 3 mg, 5.3% with plecanatide 6 mg, and 1.3% with placebo. Rates of discontinuation due to diarrhea were 2.7% for plecanatide 3 mg, 2.6% for plecanatide 6 mg, and 0.4% for placebo. No unexpected safety signals were observed in this trial and no deaths were reported.
Table 3. Summary of TEAE.
| Placebo (N=458) | Plecanatide 3 mg (N=474) | Plecanatide 6 mg (N=457) | |
|---|---|---|---|
| Patients with at least one TEAE | 150 (32.8) | 168 (35.4) | 151 (33.0) |
| Patients with at least one severe TEAE | 7 (1.5) | 13 (2.7) | 17 (3.7) |
| Patients with at least one serious TEAE | 4 (0.9) | 6 (1.3)a | 5 (1.1) |
| Patients with at least one TEAE leading to discontinuation | 6 (1.3) | 24 (5.1) | 24 (5.3) |
| TEAEs with incidence in >2% of the plecanatide patients | |||
| Diarrhea | 6 (1.3) | 28 (5.9) | 26 (5.7) |
| Nasopharyngitis | 8 (1.7) | 4 (0.8) | 11 (2.4) |
| Sinusitis | 3 (0.7) | 10 (2.1) | 3 (0.7) |
TEAE, treatment-emergent adverse events.
Values are number of patients (%).
Abdominal pain did not exceed 2% of the plecanatide patients (placebo, 0.9% plecanatide 3 mg, 0.8% plecanatide 6 mg, 1.3%).
2 of the 6 serious adverse events occurring in the plecanatide 3 mg group were non-serious pregnancies; therefore, only 4 (0.8%) serious adverse events occurred in this treatment group.
The most common AE was diarrhea (Table 3), which was of mild to moderate severity in the majority of patients. Diarrhea occurred in 5.9% of patients treated with plecanatide 3 mg, 5.7% in patients treated with plecanatide 6 mg, and 1.3% of patients who received placebo. Diarrhea was followed in incidence by nasopharyngitis and sinusitis. No plecanatide dose dependency was observed for any AE. Overall, the incidence of AEs in any preferred term was low. Laboratory findings, vital signs, and physical examination were all unremarkable, with low incidence of any clinically important changes.
Discussion
This double-blind, phase III, randomized study in patients with CIC demonstrated the efficacy of plecanatide in the treatment of CIC. Plecanatide, at both doses tested, resulted in a significantly greater percentage of patients defined as durable overall CSBM responders versus placebo. In addition, plecanatide improved the frequency of CSBMs/week and SBMs/week, stool consistency, straining, other symptomatic endpoints associated with CIC, and health-related quality of life. Patients treated with plecanatide expressed satisfaction with their treatment and an intention to continue treatment. Plecanatide treatment was associated with a low frequency of AEs. The incidence of diarrhea was no more than 5.9% and no other clinically meaningful safety findings were observed.
Plecanatide is the first uroguanylin analog to be approved for the treatment of CIC or other functional GI disorders. It is also the first treatment for CIC to be evaluated in a clinical study using the stringent criteria of a durable overall CSBM responder rate. This endpoint requires a clinical response in at least 3 of the 4 last weeks of treatment in addition to the previously required 9 out of 12 weeks overall to evaluate the durability of efficacy. In meeting this revised regulatory endpoint, plecanatide has successfully demonstrated significant durable efficacy. In addition, plecanatide demonstrated a rapid onset of efficacy (within the first week of treatment) with a significant increase in the weekly CSBM responders as well as other secondary endpoints compared to placebo. Further, the rapid onset of effect was demonstrated with a statistically greater percentage of plecanatide patients vs. placebo patients who experienced CSBMs and SBMs within the first 24 h of treatment.
The secondary and additional endpoints in this study support the utility of plecanatide in the management of CIC. Plecanatide improved stool consistency to a mean BSFS score of 4 at each of the treatment visits throughout the 12-week treatment period. Straining, common to CIC, was also reduced, which may be related to the improvement in stool consistency. There was a statistically significant reduction in straining score with plecanatide vs. placebo (−0.9 for both plecanatide 3 and 6 mg; −0.6 for placebo; P<0.001 for both doses). Abdominal symptoms such as bloating and discomfort were also significantly reduced compared to placebo. These changes were associated with an overall improvement in constipation symptoms as observed by a reduction in the constipation severity score in the Patient Global Assessment questionnaire. In this study, the ability of plecanatide to improve symptoms was confirmed independently by the use of the PAC-SYM and PAC-QoL instruments.
All parameters assessed in this study improved within the first week of treatment and were maintained for as long as plecanatide was administered. Following discontinuation of plecanatide, no worsening of these parameters to below baseline levels was observed in the follow-up period.
Side effects associated with plecanatide treatment were minimal. Diarrhea was the most common side effect, observed in 5.9% (3 mg) and 5.7% (6 mg) of plecanatide-treated patients compared with placebo (1.3%). Furthermore, the rate of discontinuation of plecanatide treatment due to diarrhea was low (2.7% for plecanatide 3 mg, 2.6% for plecanatide 6 mg, and 0.4% for placebo). Although this study provides information about the short-term safety of plecanatide, a long-term, open-label study was also completed to examine the long-term safety profile of plecanatide.
Results from the Patient Global Assessment questionnaire's Treatment Satisfaction and Treatment Continuation questions indicate a greater overall satisfaction with plecanatide compared with placebo and a greater intention to continue treatment in patients receiving plecanatide.
As an analog of uroguanylin, plecanatide-mediated activation of GC-C receptors is pH-sensitive with higher activity in the slightly acidic environment of the small intestine than in the neutral or more basic environments of the distal GI tract (12). Activation of GC-C receptors leads to fluid secretion that serves to hydrate the stool and facilitates regular bowel function, as well as decreases visceral hypersensitivity, which can relieve abdominal discomfort and accelerate stool transit through the intestine. In addition, plecanatide is not absorbed into the systemic circulation (12); therefore, it exerts its biologic activity only in the intestinal tract. It is hypothesized here that the regulated (local) biologic activity, minimal absorption, and pH-sensitive GC-C receptor activation by plecanatide may contribute to the favorable efficacy profile and the low incidence of AEs (including diarrhea) observed in this trial.
Conclusion
Plecanatide, orally administered once daily for 12 weeks, significantly increased the percentage of patients with CIC defined as durable overall CSBM responders compared with placebo. Significant improvements in BM frequency and stool consistency were accompanied by significant improvements in straining and abdominal symptoms. Plecanatide was well tolerated, exhibiting a limited AE profile, including a low incidence of diarrhea. Plecanatide appears to be a promising new treatment for patients with CIC.
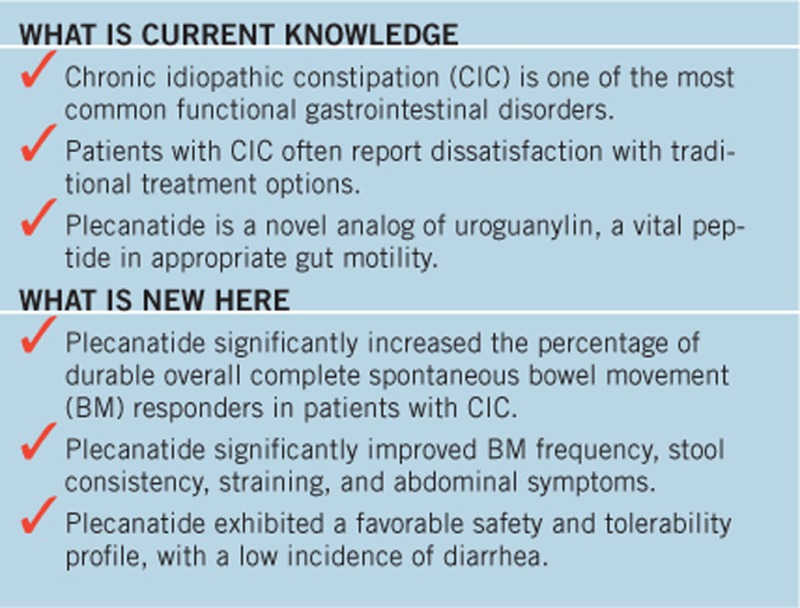
Study Highlights
Acknowledgments
We thank the patients with CIC who participated in this study and the clinicians who contributed their efforts to the conduct of the study. The contributions of S. Comiskey and R. Feng in the development of a room temperature-stable formulation and in manufacturing of plecanatide and placebo tablets used in this study are thankfully acknowledged. Medical writing support was provided by Medical Dynamics, New York, NY, with editorial and submission support from The Medicine Group, New Hope, PA.
Footnotes
Supplementary Material is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Leslie Magnus, MD.
Specific author contributions: Study concept: Drs Miner and Shailubhai; study design: Drs Miner, Barrow, and Griffin; data acquisition: Drs Miner, Koltun, Wiener, De La Portilla, and Prieto; data analysis: Drs Miner, Barrow, and Griffin and Ms Layton; data interpretation, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript: all authors.
Financial support: Funding for this trial and manuscript support were provided by Synergy Pharmaceuticals Inc. Synergy Pharmaceuticals Inc. also provided the plecanatide and placebo used for the study.
Potential competing interests: Drs Shailubhai, Barrow, Magnus, and Griffin and Ms Layton are employees and stockholders of Synergy Pharmaceuticals Inc. Drs Miner, Koltun, Wiener, De La Portilla, and Prieto were study investigators paid for their participation in this clinical trial. Dr Koltun has received funding for either advisory boards or speaking from the following institutions: Synergy Pharmaceuticals, Symiomix Theraputics, Ferring Pharmaceuticals, Enteris Pharmaceuticals, and Shionogi Inc. Dr Miner has received funding for advisory board meetings. Transcript Profiling: does not apply.
Supplementary Material
References
- Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582–1591. [DOI] [PubMed] [Google Scholar]
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–759. [DOI] [PubMed] [Google Scholar]
- American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 (Suppl 1): S1–S4. [DOI] [PubMed] [Google Scholar]
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Dorn SD, Lembo A et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144:211–217. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Approves Linzess To Treat Certain Cases Of Irritable Bowel Syndrome And Constipation [press release]. Silver Spring, MD: US Food and Drug Administration (FDA). 2012. [Google Scholar]
- FDA Approves New Prescription Drug For Adults For Treatment of Chronic "Idiopathic" Constipation [press release]. Silver Spring, MD: US Food and Drug Administration (FDA). 2006. [Google Scholar]
- Wald A. Constipation: advances in diagnosis and treatment. JAMA 2016;315:185–191. [DOI] [PubMed] [Google Scholar]
- Kita T, Smith CE, Fok KF et al. Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol 1994;266:F342–F348. [DOI] [PubMed] [Google Scholar]
- Hamra FK, Eber SL, Chin DT et al. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci USA 1997;94:2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailubhai K, Comiskey S, Foss JA et al. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci 2013;58:2580–2586. [DOI] [PubMed] [Google Scholar]
- Shailubhai K, Palejwala V, Arjunan KP et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther 2015;6:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Overbey D, Watkinson LD et al. In vivo imaging of human colorectal cancer using radiolabeled analogs of the uroguanylin peptide hormone. Anticancer Res 2009;29:3777–3783. [PubMed] [Google Scholar]
- Vaandrager AB, Smolenski A, Tilly BC et al. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl- channel activation. Proc Natl Acad Sci USA 1998;95:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriano R, Ozu M, Politi MT et al. Uroguanylin regulates net fluid secretion via the NHE2 isoform of the Na+/H+ exchanger in an intestinal cellular model. Cell Physiol Biochem 2011;28:733–742. [DOI] [PubMed] [Google Scholar]
- Busby RW, Bryant AP, Bartolini WP et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol 2010;649:328–335. [DOI] [PubMed] [Google Scholar]
- Brierley SM. Guanylate cyclase-C receptor activation: unexpected biology. Curr Opin Pharmacol 2012;12:632–640. [DOI] [PubMed] [Google Scholar]
- Field M, Graf LH Jr, Laird WJ et al. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci USA 1978;75:2800–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailubhai K. Therapeutic applications of guanylate cyclase-C receptor agonists. Curr Opin Drug Discov Devel 2002;5:261–268. [PubMed] [Google Scholar]
- Sindic A. Current understanding of guanylin peptides actions. ISRN Nephrol 2013;2013:813648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayalasekeran S, Ali H, Tsai HH. Novel therapies for constipation. World J Gastroenterol 2013;19:8247–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Harrington AM, Hughes PA et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 2013;145:1334-46.e1–11. [DOI] [PubMed] [Google Scholar]
- Miner PB, Surowitz R, Fogel R et al. 925g Plecanatide, a novel guanylate cyclase-C (GC-C) receptor agonist, is efficacious and safe in patients with chronic idiopathic constipation (CIC): results from a 951 patient, 12 week, multi-center trial [abstract]. Gastroenterology 2013;144:S-163. [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–1390. [DOI] [PubMed] [Google Scholar]
- Study SP304-20210: A Randomized, 12-Week, Double-Blind, Placebo-Controlled, Repeat-Dose, Oral, Dose-Ranging Study to Assess the Safety and Efficacy of Plecanatide in Patients With Chronic Idiopathic Constipation. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2012, Accessed on 25 August 2015. Available at https://clinicaltrials.gov/ct2/show/NCT01429987.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.