Abstract
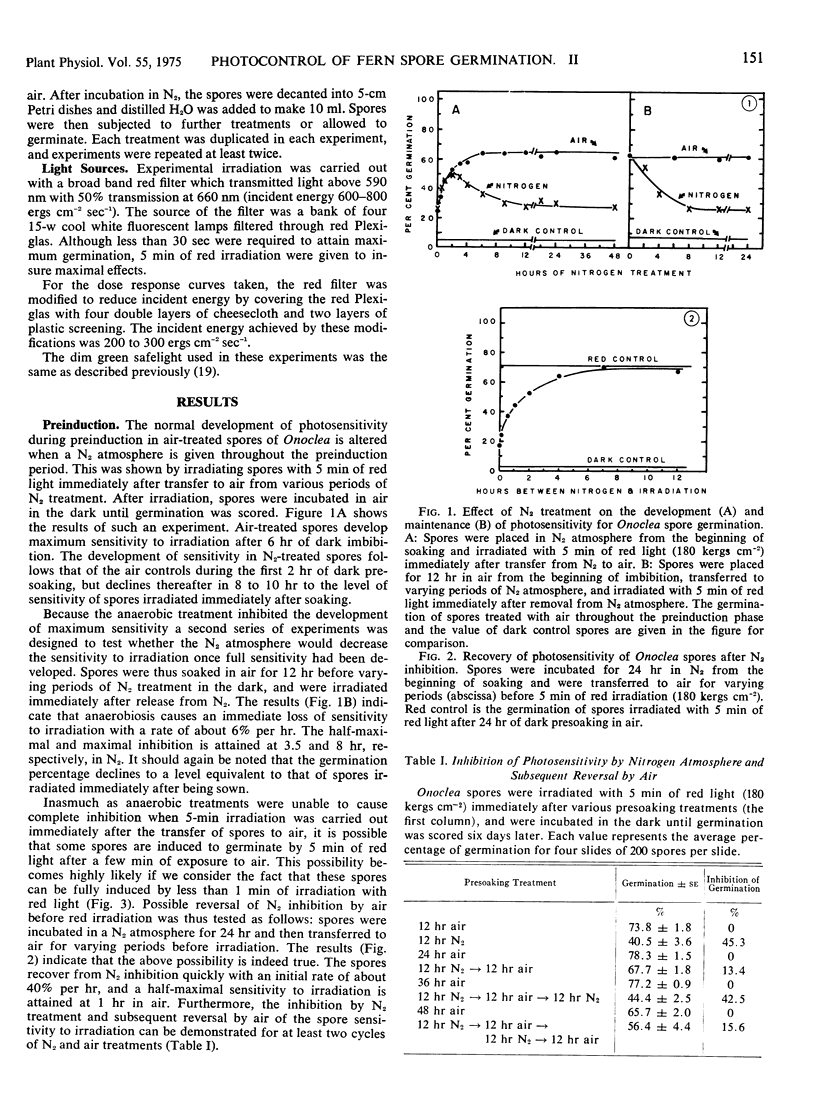
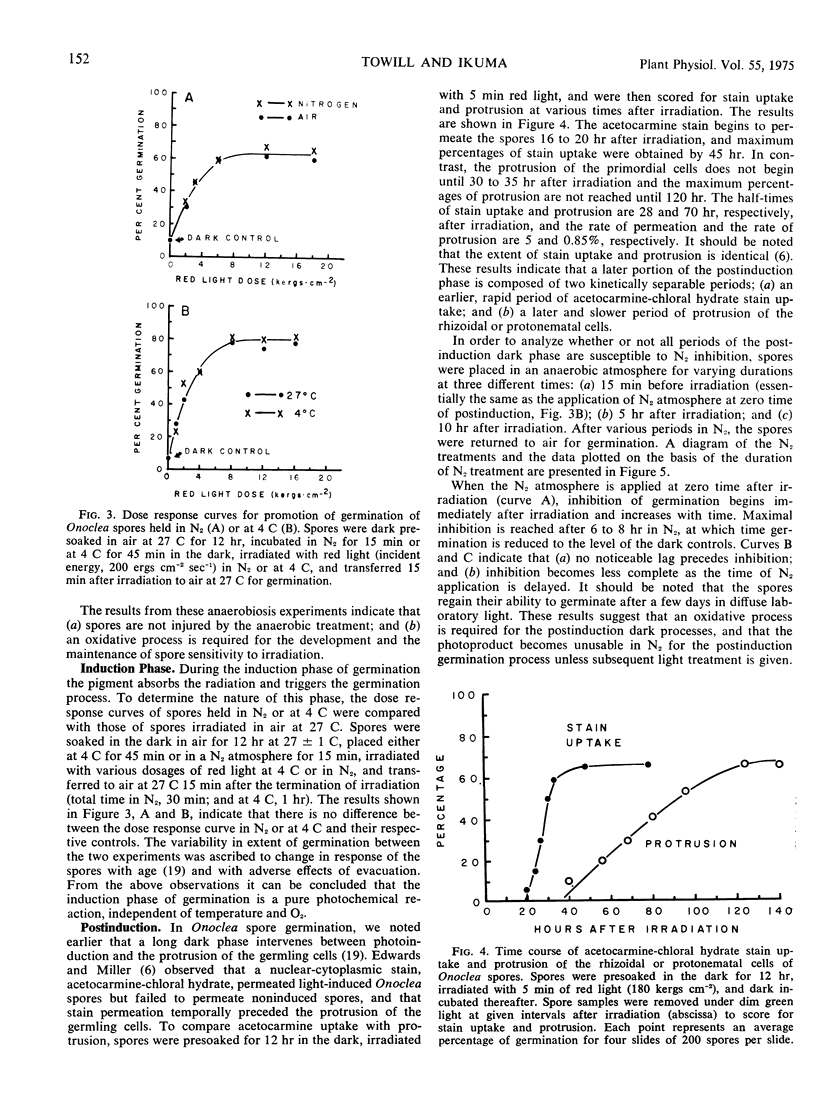
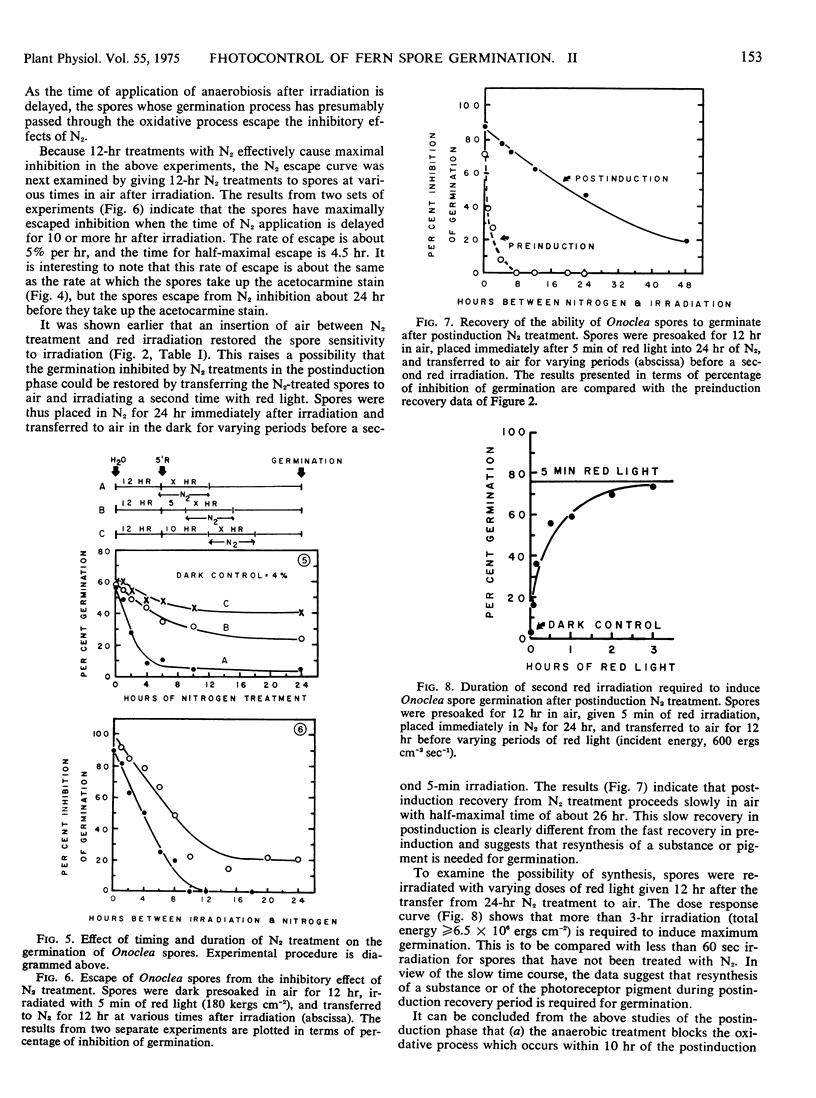
The oxygen requirements during the three phases of photoinduced germination of Onoclea sensibilis L. spores were analyzed by temporarily applying nitrogen atmosphere. The dark preinduction phase, during which the spores imbibe water and establish sensitivity to irradiation, involves an oxidative process which can be reversibly inhibited and stimulated by nitrogen and air, respectively. The induction phase of germination is characterized by a pure photochemical reaction, independent of temperature and oxygen. The postinduction phase, when the photoproduct triggers dark processes eventually leading to the protrusion of the rhizoidal or protonematal cells, involves an oxidative process which occurs within the first 10 hours of this phase. This oxidative process differs in kinetic characteristics from that in the preinduction phase. The oxidative process is inhibited by nitrogen treatment, but following nitrogen inhibition the ability of the spores to germinate can be reinstated by a long period of air intervening between the nitrogen treatment and a second irradiation. This suggests that enzymes or reactants which are needed in the postinduction process decay under anaerobic conditions and are resynthesized when the spores are transferred to air. Spores take up acetocarmine stain towards the latter part of the postinduction phase. Stain uptake is apparently succeeded very closely by cell division, and some time later by protrusion of the germling cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- GANTT E., ARNOTT H. J. SPORE GERMINATION AND DEVELOPMENT OF THE YOUNG GAMETOPHYTE OF THE OSTRICH FERN (MATTEUCCIA STRUTHIOPTERIS). Am J Bot. 1965 Jan;52:82–94. [PubMed] [Google Scholar]
- Ikuma H., Thimann K. V. Analysis of Germination Processes of Lettuce Seed by Means of Temperature and Anaerobiosis. Plant Physiol. 1964 Sep;39(5):756–767. doi: 10.1104/pp.39.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V. Germination of bracken fern spores. Regulation of protein and RNA synthesis during initiation and growth of the rhizoid. Exp Cell Res. 1970 Dec;63(2):341–352. doi: 10.1016/0014-4827(70)90222-3. [DOI] [PubMed] [Google Scholar]
- Sugai M. Photomorphogenesis in Pteris vittata. 3. Protective action of ethanol on blue-light-induced inhibition of spore germination. Dev Growth Differ. 1970 Jun;12(1):13–20. doi: 10.1111/j.1440-169x.1970.00013.x. [DOI] [PubMed] [Google Scholar]
- Towill L. R., Ikuma H. Photocontrol of the germination of onoclea spores: I. Action spectrum. Plant Physiol. 1973 May;51(5):973–978. doi: 10.1104/pp.51.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. S., Voeller B. R. Induction of fern spore germination. Proc Natl Acad Sci U S A. 1969 Nov;64(3):835–842. doi: 10.1073/pnas.64.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]


