Abstract
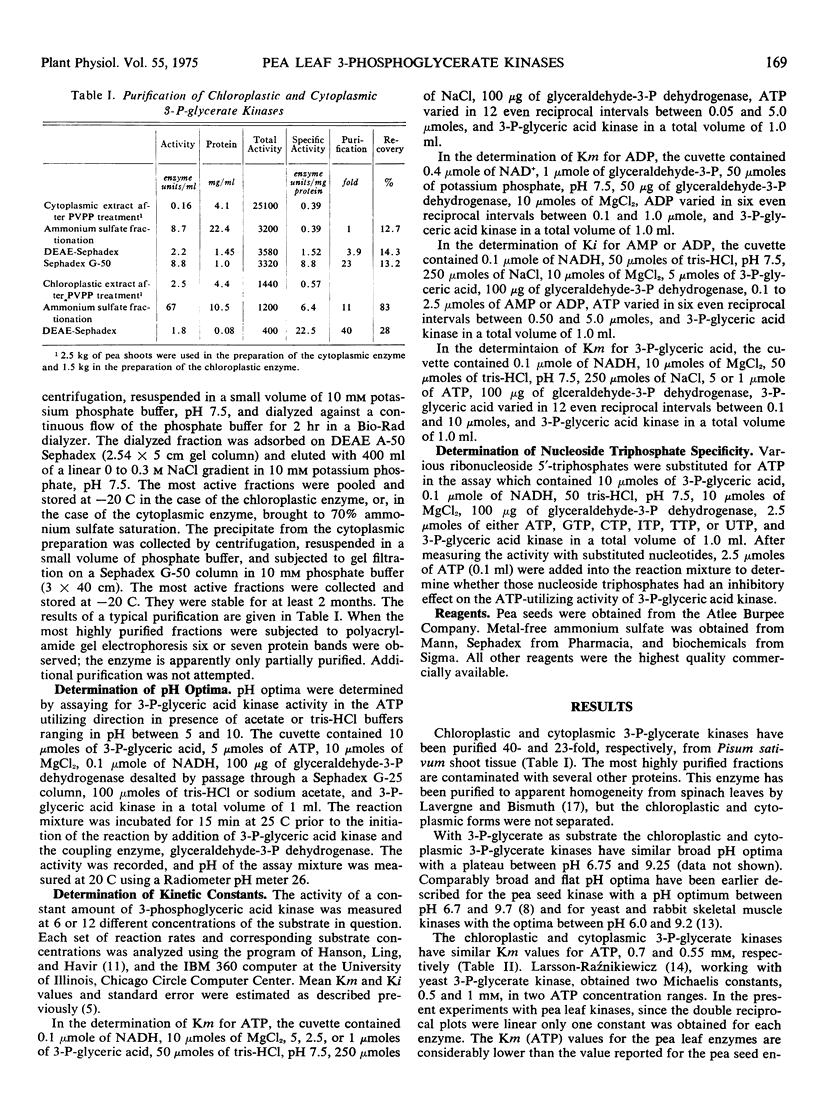
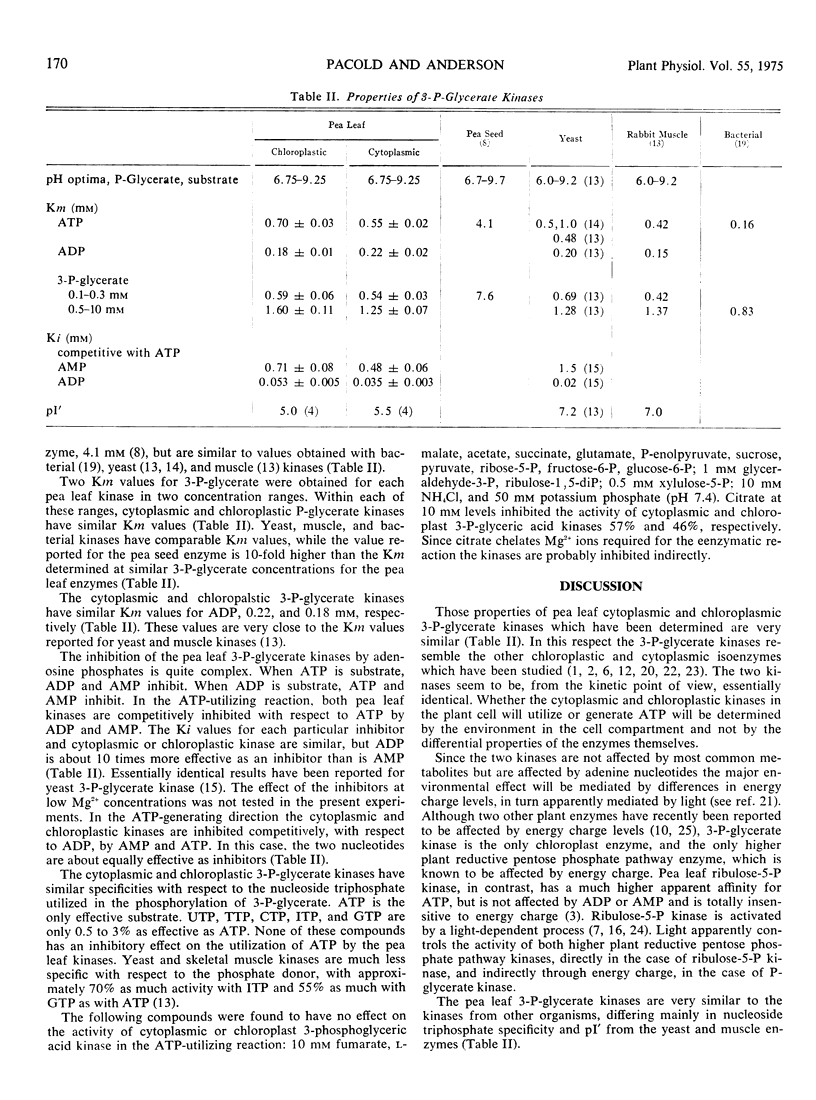
Pea (Pisum sativum) leaf chloroplastic and cytoplasmic 3-phosphoglycerate kinases (ATP: d-3-phosphoglycerate 1-phosphotransferase, EC 2.7.2.3) have similar Michaelis constants for ATP, 0.7 and 0.55 mm, for ADP, 0.18 and 0.22, and for 3-P-glycerate, 0.59 and 0.54 mm at low substrate concentrations, and 1.6 and 1.25 mm at high substrate concentrations. Both enzymes are inhibited by ADP and AMP in the ATP-utilizing direction and by ATP and AMP in the ATP-generating direction and are controlled by energy charge. Apparently, whether the cytoplasmic and chloroplastic kinases in the plant cell will participate in the reductive pentose phosphate cycle and gluconeogenesis or in glycolysis will be determined by the environment in the cell compartment and not by the differential properties of the enzymes themselves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., BANDURSKI R. S. Phosphoglyceryl kinase in higher plants. J Biol Chem. 1953 Oct;204(2):939–948. [PubMed] [Google Scholar]
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Fuller R. C. Photosynthesis in Rhodospirillum rubrum. IV. Isolation and characterization of ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1969 Jun 25;244(12):3105–3109. [PubMed] [Google Scholar]
- Anderson L. E., Pacold I. Chloroplast and Cytoplasmic Enzymes: IV. Pea Leaf Fructose 1,6-Diphosphate Aldolases. Plant Physiol. 1972 Mar;49(3):393–397. doi: 10.1104/pp.49.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Regulation of pea leaf ribulose-5-phosphate kinase activity. Biochim Biophys Acta. 1973 Oct 10;321(2):484–488. doi: 10.1016/0005-2744(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Properties of phosphoribulokinase of whole chloroplasts. Plant Physiol. 1974 Feb;53(2):136–139. doi: 10.1104/pp.53.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D., Patil K. D. Regulation of 'malic' enzyme of Solanum tuberosum by metabolites. Biochem J. 1974 Jan;137(1):45–53. doi: 10.1042/bj1370045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R., Ling R., Havir E. A computer program for fitting data to the Michaelis-Menten equation. Biochem Biophys Res Commun. 1967 Oct 26;29(2):194–197. doi: 10.1016/0006-291x(67)90586-4. [DOI] [PubMed] [Google Scholar]
- Krietsch W. K., Bücher T. 3-phosphoglycerate kinase from rabbit sceletal muscle and yeast. Eur J Biochem. 1970 Dec;17(3):568–580. doi: 10.1111/j.1432-1033.1970.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Larsson-Raźnikiewicz M., Arvidsson L. Inhibition of phosphoglycerate kinase by products and product homologues. Eur J Biochem. 1971 Oct 26;22(4):506–512. doi: 10.1111/j.1432-1033.1971.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Schuster E. 3-phosphoglycerate kinase from Hydrogenomonas facilis. J Bacteriol. 1972 Feb;109(2):751–756. doi: 10.1128/jb.109.2.751-756.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y., Harris B. G., Gracy R. W. Triosephosphate isomerases and aldolases from light- and dark-grown Euglena gracilis. Arch Biochem Biophys. 1973 Aug;157(2):580–587. doi: 10.1016/0003-9861(73)90677-2. [DOI] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Energy charge control of the Calvin cycle enzyme 3-phosphoglyceric acid kinase. Biochem Biophys Res Commun. 1973 Mar 5;51(1):139–143. doi: 10.1016/0006-291x(73)90519-6. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Wong K. F., Davies D. D. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J. 1973 Mar;131(3):451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]


