Abstract
Organic contaminants in shale gas flowback and produced water (FPW) are traditionally expressed as total organic carbon (TOC) or chemical oxygen demand (COD), though these parameters do not provide information on the toxicity and environmental fate of individual components. This review addresses identification of individual organic contaminants in FPW, and stresses the gaps in the knowledge on FPW composition that exist so far. Furthermore, the risk quotient approach was applied to predict the toxicity of the quantified organic compounds for fresh water organisms in recipient surface waters. This resulted in an identification of a number of FPW related organic compounds that are potentially harmful namely those compounds originating from shale formations (e.g., polycyclic aromatic hydrocarbons, phthalates), fracturing fluids (e.g., quaternary ammonium biocides, 2-butoxyethanol) and downhole transformations of organic compounds (e.g., carbon disulfide, halogenated organic compounds). Removal of these compounds by FPW treatment processes is reviewed and potential and efficient abatement strategies are defined.
1. Introduction
The worldwide depletion of conventional oil and gas reserves inspired growing attention to alternative energy sources. However, the current gap between nonrenewable (81.6%) and renewable (13.4%) energy sources in the global total primary energy supply remains too wide to be closed in the coming decennia, as stated by market analysts.1 Shale gas is here considered as an additional nonrenewable energy source for the transition period.2 It can partly substitute the depleting conventional gas resources and thus decrease the dependency of many countries on these traditional supplies.3,4 At the same time, shale gas development has become a source of controversies among researchers, policy makers and the general public with respect to largely unknown environmental impacts.2,5−9 The suspected deterioration of quality and quantity of water resources, air pollution, increased traffic, noise, and light pollution, induced seismic activity and adverse health impacts are the subjects of concern.9
The water-related issues deserve special attention. Hydraulic fracturing, which is a basic technology for gas production from shale gas reservoirs, involves pumping of the fracturing fluid into the well under high pressure in order to increase permeability of the shale.10,11 The fracturing fluid contains chemicals added to facilitate shale gas recovery, including gelling agents, surfactants, friction reducers, corrosion and scale inhibitors, biocides, acids and pH adjusting agents.12−16 A mixture of the fracturing fluid and formation water, generally known as flowback water, returns to the surface within several days after fracturing with the daily flows sometimes exceeding 1000 m3.17,18 Flowback water is less saline than formation water and contains chemicals from fracturing fluid as well as their transformation products.11,19 Formation and connate water, generally known as produced water, continues flowing from the well for months to years after fracturing, though its daily flow constitutes only a few cubic meters. Produced water is generally more saline than flowback water and contains released methane and constituents of the shale, such as organic hydrocarbons and naturally occurring radioactive material (NORM).19
Options for flowback and produced water (FPW) management include disposal in the subsurface through deep injection wells, reuse for the next fracturing activity and/or treatment with subsequent discharge to injection wells or surface waters.20 Whereas disposal in the deep injection wells was the main technology for FPW management in the initial years of the shale gas boom in the U.S., reuse of flowback and discharge of produced water after appropriate treatment has become more often utilized recently.21,22 The development of the Marcellus shale in the U.S. lacking deep injection wells in proximity of the shale gas play was the main driver for searching for reuse alternatives.21,23
The choice of the FPW management strategy in favor of treatment followed by reuse or discharge is also expected in Europe due to the lack of deep injection wells and legislative constraints.24,25 European legislative instruments, including among others the EU Water Framework Directive, the associated Groundwater Directive and the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR convention) aim at zero environmental harmful discharge of oil and gas wastewater.26 To date only general water quality parameters and inorganic constituents of shale gas FPW are extensively characterized, whereas knowledge on presence and effects of individual organic compounds is rather scarce.16,20,27−29
Characteristics, potential toxicity and treatability of compounds, which are present in fracturing fluids are evaluated in recent reviews.12,30 However, these compounds may and are partly known to undergo structural changes downhole under high pressure, temperature and salinity.15,31 Whereas fracturing fluids will reach the environment only as a result of accidental events, such as surface spills, fracturing of the well casing or interconnection with neighboring wells, at least part of shale gas FPW will be inevitably discharged to open water bodies after certain level of treatment. FPW treatment strategies focus on removal of oil, grease and salts, whereas specific contaminants originating from fracturing fluids remain largely unaddressed and may negatively influence and surface water ecosystems and drinking water sources.30 The aim of this paper is to identify organic contaminants in FPW potentially hazardous to environment using a risk quotient approach. The paper presents the case of the Dommel river in The Netherlands as an example calculation of the FPW impact on the ecosystems of small rivers and streams, thus giving a wider applicable framework for further calculations of such impacts for other watersheds. Furthermore, the efficiency of the FPW treatment methods toward removal of the identified potentially hazardous compounds is reviewed and the strategies for their elimination are proposed. Because the existing literature on the composition of flowback and produced water currently does not provide sufficient data on the concentrations of a vast number of compounds with proven human toxicity, which are regulated by U.S. National Primary Drinking Water Regulations, WHO guidelines and EU regulations (e.g., many chlorinated organic compounds), impact of FPW on drinking water sources deserves additional research and is not covered by this review.
2. Identification of Individual Organic Compounds in Flowback and Produced Waters
The organic contaminants of shale gas FPW include compounds injected in the fracturing fluid, compounds of the formation which are mobilized when being brought in contact with fracturing fluid, and products of physicochemical and biological transformation of organic fracturing chemicals.32 It is anticipated that fracturing chemicals dominate in early flowback, whereas chemicals of formation dominate in late flowback and produced water.17,32,33 In this paper, ultimately the organic chemicals of both flowback and produced water are assessed.
Chemical characterization of FPW addresses mostly inorganic chemicals including salts, scalants and heavy metals. With respect to organic components, FPW is usually characterized using general parameters, such as total and dissolved organic carbon (TOC and DOC), oil and grease and the sum of concentrations of benzene, toluene, ethylbenzene, and xylenes (BTEX). For example, the U.S. Geological Survey National Produced Water Database contains data on TOC, DOC, biological oxygen demand (BOD), chemical oxygen demand (COD) and several individual organic compounds (methane, acetone, acetate, BTEX, phenols, ethylene glycol) but only for limited number of shale gas producing wells.34 At the same time reporting exact composition of fracturing fluids is done by many shale gas production companies and the vast amount of data are available online in a FracFocus database.35 The presence of potentially hazardous organic compounds in the fracturing fluids became one of the main reasons for negative public and policy attitude in many countries toward shale gas production.4,36,37 Much uncertainty about the fate of this chemicals in the well under high pressure and high temperature conditions exists. Data on the exact FPW composition with respect to organic compounds are still scarce, mostly because the complex nature of these liquids remains a challenge for analytical chemistry. The overview of original data on analytical detection of individual organic compounds in shale gas FPW available in the open literature sources is given in Table 1.
Table 1. Overview of Original Literature Data on Analysis of Individual Organic Compounds in Shale Gas FPW.
| source | shale basins | water | analyzed compounds | extraction method | analytical method | detection |
|---|---|---|---|---|---|---|
| Hayes, 2009; Hayes and Severin, 2012 | Marcellus shale (19 wells); Barnett shale (5 wells) | flowback and produced (Marcellus shale); Flowback (Barnett shale) | 70 VOC; 116 sVOC; 22 pesticides; 7 PCBs; ethylene glycol | purge-and-trap | GC-MSa | quantitative |
| Maguire-Boylee and Barron, 2014 | Marcellus shale; Eagle Ford shale; Barnett shale | produced | identification of organic compounds using NIST library | liquid\liquid extraction with chloroform (acidified and nonacidified conditions) | GC-MSa | qualitative |
| Orem et al., 2014 | Marcellus shale;New Albany shale | flowback and produced | identification of organic compounds using NIST library and standard compounds | liquid\liquid extraction with dichloromethane | GC-MSa | semiquantitative |
| Thurman et al., 2014 | Denver-Julesburg basin; Barnett shale; Marcellus shale; Haynesville basin | flowback and produced | ethoxylated surfactants | direct injection | HPLC/Q-TOF-MSb | qualitative |
| Ferrer and Thurman, 2015 | Denver-Julesburg basin | flowback and produced | biocides (ADBAC, glutaraldehyde); surfactant (cocamidopropyl dimethylamine); thickener (guar gum) | direct injection | HPLC/Q-TOF-MSb | qualitative |
| Lester et al., 2015 | Denver-Julesburg basin | flowback | VOC; sVOC; surfactants | purge and trap (VOC, sVOC); direct injection (trace organics) | GC-MSa; HPLC/Q-TOF-MSb | quantitative (VOC, sVOC); qualitative (trace organics) |
| Thacker et al., 2015 | Barnett shale | not specified | VOC; sVOC;nonvolatile organics | purge and trap (VOC, sVOC); Direct injection (trace organics) | GC-MS;a HPLC-HRMSc | qualitative |
| Regnery et al., 2016 | Denver-Julesburg basin | produced | linear aliphatic hydrocarbons (n-C10 to n-C32); PAH-16 | solid-phase extraction | GC-MSa | quantitative |
Gas chromatography coupled with mass spectrometry.
High pressure liquid chromatography coupled with quadrupole time-of-flight mass spectrometry.
High performance liquid chromatography coupled to high resolution mass spectrometry.
2.1. Detection of Volatile and Semivolatile Organic Compounds Using Gas Chromatography
To date, the largest sources of information on quantitative determination of particular organic compounds in shale gas FPW are the reports of Hayes (2009) and Hayes and Severin (2012).17,38 The reports cover sampling of 19 wells of Marcellus shale (Pennsylvania and West Virginia, USA) and 5 wells of the Barnett Shale (Texas, U.S.) (Table 1). Fifteen volatile organic compounds (VOCs) and 17 semivolatile organic compounds (sVOCs) were detected by the authors in more than one FPW sample. The detected VOCs included BTEX and their alkyl derivatives, carbon disulfide, acetone and halogenated compounds, including trichlorobenzene and bromoform. Polycyclic aromatic hydrocarbons (PAHs), phthalates, phenols, and pyridine were present among analyzed sVOCs. Most of the compounds were found in μg/L levels, whereas BTEX, carbon disulfide and pyridine were determined in low mg/L in the FPW from several wells. Pesticides, polychlorinated biphenyls (PCBs) and a number of VOCs and sVOCs were occasionally detected in single samples. The authors concluded that their detection is attributed rather to the sampling and analytical errors then to the actual presence of these compounds in the FPW.
Lester et al. (2014) showed similar composition of flowback water from Denver-Julesburg basin with respect to sVOCs.39 Acetone was the dominating VOC in flowback water with a concentration of 16 mg/L, thus 2 orders of magnitude higher than concentration reported by Hayes (2009).17 Other VOCs, present in the FPW analyzed by Hayes (2009), were not reported by Lester et al. (2014), except for xylenes and 2-butanone.17,39 This difference can be explained by the less sensitive sampling and analytical methods used in the latter study.
Several recent peer-reviewed publications were aiming at qualitative or semiquantitative detection of organic pollutants in shale gas FPW by nontarget analysis.32,29 The authors identified a variety of saturated aliphatic and aromatic hydrocarbons present in the FPW. Maguire-Boyle and Barron (2014) applying acidified conditions for polar compound extraction from FPW detected large number of alkanes, resins, asphaltenes, heterocyclic and halogenated compounds, which were not reported by Orem et al. (2014).29,32 Also Maguire-Boylee and Barron (2014) analyzed produced rather than flowback water, where compounds of formation typical for oil and gas produced waters are present in higher variety and concentrations.29 In contrast, Orem et al. (2014) reported presence of compounds originating from fracturing fluids (solvent 2,2,4-trimethyl-1,3-pentanediol, biocide hexahydro-1,3,5-trimethyl-1,3,5-triazine-2-thione, friction reducer ethylene glycol), which were not detected by Maguire-Boylee and Barron (2014).32 Whereas some compounds were present in early flowback samples in concentrations reaching low mg/L levels, the rapid decrease of concentrations in samples taken during first 20 days after fracturing operation was observed.
2.2. Detection of Nonvolatile Organic Compounds Using Liquid Chromatography
Several authors used HPLC/Q-TOF-MS for qualitative detection of nonvolatile organic compounds in shale gas FPW.39−42 A number of constituents of fracturing fluids were detected by these authors. Thus, presence of ethoxylated surfactants in flowback waters from different shale gas basins in the U.S. was proven in two studies.39,41 Representative compounds of the cocamidopropyl family of surfactants were detected in FPW samples by several authors.40,42 Biocide alkyl dimethyl benzyl ammonium chloride (ADBAC) was found in 54% of analyzed FPW samples by Ferrer and Thurman (2015), whereas another biocide, glutaraldehyde, was found in none of the samples.40 Thacker et al. (2015) reported presence of alkyl amines (acid dispersants/surfactants) and 2-butoxyethanol (solvent/acid dispersant/surfactant) in shale gas wastewater without specifying the water type.42
2.3. Knowledge Gaps in Detection of Organic Compounds in FPW
Both quantitative and qualitative data on the presence of organic pollutants in shale gas FPW are scarce, especially concerning the compounds used in fracturing fluids for shale gas production. Thus, no research on the presence of corrosion inhibitors and clay stabilizers, including toxic compounds such as propargyl alcohol, thiourea and tetramethylammonium chloride, in FPW has been conducted yet. The knowledge on biocides presence and concentrations in FPW is limited to few compounds (ADBAC, hexahydro-1,3,5-trimethyl-1,3,5-triazine-2-thione and glutaraldehyde).32,40 Little is known on the transformation products of fracturing fluid additives that are formed downhole under high temperature and high pressure and return to the surface with FPW. The low molecular weight organics such as acetone, acetate and pyridine, which is not attributed to the compounds commonly present in fracturing fluids and formation waters, were found in the FPW in high concentrations as possible transformation products of more complex organic molecules.17,39 Formation of halogenated organic compounds in FPW is predicted by several authors, given high chloride and bromide content of FPW and possible decomposition of fracturing fluid additives that contain Cl and Br atoms in their molecular structure.29 However, knowledge on halogenated organics in FPW is still limited to identification of few aliphatic structures.17,29
3. Risk Screening of Individual Organic Compounds
The available literature data on concentrations of individual organic compounds in the shale gas FPW were used for their environmental risk screening via Predicted Effect Concentration/Predicted No Effect Concentration (PEC/PNEC) approach.43 Briefly, the environmental concentrations in the receiving surface water (PECs) were predicted for the worst case scenario of FPW discharge with no removal of individual organic compounds because of inadequate treatment. They were compared with PNECs for fresh water organisms derived from literature or calculated from toxicity data using assessment factors. A resulting risk quotient (RQ) above the value of 1 indicates potential risk of an organic compound to the surface water ecosystems and implies the necessity of its removal prior to wastewater discharge.
3.1. Determination of PEC for Surface Fresh Waters
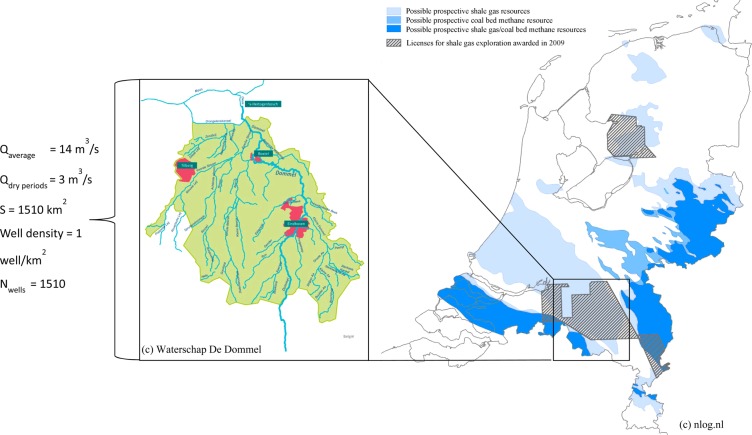
Small streams can be heavily impacted by the discharge of shale gas FPW, even after FPW treatment. The river Dommel located in the province of Noord Brabant (The Netherlands) was used as a model stream for calculation of the impact of FPW chemicals on aquatic environment (Figure 1). The Dommel has a total length of 120 km, with 85 km of downstream flow located within The Netherlands. The average flow at the river mouth is 14 m3/s, dropping to 3 m3/s in the dry periods.44 The water is not directly abstracted for drinking water production, nor intensively used for recreational purposes, therefore, direct negative impact of treated produced water discharged to Dommel on human health is considered to be minimal and is not reviewed further.
Figure 1.
River Dommel as a model stream for calculation of environmental impact of the FPW chemicals on aquatic environment.
A possible shale gas production field located in the Dommel catchment basin within The Netherlands has an area of 1510 km2.44 The development of shale gas resources within the whole catchment basin was assumed, using a scenario with a well density of 1 well/km2.45 In this scenario 329 wells are drilled over an area of 330.5 km2 within the Dommel catchment basin. Though the drilling-free zones, such as nature and groundwater protection areas and urban areas in the province of Noord Brabant are numerous, the drilling-free parcels are dispersed with a radius of individual parcels not exceeding 2 km. The horizontal laterals of each borehole can stretch up to 3 km, therefore, drilling-free areas were not considered as a constrain for development of the whole field. An estimate number of 1510 wells was obtained assuming the same well density in the whole Dommel catchment basin.
Average FPW volume of 2900 m3 is collected during 90 days after the fracturing event according to data from Marcellus shale gas production facilities.17 The largest volumes of flowback water are collected on the first day after the fracturing event with an average flow of 700 m3/day. However, on-site storage capacities for flowback water allows for equalization of the FPW discharge. Therefore, an average daily flow of 32 m3/well was calculated assuming complete equalization of the FPW flow during initial 90 days of well operation.
The time-evolution of the shale gas production includes the rapid development of the field during first 10–15 years followed by the decrease of the number of wells. Nicot et al. (2012) estimated the total water use in the peak year of the shale development equal to 3.6% of the total water use for Haynesville Shale, 3.1% for Eagle Ford Shale and 5.7% for Barnett Shale.18 The average peak year water use for the three shales is equal to 4.1% of the total water demand for shale gas production. This figure can be also extrapolated into the number of wells within a shale, hence Nicot et al. (2012) assumed the equal water use throughout a shale.18 With an estimate number of 1510 wells within the Dommel catchment basin fracturing of 62 wells during a peak year is expected. A total daily generation of 1980 m3 of FPW is estimated for these 62 wells based on the average daily flow of 32 m3/well. Possible refracturing of the wells was not accounted for. The water reuse ratio of 70% similar to that reported for Marcellus Shale was assumed,21 therefore, decreasing predicted discharge of treated FPW to Dommel to 600 m3/d. This value was compared with the lowest average river flow of 3 m3/s (2.6 · 105 m3/d), resulting in a dilution factor of 435 for discharged FPW.
The maximal concentrations of organic compounds in FPW reported in the literature (see Section 2) were divided by dilution factor to obtain their mean PECs. In total, PEC of 22 VOCs, 37 sVOCs and 3 nonvolatile organic compounds were calculated (Table 2).
Table 2. Risk Screening of Organic Compounds Detected in FPW.
| compound | CAS no. | CMAX, μg/L | predicted CMAX river, μg/L | PNEC, μg/L | assessment factor | RQ | end point |
|---|---|---|---|---|---|---|---|
| Volatile Organic Compounds, μg/L | |||||||
| 1,2,4-trimethylbenzene | 95–63–6 | 400015 | 9.2 | 445 | 100 | 2.3 | reproduction of Daphnia magna, NOEC (21 d) |
| 1,3,5-Trimethylbenzenea | 108–67–8 | 190015 | 4.4 | 445 | 100 | 1.1 | reproduction of Daphnia magna, NOEC (21 d) |
| 2-butanone | 78–93–3 | 24037 | 0.6 | 55 80044 | 1 (SSD) | 0.00001 | |
| acetone | 67–64–1 | 1600037 | 36.7 | 10 60044 | 50 | 0.003 | growth of Microcystis aeruginosa, NOEC (8 d) |
| benzene | 71–43–2 | 530015 | 12.2 | 190044 | 1 (SSD) | 0.006 | |
| benzothiazole | 95–16–9 | <2530 | <0.1 | 1545 | 100 | <0.007 | reproduction of Daphnia magna, NOEC (21 d) |
| bromoform | 75–25–2 | 9.315 | 0.0 | 2947 | 1000 | 0.001 | Lepomis macrochirus, LC50 |
| carbon disulfide | 75–15–0 | 730015 | 16.8 | 1044 | 100 | 1.7 | number of hatched eggs of Danio rerio, NOEC (8 d) |
| chloromethane (methyl chloride) | 74–87–3 | 15015 | 0.3 | 20044 | 1000 | 0.002 | mobility of Daphnia magna, EC50 (48h) |
| ethylbenzene | 100–41–4 | 67036 | 1.5 | 10044 | 10 | 0.02 | reproduction of Ceriodaphnia dubia, NOEC (7 d) |
| isopropylbenzene | 98–82–8 | 16015 | 0.4 | 3544 | 10 | 0.01 | reproduction of Daphnia magna, NOEC (21 d) |
| Methylene Chloride | |||||||
| (dichloromethane) | 75–09–2 | 5.515 | 0.0 | 31044 | 20 (QSAR) | 0.00004 | QSAR case study: prediction of long-term NOEC for Daphnia magna (21 d) |
| n-isopropyltoluene | 99–87–6 | 5415 | 0.1 | 6.546 | 1000 | 0.02 | Daphnia magna, LC50 (48h) |
| toluene | 108–88–3 | 810036 | 18.6 | 68044 | 1 (SSD) | 0.03 | |
| xylene | 1330–20–7 | 650015 | 14.9 | 32744 | 1 (SSD) | 0.05 | |
| Semivolatile Organic Compounds, μg/L | |||||||
| 1,4-dioxane | 123–91–1 | 6037 | 0.1 | 10 00044 | 10 | 0.00001 | mortality of Pimephales promelas, NOEC (32 d) |
| 2-butoxyethanol (EGBE)b | 111–76–2 | 6600051 | 151.5 | 88044 | 100 | 0.2 | growth of Pseudokirchneriella subcapitata, NOEC (72 h) |
| 2,4-dimethylphenol | 105–67–9 | 79037 | 1.8 | 2.1246 | 1000 | 0.9 | Daphnia magna, LC50 (48 h) |
| 2-methylnaphthalene | 91–57–6 | 2000036 | 45.9 | 2.345 | 100 | 20 | reproduction of Daphnia magna, NOEC (21 d) |
| 2-methylphenol (o-cresol) | 95–48–7 | 15037 | 0.3 | 10044 | 10 | 0.003 | mortality of Daphnia magna, NOEC (21 d) |
| 3- and 4-methylphenol (m-cresol and p-cresol) | 17037 | 0.4 | 10044 | 10 | 0.004 | mortality of Daphnia magna, NOEC (21 d) | |
| benzyl alcohol | 100–51–6 | 75015 | 1.7 | 100044 | 50 | 0.002 | reproduction of Daphnia magna, NOEC (21 d) |
| bis(2-ethylhexyl) phthalate (DEHP) | 117–81–7 | 87015 | 2.0 | 0.249 | 1 (SSD) | 10 | |
| butyl benzyl phthalate | 85–68–7 | 11036 | 0.3 | 6.544 | 10 | 0.04 | Pimephales promelas, NOEC (42 d) |
| dimethyl phthalate | 131–11–3 | 1537 | 0.03 | 22044 | 50 | 0.0002 | mortality of Oncorhynchus mykiss, NOEC (102 d) |
| di-n-butyl phthalate | 84–74–2 | 13015 | 0.3 | 1044 | 10 | 0.03 | length of Oncorhynchus mykiss, NOEC (99 d) |
| di-n-octyl phthalate | 117–84–0 | 560030 | 12.9 | 6.446 | 50 | 19 | Daphnia magna, LC50 (48 h) |
| ethylene glycol | 107–21–1 | 5815 | 0.1 | 1000044 | 10 | 0.00001 | growth of Pseudokirchneriella subcapitata, NOEC (72 h) |
| fluorene | 86–73–3 | 40.715 | 0.1 | 0.2546 | 100 | 0.22 | Ictalurus punctatus, LC50 (48 h) |
| naphthalene | 91–20–3 | 140015 | 3.2 | 2.444 | 50 | 1.3 | growth of Oncorhynchus gorbuscha NOEC (40 d) |
| phenanthrene | 85–01–8 | 140036 | 3.2 | 2.5746 | 100 | 65.6 | changes in general physiology of Lepomis macrochirus, EC50 (96 h) |
| phenol | 108–95–2 | 83037 | 1.9 | 844 | 10 | 0.2 | growth of Cirrhina mrigala, NOEC (60 d) |
| propylene glycol | 57–55–6 | 16015 | 0.4 | 260 00044 | 50 | 0.000001 | growth of Ceriodaphnia dubia, NOEC (7 d) |
| pyrene | 129–00–0 | 1315 | 0.03 | 0.62646 | 100 | 0.41 | growth of Daphnia magna, EC50 (24 h) |
| pyridine | 110–86–1 | 260015 | 6.0 | 30044 | 1000 | 0.02 | mobility of Daphnia magna, EC50 (48 h) |
| Nonvolatile Organic Compounds, μg/L | |||||||
| alkyl dimethyl benzyl ammonium chloride (ADBAC)c | 68424–85–1 | >1038 | >0.02 | 0.41548 | 10 | >0.05 | reproduction of Daphnia magna, NOEC (21 d) |
| hexahydro-1,3,5-Trimethyl-1,3,5-triazinane-2-thioned | 59887–80–8 | 150030 | 3.4 | 0.11446 | 1000 | 11.1 | immobilization of Daphnia magna, EC50 (48 h) |
| 2,2,4-trimethyl-1,3-pentanediole | 144–19–4 | 47030 | 1.1 | 10944 | 1000 | 0.01 | growth of Pseudokirchneriella subcapitata, EC50 (72 h) |
Compounds with PEC < 1 are highlighted in bold.
The maximal concentration of 2-butoxyethanol in the effluent of wastewater treatment plant treating unconventional oil and gas wastewater by combination of settling and chemical precipitation is used as CMAX42
ADBAC was detected in 54% of FPW samples at concentrations above the LOD = 10 μg/L38
EC50 data for 3,5-dimethyl-1,3,5-thiadiazinane-2-thione (dazomet) were used for PNEC calculation
EC50 data for 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate were used for PNEC calculation
3.2. PNEC Assessment and RQ Calculation
PNEC of a chemical compound in aquatic environment is an indicatory parameter calculated by extrapolation of toxicological laboratory data for the most sensitive species to possible field impact of the compound, using the assessment factors.43 The PNECs for individual organic compounds in FPW were either obtained from online databases or from peer-reviewed literature sources.46−51 Most of the PNECs in these databases were calculated from chronic and acute toxicity data with assessment factors of 10–1000. PNECs of benzene, toluene, xylenes, and 2-butanone were calculated using the fifth percentile of a species sensitivity distribution (SSD).
Risk quotients of three VOCs (1,2,4-trimethylbenzene, 1,3,5-trimethylbenzene, and carbon disulfide), three PAHs (naphthalene, 2-methylnaphthalene and phenanthrene), two phthalates (DEHP and di-n-octyl phthalate (DnOP)), and the transformation product of biocide dazomet (hexahydro-1,3,5-trimethyl-1,3,5-triazinane-2-thione) exceeded 1. These compounds will be further referred to as “prioritized shale gas-related organic compounds”.
PAHs and phthalates may originate from the connate water of formation, as well as the fracturing fluid.17,29 Methylation of benzene rings which leads to the formation of 1,2,4-trimethylbenzene and 1,3,5-trimethylbenzene possibly takes place downhole under high temperature and high pressure conditions. Carbon disulfide is not used in fracturing fluid, but is a degradation product of some components of fracturing fluid, such as friction reducers and biocides.17,52
Removal of 2-butoxyethanol is reviewed in this paper despite its comparatively low RQ (0.2) because of its abundant utilization in fracturing fluids and numerous cases of groundwater and surface water contaminations reported in the USA.12,53 ADBAC was detected in FPW only semiquantitatively with RQ > 0.05. Giving the low acute toxicity of ADBAC and uncertainty over the maximal concentrations of this biocide in FPW it is reviewed as prioritized shale gas-related organic compound. Removal of halogenated organics is also considered in this study. Despite 1,2,3-trichlorobenzene was the only organohalogen in FPW with RQ above 1, a number of authors confirmed the presence of halogenated aliphatic and aromatic organics and predicted their formation from high concentrations of Cl– and Br– in FPW.29,32 Removal of hexahydro-1,3,5-trimethyl-1,3,5-triazinane-2-thione is not further reviewed as the data on its behavior in wastewater treatment systems were not found in the literature.
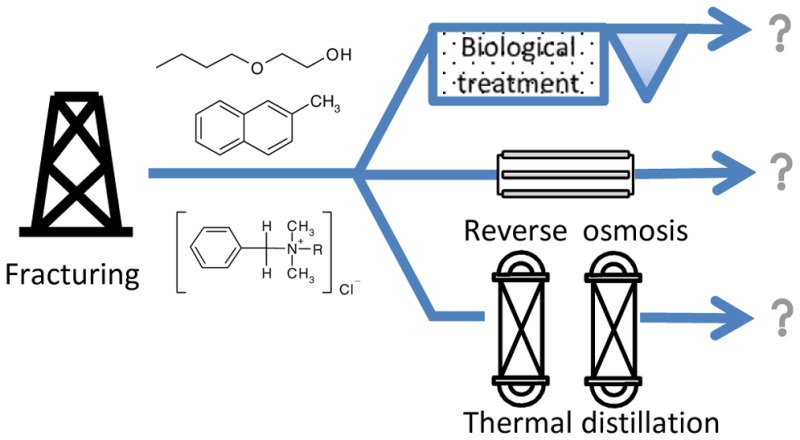
4. Treatment Strategies for Removal of Prioritized Shale Gas-Related Organic Compounds
The treatment methods used for shale gas FPW include basic separation technologies designed for removal of TSS, oil and grease, and advanced treatment technologies designed for removal of dissolved organic compounds, inorganic ions, and radioactive materials.26,54,55 Phase separation underlies basic separation technologies, which include centrifugation, hydrocyclones, flotation, media filtration, coagulation/flocculation, and evaporation. Centrifugation and hydrocyclones provide separation of suspended solids from water by gravity, but are less effective toward oil and grease removal.55 Flotation and coagulation can efficiently remove dispersed oil and grease, therefore, they are often utilized for produced water treatment.
The most common advanced treatment technologies utilized for oil and gas produced water treatment include membrane filtration, thermal distillation, adsorption, ion exchange and advanced oxidation. Nanofiltration and reverse osmosis (RO) units are utilized for removal of inorganic ions from produced water with TDS below 40 g/L.56 However, TDS of shale gas produced water can reach 200 g/L, therefore other technologies such as thermal distillation are used instead of RO.26 Mechanical vapor recompression is particularly suitable for demineralized water recovery from shale gas produced water with high TDS.57 Sorption to activated carbon, advanced oxidation and biological treatment are used for removal of dissolved organic compounds, often preceding reverse osmosis in order to remove potential foulants from feedwater.58
The detailed description of treatment technologies, their advantages and drawbacks, energy consumption and cost efficiency can be found in a number of reviews on produced water treatment.26,54,55,59−62 This review is focused instead on the ability of produced water treatment technologies to remove prioritized shale gas-related organic compounds as defined in Section 3. Influence of operational parameters and matrix effects, especially high salt content, on organic compounds removal is also discussed.
4.1. Basic Separation Technologies
Basic separation technologies are not designed for removal of individual organic contaminants. However, some of the compounds are removed through volatilization or sorption to suspended particles. Stripping of VOCs and, to lesser extent, sVOCs, occurs in dissolved air flotation (DAF) units63 (Table 3). In contrast, low (10%) removal of volatile and semivolatile chlorinated organic compounds, such as halomethanes and chloroethylenes in a DAF unit was observed.64 Removal of VOCs decreases when oil and grease content of refinery wastewater increases from 100 to 500 mg/L, possibly because oil and grease accumulation on the water surface reduces surface volatilization of these compounds.64 With oil and grease content being on average below 50 mg/L11 significant fraction of VOCs is expected to be removed from shale gas FPW in DAF units.
Table 3. Removal of Prioritized Shale Gas-Related Organic Compounds via Selected FPW Treatment Processesa.
| technology | VOCs | PAHs | phthalates | ADBAC | 2-BE | organohalogens |
|---|---|---|---|---|---|---|
| flotation | + to + +61 | + to + +64 | ND | ND | ND | +62 |
| coagulation/flocculation | ++65 | ++64,65 | ++66 | ND | –51 | –69 |
| electrocoagulation | ND | ++73 | +++72 | ND | ND | –71 |
| membrane separation (nanofiltration & reverse osmosis) | + to + +75 | +++78 | +++77 | +++79 | -80 | + to + ++75,76 |
| thermal distillation (VCD, MSF, dewvaporation) | ++55 | ++84 | ND | ND | ND | +++83 |
| MPPE | +++96,97 | +++96,97 | ND | ND | ND | +++96,97 |
| aerobic biodegradation | + to + +65,114 | + to + +65,115,116 | +118−120 | ++ to + ++121−123 | +++125,126 | ++127−129b |
| anaerobic biodegradation | + to + +113 | - to + +115 | +119 | ++121−123 | +++125 | |
| MBR | +++132,133,136 | +++132,133 | +120 | ND | ND | + to + ++134 |
– removal <20%; + removal 20–50%; + + removal 50–90%; + ++ removal >90%.
Removal attributed to the subsequent application of anaerobic and aerobic treatment.
Partial removal of hydrophobic semivolatile and nonvolatile compounds, such as high molecular weight PAHs, can be accomplished by their distribution to dispersed oil droplets with subsequent skimming of the droplets from water surface.65 However, removal of PAHs via this mechanism is quite low, for example, Younker and Walsh (2014) reported naphthalene removal of <15% in the DAF unit with initial naphthalene concentration of 1 mg/L.66 Phenol removal was not observed in the same study, therefore, removal of water-soluble organic compounds, including 2-butoxyethanol, is not expected, if absorption to dispersed oil with subsequent skimming is the major mechanism of their removal.66
Removal of PAH reached 20% (initial concentration -19.1 μg/L) in the pilot-scale coagulation-flocculation unit treating oil plant produced water with comparatively low salinity (5 g/L of NaCl) with aluminum sulfate used as a coagulant.67 Younker and Walsh (2014) observed increase of naphthalene removal from 20% to 50% after addition of FeCl3 to DAF unit as a coagulant66 Removal of phthalates (DEHP, dibutyl-phthalate, diethyl-phthalate) in the full-scale installation treating landfill leachate by coagulation with FeCl3 was below 50%.68 Correlation between removal of phthalates and removal of DOC exists, with higher phthalate removal observed when higher fraction of DOC is removed.69 Higher phthalate removal is achieved with aluminum ions used as coagulant instead of iron.70 Gopal (2007) reported low removal of halogenated disinfection byproducts in coagulation processes used in drinking water treatment.71 Combination of charge neutralization, adsorption, entrapment, and complexation with coagulant ions are responsible for removal of organic compounds in coagulation unit. Therefore, removal efficiencies depend on physicochemical characteristics of compounds but also on operational parameters of the process.72
Electrocoagulation is the variation of coagulation process, where coagulant is generated in situ by oxidation of electrode material. Besides generation of coagulant, the process promotes degradation of organic matter by generation of oxidative species, such as hydroxyl ions, hypochlorite and hypochlorous acid.73 Kabdasli et al. (2009) reported complete mineralization of aqueous DEHP (50–100 mg/L) in NaCl solution.74 Nassef (2014) achieved complete removal of PAHs (β-naphthols) from NaCl solution of 1–7 g/L by electrocoagulation, with higher removal efficiencies at higher NaCl concentrations.75 However, the author did not study the mechanisms of β-naphthols removal and formation of halogenated transformation products. At the same time, halogenation of organic compounds in highly saline produced water can be expected during electrocoagulation73
4.2. Advanced Treatment Technologies
4.2.1. Membrane Separation
Membrane separation is often employed for produced water treatment in oil and gas industry, either as a combination of membranes with different retention properties (microfiltration and ultrafiltration for removal of suspended matter followed by nanofiltration and reverse osmosis for desalination) or as a desalination technology (reverse osmosis). The membranes in oil and gas wastewater are preceded by different pretreatment technologies (media filtration, dissolved air flotation or biological treatment), because they are not resistant to oil constituents, such as BTEX.56 Microfiltration and ultrafiltration membranes are designed for removal of suspended and colloidal matter, bacteria, viruses and high molecular weight hydrocarbons and have low to moderate efficiency toward removal of low molecular weight organic contaminants.76
Removal of VOCs, chlorinated organics and phthalates by nanofiltration and reverse osmosis (RO) is reviewed by Agenson et al. (2003) and Rodriguez et al. (2012). Rejection of these organic compounds is more effective with high desalting membranes with molecular weight cutoff (MWCO) ≤ 100 Da, then low desalting membranes with MWCO = 200 Da.77,78 Consistently low removal of dichloroethylenes (<40%), halogenated methane (<50%) and benzene (<55%) was observed with different types of high desalting RO membranes. Removal of molecules with higher molecular weight, such as toluene (>65%) and xylenes (>90%) was considerably higher.77 The authors concluded that membrane material, as well as size, dimensions and logKow of organic molecules appeared to be important parameters governing removal of organic compounds via RO with low rejection of volatile nonbranched low molecular weight compounds.
Removal of high molecular weight phthalates, including DEHP, exceeded 90% with RO, but also nanofiltration (MWCO = 300 Da) and ultrafiltration (MWCO = 8000 Da) membranes independent of the concentrations of phthalates79 Sorption to membrane surface is possible mechanism for phthalate removal by ultrafiltration. Removal of eight PAHs, including naphthalene and phenanthrene exceeded 95% in a pilot-scale RO system applied to shale gas produced water.80 DBNPA, a biocide, often used in hydraulic fracturing operations, is also efficiently removed by RO membranes (rejection of 98.5–99.5%).81 DBNPA is even recommended for usage as an antifouling agent in drinking water treatment.81 At the same time, low molecular weight semivolatile 2-butoxyethanol was not rejected with ASF-99 RO membrane characterized by high NaCl rejection.82
In summary, reverse osmosis can remove most of the organic compounds of concern from FPW, except of low molecular weight chlorinated hydrocarbons and 2-butoxyethanol, which are not rejected even by high-desalting RO membranes (Table 3).
4.2.2. Thermal Distillation
The operational costs of thermal distillation technologies are several times higher than that of reverse osmosis because of the high energy consumption.26 At the same time, thermal distillation is often utilized for FPW treatment in the full-scale installations, though it is justified rather by the need to handle produced water with high salinity (TDS > 40 g/L) then by cost efficiency.26,83,84 Multistage flash (MSF), multieffect distillation (MSD), mechanical vapor recompression (MVR) and dew-vaporation are four most common thermal distillation technologies, with MVR being most often used for shale gas FPW treatment.26,57
High removal of VOCs in thermal distillation units is achieved mainly due to volatilization with vapor being removed from the system during degassing events (Table 3). Thus, Hayes et al. (2014) observed 94% removal of VOCs in full scale MVR plant treating shale gas FPW through degassing and pressure-relief valves throughout the separator tank. The rest of VOCs (6% of the initial concentration) was found in distillate, being condensed from vapor together with distilled water, while brine did not contain VOCs.57 Removal of chlorinated disinfection byproducts (trihalomethanes and haloacetic acids) from seawater with high bromide content reached 99% in a MSF unit.85 Whereas trihalomethanes were completely vented to the air, 82% of less volatile haloacetic acids were found in brine. PAH removal by distillation is less effective, with 8% of naphthalene and >30% of methylnaphthalene recovered in the distillate of the circulation evaporator treating oil field produced water.86 No data on removal of phthalates, 2-butoxyethanol and quaternary ammonium compounds were found in the literature, though high solubility and moderate volatility of 2-butoxyethanol indicate that this compound can be partly condensed from vapor.
The available literature implies that thermal distillation technologies do not abate VOCs and sVOCs, but rather transform them from liquid to gas phase, followed by partial condensation in treated distillate (Table 3). The vapor thus needs to be treated before being released to the atmosphere in order to prevent environmental and human health risks related to VOCs.
4.2.3. Adsorption to Activated Carbon
Adsorption to activated carbon is usually utilized as a polishing step in wastewater treatment, because carbon can be easily overloaded with organics, especially with oil and grease.26 Granular activated carbon (GAC) is preferred to powdered activated carbon (PAC) for oil and gas industry applications because of the lower carbon usage rate and associated operational costs.87 Adsorption to activated carbon is efficient toward wide variety of organic compounds, but strongly depends on many factors, including dose and contact time, intrinsic properties of activated carbon, size, chemical structure and polarity of the adsorbates and solution properties, such as presence of competing organic matter, pH, and salinity.
Efficient removal of PAHs, including naphthalene and phenanthrene, quaternary ammonium compounds, 2-butoxyethanol, and chlorinated organic compounds via sorption to activated carbon was reported in the literature88−92 However, removal of phthalates by activated carbon is inefficient, especially at basic pH, because of the increased electrostatic resistance between negatively charged activated carbon surface and phthalate anions, produced under basic conditions.93,94 Low affinity of phthalates to activated carbon will ultimately result in low performance of full scale installation, as shown by Asakura and Matsuto (2009), who predicted 50–70% removal of DEHP from leachate, based on carbon utilization rate of 1 kg/m3·d and initial DEHP concentration of 50 μg/L.68
It should be noted, that most of the experiments on sorption of organic contaminants were performed in distilled water or distilled water with artificial addition of organic matter or NaCl, therefore, knowledge on sorption of individual organic contaminants in real FPW is missing. Moreover, different conditions of the experiments, reported in the literature, did not allow comparison of the adsorption capacity of activated carbon for all prioritized shale gas-related compounds from shale gas FPW.
High water salinity has several opposite effects on the adsorption of organic compounds. It is widely accepted that increased salinity improves sorption of organic compounds to activated carbon decreasing their solubility in the solution (so-called “salting out effect”) and suppressing electrostatic repulsions between organic compounds and activated carbon.89,95 Thus, adsorption of benzyltriethylammonium chloride in the presence of NaCl (Freundlich adsorption constant Kf = 285 mg/g) is higher than its adsorption in fresh water solution (Kf = 235 mg/g).90 Increased adsorption of PAH to different adsorbents with increased salt concentration was reported by several authors.95 At the same time, high salinity does not influence or may even enhance the adsorption of strongly hydrophobic compounds with low solubility, such as naphthalene or di-n-butyl phthalate, at low concentrations.89,96 Negative effect of increased salinity was also observed by some authors. Thus, adsorption of phenanthrene at low concentrations (3–10 μg/L) is lower in salt water, than in fresh water, whereas at high concentration (50–200 μg/Ll) the effect is opposite.88 High salinity may induce formation of water clusters on surface hydrophilic oxygen and carbonyl group, thus, directly competing with sorption of organics and hindering access to the graphitic basal planes of activated carbon.97 Thus, sorption of toluene in controlled pH solution decreased from 750 to 350 mg/g with increase of KCl concentration from 0.05 to 0.7 M.97
Other parameters, influencing sorption of organic compounds from FPW to activated carbon include dispersed oil content, temperature and pH. Dispersed oil decreases sorption of low molecular weight organic matter by competing with sorption sites on the surface of activated carbon and blocking access of low molecular weight organics to the inner micropores.89 Thus, addition of 100 mg/L emulsified crude oil decreased BET surface area of powdered activated carbon from 907.7 m2/g to 3.8 m2/g.89 Consequently, adsorption to GAC in oil and gas produced water treatment always follows pretreatment step designed for oil and grease removal, for example, DAF or coagulation.
4.2.4. Macro Porous Polymer Extraction (MPPE)
MPPE is a liquid–liquid extraction technique developed for hydrocarbon removal from oil and gas produced water with extraction liquid immobilized in porous polymer particles.98 The particles are immobilized in columns and periodically regenerated in situ by stripping with low pressure steam followed by condensation of recovered hydrocarbons in a concentrated solution. The pilot field tests and results obtained from full scale installations proved that 95–99% removal of BTEX, PAHs, CS2, aliphatic and aromatic chlorinated hydrocarbons, including volatile chlorinated hydrocarbons with low molecular weight, is achieved with this technology (Table 3).98,99 MPPE performance toward removal of fracturing fluid chemicals has not been studied so far.
4.2.5. Advanced Oxidation
Advanced oxidation processes are used in oil and gas produced water treatment for removal of organic and some inorganic compounds, disinfection and removal of odor and color.26 Commonly used oxidants include ozone, hydrogen peroxide, chlorine and Fenton’s reagent (combination of hydrogen peroxide with ferrous iron). Flowback water treatment technology based on the combination of hydrodynamic cavitation, ozonation, acoustic cavitation and electrochemical oxidation is used for removal of organic matter, bacteria and scalants either for flowback reuse or as a pretreatment for reverse osmosis aiming at water discharge.100
Advanced oxidation processes remove vast majority of organic compounds present in FPW (Table 3).70,101 PAHs, including naphthalene and phenanthrene are efficiently removed by ozonation with reaction rate being higher at acidic and neutral conditions.102,103 Combination of ozonation with hydrogen peroxide, ultraviolet (UV) or both does not enhance PAH removal,102 Photocatalytic oxidation of PAH in produced water is much slower than ozonation, probably, because of the light scavenging effect by turbidity.103 Contrary to PAH removal, only moderate removal of phthalates (30%) is achieved with ozone70 Combination of ozone with catalysts (Cr3+, GAC) or alkalination of solution increases removal efficiency of phthalates to 65–80% due to the higher decomposition of ozone into hydroxyl radicals.70
Ozonation is efficient toward removal of quaternary ammonium compounds, as shown by Hernandez Leal et al. (2011), who achieved >98% removal of benzalkonium chloride (initial concentration 1 mg/L) in aerobically treated gray water after application of 1.22 mg/L of ozone.104 Removal of 2-butoxyethanol by ozonation is not reported in the literature. However, its removal by Fenton oxidation (combination of H2O2 and Fe(II)) from water-based food paint wastewater (COD = 18 g/L, Cl– = 3.9 g/L) was above 95%.105
Halogenated aliphatic compounds (e.g., trichloromethane, trichloroethylene tetrachloroethylene), as well as halogenated aromatic compounds and phthalates do not react readily with ozone, therefore, combination with other oxidation processes, such as O3–UV and O3–H2O2 systems generating OḢ and other strong oxidants, are required for their destruction.106,107 Additionally, ozonation of FPW with high chloride and bromide concentration will promote formation of chlorinated and brominated organic compounds that can be more toxic than parent compounds since ozone enhances formation of Cl̇ and Bṙ radicals.107
The rate constant of OḢ reaction with chloride (kOH = 7.5 · 104 M–1 s–1) is significantly lower than the rate constants of OḢ reaction with organic compounds (kOH > 1 · 107 M–1 s–1).39,108 Therefore, increase of Cl– concentrations from 0 to 50 g/L only slightly decreases ozonation efficiency catalyzing ozone destruction by high salinity.109 The presence of chloride also slightly enhances ozone decomposition and radical formation, thus, decreasing ozone selectivity.110,111 In contrast, significant negative influence of salinity on Fenton’s oxidation process was found with Fenton’s reaction being inhibited by complexation of Fe3+ with Cl– and scavenging of OḢ radicals by chloride.112
Contrary to chloride, bromide with kOH of 8.5 · 108 M–1 s–1 may compete with organic compounds significantly decreasing efficiency of ozonation in FPW by high concentrations of bromide.39 Whereas organic compounds with kOH > 1 × 109 M–1 s–1 including BTEX, PAHs, and chloroethylenes will be targeted by hydroxyl radicals, oxidation of common low molecular weight degradation products, such as acetate (kOH = 1.9 × 107 M–1 s–1), methanol (kOH = 4.6 × 108 M–1 s–1) and trichloroacetate (kOH = 6 × 107 M–1 s–1) will be scavenged by bromides.39,108 Application of ozonation for partial destruction of high molecular weight organic compounds with high kOH followed by biological treatment or adsorption to activated carbon can be implemented for removal of intermediates from FPW with high bromide content.113
4.3. Biological Treatment
Biological aerobic and anaerobic treatment of FPW was successfully implemented both in the lab and industrial scale.39,58,114 Aerobic biological treatment in sequencing batch reactor is suitable for shale gas FPW with moderate salinity (TDS = 22.5 g/L) and high acetic acid content (16 mg/L).39 Combination of anaerobic and aerobic treatment and a membrane bioreactor is used as pretreatment for organic matter removal prior to a membrane unit at the Pinedale Anticline FPW treatment facility.58,114
Low to moderate removal of VOCs, PAHs and phthalates during aerobic and anaerobic treatment of industrial wastewaters is reported by several authors.67,115−117 Thus, 40% of the sum of PAHs present at initial concentrations of 15.2 μg/Lwere degraded in pilot-scale aerobic SBR treating petrochemical wastewater at long hydraulic retention time (HRT = 22 d.).67 Two to three ring PAHs are more amenable to biodegradation under both aerobic and anaerobic conditions when compared to PAH with >3 rings.117 Thus, naphthalene removal reached 61% in the aeration tank of the municipal sewage treatment plant (HRT = 3 h), whereas removal of phenanthrene was <40% and removal of benzo(a)pyrene did not exceed 15%.118 Selection of bacteria responsible for degradation of PAH might occur with increased SRT after long-term reactor operation115,119
Biodegradation of phthalates depends on their molecular weight, with high removal observed for low molecular weight phthalates, such as dimethyl phthalate and diethyl phthalate, under both aerobic and anaerobic conditions.120,121 Aerobic biological removal of high molecular weight phthalates, such as DEHP, is negligible at different HRT and SRT.120,122 DEHP and DnOP are not degraded under anaerobic conditions either.121
Aerobic conditions are more favorable for biodegradation of quaternary ammonium compounds, including ADBAC, when compared to anaerobic conditions.123−125 Apart from biodegradation, sorption to sludge contributes significantly to removal of QAC.126 Decrease of benzalkonium chloride concentrations in sludge of UASB reactor, operated on a mixture of black water and kitchen waste for more than 2 years without sludge removal, was observed over one year period, indicating that adaptation of anaerobic bacterial consortium with subsequent increase of QAC biodegradation might occur.126
2-butoxyethanol was readily biodegradable both under aerobic and anaerobic conditions in batch experiments with saline water and marine sediments.127 Similar results were obtained in an aeration tank of sewage treatment plant, with 2-butoxyethanol concentrations decreasing from 140.6 μg/L to <0.1 μg/L.128
Biological removal of halogenated organic compounds under aerobic conditions is generally low.129 Co-metabolic removal by several groups of aerobic bacteria, including methane-oxidizing methanotrophs, toluene oxidizers and phenol oxidizers is achieved, therefore, high concentrations of methane, toluene and phenols observed in FPW will promote aerobic degradation of halogenated organic compounds.130 Combination of subsequent anaerobic treatment for dehalogenation and aerobic treatment for ring cleavage of aromatic structures and complete mineralization is the most efficient strategy for mineralization of haloorganic compounds.129−131
Removal of organic pollutants is generally higher in membrane bioreactors (MBR) than in conventional activated sludge treatment systems.132 Membrane bioreactors (MBR) are effective toward polar organic compounds that are removed by combination of sorption and biodegradation, whereas removal of hydrophilic compounds is variable and depends on their structure.133 Removal of six PAHs, including naphthalene and phenanthrene, from synthetic petrochemical wastewater in two aerobic MBR with sidestream filtration based on cross-flow and semi dead-end filtration systems respectively, exceeded 90%.134 Biological degradation was the main removal mechanisms, responsible for at least 50% removal of each investigated compound. Volatilization was responsible for removal of <15% of low molecular weight PAH, whereas >20% of high molecular weight PAH were removed by adsorption.134 Aerobic submerged MBR is also suitable for removal of aliphatic hydrocarbons, monoaromatic hydrocarbons, aliphatic acids, aromatic acids, and PAH from oil produced water with high salinity.135 However, phthalates are poorly removed in membrane bioreactor, as shown by Llop et al. (2009), who observed negligible removal of DEHP in the submerged-filter MBR treating wastewater from chemical industry.122 The data on QAC and 2-butoxyethanol removal in MBR were not found, though high removal of these compounds is expected based on the results achieved in conventional aerobic activated sludge reactors.
Removal of halogenated organic compounds in MBR depends not only on biodegradation and/or volatilization, but also on sorption. Compounds with high log D (>3.2) and high halogen content (>0.3), as well as compounds with low log D (<3.2) and low halogen content (<0.1) are well removed (>85%) when compared to other organohalogens.136 Combination of anaerobic and aerobic MBR promote biodegradation of halogenated compounds, as mentioned earlier.129,130
High salinity of produced water is the main challenge for application of biological processes for FPW treatment, because TDS concentrations above 10 g/L can negatively influence biological activity by plasmolysis and loss of cell activity.62 However, removal of organic compounds in anaerobic and aerobic treatment systems are observed even at much higher salinity. Thus, Belkin et al. (1993) reported DOC removal of 70% at TDS of 32 g/L and 40% at TDS of 100 g/L in the combination of anaerobic and aerobic SBRs with HRTs of 20 days and 6 days respectively treating wastewater from chemical industry.131 TOC removal decreased from 90% to 40% and COD removal–from 90% to 17% in the MBR treating real oilfield produced water at 16 and 250 g/L TDS respectively.137 The impact of FPW salinity on biological removal of PAH, phthalates and QAC in activated sludge systems and MBR requires further investigation, since available literature mainly reports biological removal of these compounds from municipal and industrial wastewaters with low salinity.
5. Prospects for Removal of Prioritized Shale Gas-Related Organic Compounds with Selected Technologies
The main objectives for FPW treatment aimed at discharge to surface water include removal of suspended matter, oil and grease, dissolved organic compounds and salts. Removal of NORM is also required for the FPW with enhanced NORM content. The review of technologies supports the choice in favor of DAF with coagulation followed by MBR, ultrafiltration and reverse osmosis for FPW with low salinity and ozonation, GAC filtration and thermal distillation for FPW with high salinity.
DAF assisted with coagulant addition achieves the best performance among the basic separation technologies removing particles down to 0.3 μm, though removing only a minor fraction of prioritized shale gas-related organic compounds mostly by stripping. Collection and treatment of the generated off-gases is required to minimize negative impacts on the environment and human health.4
Removal of organic compounds should precede the salt removal, because main methods utilized for desalination are either negatively influenced by organic matter (reverse osmosis) or cannot provide adequate removal of organic compounds with low molecular weight (thermal distillation). Application of subsequent anaerobic and aerobic biological treatment, where aerobic treatment stage can be designed as a submerged-filter MBR, is desired for removal of organohalogens, naturally present in the produced water. MBR is more efficient in removal of PAHs then conventional activated sludge, whereas its submerged-filter configuration is characterized by lower volatilization of organic compounds, when compared to the sidestream filtration.134,138 MBR can be followed by ultrafiltration and RO for removal of TDS and, eventually, radioactive materials, such as radium-226.139
Fraction of low molecular weight hydrophilic organic compounds is often dominating DOM of FPW making biological treatment feasible.32,39 However, presence of refractory organic compounds, such as phthalates and certain transformation products of fracturing chemicals may require application of alternative treatment strategies, such as adsorption to activated carbon or ozonation either in combination with biological treatment or as a stand-alone processes.
Treatment of FPW with high salinity (TDS > 40g/L) requires another strategy, as both biological treatment and RO are not applicable at such salt concentrations. Thermal distillation is normally used for treatment of FPW with high salinity. Apart from high energy costs and significant amount of solid waste created, thermal distillation processes have several disadvantages with respect to removal of prioritized shale gas-related organic compounds. They transfer the VOCs and sVOCs mostly to the gas phase, concentrate organic compounds with high molecular weight in brine, which can constitute up to 30% of initial water volume, whereas low-molecular weight VOCs and sVOCs end up in the distillate. Preozonation of high-molecular weight organics coupled to adsorption to activated carbon or adsorption to activated carbon alone can be used as a pretreatment step before thermal distillation.
Source control measures, such as substitution of prioritized shale gas-related organic compounds in frack fluids by less hazardous alternatives should be considered.9 Thus, QAC biocides should be substituted by easily biodegradable and less hazardous compounds, such as glutaraldehyde.52 The separation and multiple reuse of early flowback may be an effective measure for prevention of environmental contamination, because prioritized shale gas-related organic compounds originating from frack fluid will remain within the shale gas production cycle, with only minor portion entering the environment.5,8,9
Finally, the impact of organic compounds from shale gas FPW on drinking water sources deserves special attention. The existing uncertainties include the human toxicity thresholds, the fate of organic contaminants in surface waters, their dilution before water intake for drinking water production and capacity of various drinking water treatment technologies for removal of these specific compounds. More elaborated studies on human toxicity assessment of FPW chemicals, including analysis of organic compounds with proven human toxicity in FPW and potential formation of disinfection byproducts from FPW-originated precursors in drinking water sources are needed.
Acknowledgments
This work was funded by The Netherlands Organisation for Scientific Research (NWO) Earth and Life Sciences (ALW), project number 859.14.001, and the water utilities Brabant Water, Oasen and WML.
The authors declare no competing financial interest.
References
- Melikoglu M. Shale gas: Analysis of its role in the global energy market. Renewable Sustainable Energy Rev. 2014, 37, 460–468. 10.1016/j.rser.2014.05.002. [DOI] [Google Scholar]
- Howarth R. W.; Ingraffea A.; Engelder T. Natural gas: Should fracking stop?. Nature 2011, 477 (7364), 271–273. 10.1038/477271a. [DOI] [PubMed] [Google Scholar]
- Sovacool B. K. Cornucopia or curse? Reviewing the costs and benefits of shale gas hydraulic fracturing (fracking). Renewable Sustainable Energy Rev. 2014, 37, 249–264. 10.1016/j.rser.2014.04.068. [DOI] [Google Scholar]
- Wang Q.; Chen X.; Jha A. N.; Rogers H. Natural gas from shale formation - The evolution, evidences and challenges of shale gas revolution in United States. Renewable Sustainable Energy Rev. 2014, 30, 1–28. 10.1016/j.rser.2013.08.065. [DOI] [Google Scholar]
- Ziemkiewicz P. F.; Quaranta J. D.; Darnell A.; Wise R. Exposure pathways related to shale gas development and procedures for reducing environmental and public risk. J. Nat. Gas Sci. Eng. 2014, 16, 77–84. 10.1016/j.jngse.2013.11.003. [DOI] [Google Scholar]
- Bergmann A.; Weber F. A.; Meiners H. G.; Müller F. Potential water-related environmental risks of hydraulic fracturing employed in exploration and exploitation of unconventional natural gas reservoirs in Germany. Environ. Sci. Eur. 2014, 26 (1), 1. 10.1186/2190-4715-26-10. [DOI] [Google Scholar]
- Finkel M. L.; Hays J. The implications of unconventional drilling fornatural gas: A global public health concern. Public Health 2013, 127 (10), 889–893. 10.1016/j.puhe.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Rahm B. G.; Riha S. J. Evolving shale gas management: Water resource risks, impacts, and lessons learned. Environmental Sciences: Processes and Impacts 2014, 16 (6), 1400–1412. 10.1039/c4em00018h. [DOI] [PubMed] [Google Scholar]
- Small M. J.; Stern P. C.; Bomberg E.; Christopherson S. M.; Goldstein B. D.; Israel A. L.; Jackson R. B.; Krupnick A.; Mauter M. S.; Nash J.; North D. W.; Olmstead S. M.; Prakash A.; Rabe B.; Richardson N.; Tierney S.; Webler T.; Wong-Parodi G.; Zielinska B. Risks and risk governance in unconventional shale gas development. Environ. Sci. Technol. 2014, 48 (15), 8289–8297. 10.1021/es502111u. [DOI] [PubMed] [Google Scholar]
- Vengosh A.; Jackson R. B.; Warner N.; Darrah T. H.; Kondash A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48 (15), 8334–8348. 10.1021/es405118y. [DOI] [PubMed] [Google Scholar]
- Gregory K. B.; Vidic R. D.; Dzombak D. A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 2011, 7 (3), 181–186. 10.2113/gselements.7.3.181. [DOI] [Google Scholar]
- Stringfellow W. T.; Domen J. K.; Camarillo M. K.; Sandelin W. L.; Borglin S. Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J. Hazard. Mater. 2014, 275, 37–54. 10.1016/j.jhazmat.2014.04.040. [DOI] [PubMed] [Google Scholar]
- Ferrer I.; Thurman E. M. Chemical constituents and analytical approaches for hydraulic fracturing waters. Trends Environ. Anal. Chem. 2015, 5, 18–25. 10.1016/j.teac.2015.01.003. [DOI] [Google Scholar]
- Pakulska D. Chemical hazards arising from shale gas extraction. Med. Pr. 2015, 66 (1), 99–117. 10.13075/mp.5893.00147. [DOI] [PubMed] [Google Scholar]
- Yost E. E.; Stanek J.; Dewoskin R. S.; Burgoon L. D. Overview of Chronic Oral Toxicity Values for Chemicals Present in Hydraulic Fracturing Fluids, Flowback, and Produced Waters. Environ. Sci. Technol. 2016, 50 (9), 4788–4797. 10.1021/acs.est.5b04645. [DOI] [PubMed] [Google Scholar]
- EPA. Hydraulic Fracturing for Oil and Gas: Impacts from the Hydraulic Fracturing Water Cycle on Drinking Water Resources in the United States (Final Report). 2016.
- Hayes T. D.Sampling and Analysis of Water Streams Associated with the Development of Marcellus Shale Gas; Gas Technology Institute: December 2009. [Google Scholar]
- Nicot J. P.; Scanlon B. R. Water use for shale-gas production in Texas, U.S. Environ. Sci. Technol. 2012, 46 (6), 3580–3586. 10.1021/es204602t. [DOI] [PubMed] [Google Scholar]
- Shih J. S.; Saiers J. E.; Anisfeld S. C.; Chu Z.; Muehlenbachs L. A.; Olmstead S. M. Characterization and Analysis of Liquid Waste from Marcellus Shale Gas Development. Environ. Sci. Technol. 2015, 49 (16), 9557–9565. 10.1021/acs.est.5b01780. [DOI] [PubMed] [Google Scholar]
- Annevelink M. P. J. A.; Meesters J. A. J.; Hendriks A. J. Environmental contamination due to shale gas development. Sci. Total Environ. 2016, 550, 431–438. 10.1016/j.scitotenv.2016.01.131. [DOI] [PubMed] [Google Scholar]
- Rahm B. G.; Bates J. T.; Bertoia L. R.; Galford A. E.; Yoxtheimer D. A.; Riha S. J. Wastewater management and Marcellus Shale gas development: Trends, drivers, and planning implications. J. Environ. Manage. 2013, 120, 105–113. 10.1016/j.jenvman.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Nicot J. P.; Scanlon B. R.; Reedy R. C.; Costley R. A. Source and fate of hydraulic fracturing water in the barnett shale: A historical perspective. Environ. Sci. Technol. 2014, 48 (4), 2464–2471. 10.1021/es404050r. [DOI] [PubMed] [Google Scholar]
- Rahm B. G.; Riha S. J. Toward strategic management of shale gas development: Regional, collective impacts on water resources. Environ. Sci. Policy 2012, 17, 12–23. 10.1016/j.envsci.2011.12.004. [DOI] [Google Scholar]
- Vandecasteele I.; Marí Rivero I.; Sala S.; Baranzelli C.; Barranco R.; Batelaan O.; Lavalle C. Impact of Shale Gas Development on Water Resources: A Case Study in Northern Poland. Environ. Manage. 2015, 55 (6), 1285–1299. 10.1007/s00267-015-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson O.; Weichgrebe D.; Rosenwinkel K. H. Hydraulic fracturing wastewater in Germany: Composition, treatment, concerns. Environ. Earth Sci. 2013, 70 (8), 3895–3906. 10.1007/s12665-013-2535-4. [DOI] [Google Scholar]
- Igunnu E. T.; Chen G. Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9 (3), 157–177. 10.1093/ijlct/cts049. [DOI] [Google Scholar]
- Ziemkiewicz P. F.; Thomas H. Y. Evolution of water chemistry during Marcellus Shale gas development: A case study in West Virginia. Chemosphere 2015, 134, 224–231. 10.1016/j.chemosphere.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Kantor M.; Konieczyńska M.; Lipińska O. Shale gas exploration - The results of environmental field studies. Przeglad Geologiczny 2015, 63 (7), 404–409. [Google Scholar]
- Maguire-Boyle S. J.; Barron A. R. Organic compounds in produced waters from shale gas wells. Environmental Sciences: Processes and Impacts 2014, 16 (10), 2237–2248. 10.1039/C4EM00376D. [DOI] [PubMed] [Google Scholar]
- Camarillo M. K.; Domen J. K.; Stringfellow W. T. Physical-chemical evaluation of hydraulic fracturing chemicals in the context of produced water treatment. J. Environ. Manage. 2016, 183 (Part 1), 164–174. 10.1016/j.jenvman.2016.08.065. [DOI] [PubMed] [Google Scholar]
- Kahrilas G. A.; Blotevogel J.; Corrin E. R.; Borch T. Downhole Transformation of the Hydraulic Fracturing Fluid Biocide Glutaraldehyde: Implications for Flowback and Produced Water Quality. Environ. Sci. Technol. 2016, 50 (20), 11414–11423. 10.1021/acs.est.6b02881. [DOI] [PubMed] [Google Scholar]
- Orem W.; Tatu C.; Varonka M.; Lerch H.; Bates A.; Engle M.; Crosby L.; McIntosh J. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int. J. Coal Geol. 2014, 126, 20–31. 10.1016/j.coal.2014.01.003. [DOI] [Google Scholar]
- Haluszczak L. O.; Rose A. W.; Kump L. R. Geochemical evaluation of flowback brine from Marcellus gas wells in Pennsylvania, USA. Appl. Geochem. 2013, 28, 55–61. 10.1016/j.apgeochem.2012.10.002. [DOI] [Google Scholar]
- Blondes M. S.; Gans K. D.; Rowan E. L.; Thordsen J. J.; Reidy M. E.; Engle M. A.; Kharaka Y. K.; Thomas B., National Produced Waters Geochemical Database v2.2. In USGS, Ed. 2016. [Google Scholar]
- FracFocus Chemical Disclosure Registry, Ground Water Pro-tection Council and Interstate Oil and Gas Compact Commission, 2016, (http://fracfocus.org) (accessed March 17, 2016). [Google Scholar]
- Soeder D. J.; Kappel W. M.. Water Resources and Natural Gas Production from the Marcellus Shale. U.S. Geological Survey Fact Sheet 2009–3032, 6p; 2009.
- Elsner M.; Hoelzer K. Quantitative Survey and Structural Classification of Hydraulic Fracturing Chemicals Reported in Unconventional Gas Production. Environ. Sci. Technol. 2016, 50 (7), 3290–3314. 10.1021/acs.est.5b02818. [DOI] [PubMed] [Google Scholar]
- Hayes T. D.; Severin B. F.. Characterisation of Flowback Waters from the Marcellus and the Barnett Shale Regions. RPSEA report No. 08122–05.09; Gas Technology Institute: 2012. [Google Scholar]
- Lester Y.; Ferrer I.; Thurman E. M.; Sitterley K. A.; Korak J. A.; Aiken G.; Linden K. G. Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment. Sci. Total Environ. 2015, 512–513, 637–644. 10.1016/j.scitotenv.2015.01.043. [DOI] [PubMed] [Google Scholar]
- Ferrer I.; Thurman E. M. Analysis of hydraulic fracturing additives by LC/Q-TOF-MS. Anal. Bioanal. Chem. 2015, 407 (21), 6417–6428. 10.1007/s00216-015-8780-5. [DOI] [PubMed] [Google Scholar]
- Thurman E. M.; Ferrer I.; Blotevogel J.; Borch T. Analysis of hydraulic fracturing flowback and produced waters using accurate mass: Identification of ethoxylated surfactants. Anal. Chem. 2014, 86 (19), 9653–9661. 10.1021/ac502163k. [DOI] [PubMed] [Google Scholar]
- Thacker J.; Carlton D.; Hildenbrand Z.; Kadjo A.; Schug K. Chemical Analysis of Wastewater from Unconventional Drilling Operations. Water 2015, 7 (4), 1568. 10.3390/w7041568. [DOI] [Google Scholar]
- Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances, Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances, Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market.; European Comission - Joint Research Centre Institute for Health and Consumer Protection European Chemical Bureau (ECB): 2003. [Google Scholar]
- Waterschap De Dommel. Webpage (accessed February 12, 2016). www.dommel.nl.
- EBN Notional Field Development Plan: Final Report; Halliburton: 2011. [Google Scholar]
- European Chemical Agency. Database of substances registered under REACH regulation.https://echa.europa.eu/information-on-chemicals/registered-substances (accessed August 28, 2016)..
- Ministry of the Environment of Japan. Profiles of the Initial Environmental Risk Assessment of Chemicals. https://www.env.go.jp/en/chemi/chemicals/profile_erac/index.html (accessed August 28. 2016).
- Hazardous Substances Data Bank: A Toxnet Database. http://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm (accessed on 28 August 2016).
- Verbruggen E. M. J.; Traas T. P.; Fleuren R. H. L. J.; Ciarelli S.; Posthumus R.; Vos J. H.; Scheepmaker J. W. A.; van Vlaardingen P. L. A.. Environmental Risk Limits for alcohols, glycols, and some other relatively soluble and/or volatile compounds. Part 1. Ecotoxicological evaluation.; Dutch National Institute for Public Health and the Environment (RIVM). Report #601501016/2005:: 2005. [Google Scholar]
- Directive 98/8/EC concerning the placing biocidal products on the market. Inclusion of active substances in Annex I to Directive 98/8/EC. Assessment Report for Alkyl (C12–16) dimethylbenzyl ammonium chloride. Product-type 8 (Wood preservative); European Comission: 2015. [Google Scholar]
- Liu N.; Wang Y.; Yang Q.; Lv Y.; Jin X.; Giesy J. P.; Johnson A. C. Probabilistic assessment of risks of diethylhexyl phthalate (DEHP) in surface waters of China on reproduction of fish. Environ. Pollut. 2016, 213, 482–488. 10.1016/j.envpol.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Kahrilas G. A.; Blotevogel J.; Stewart P. S.; Borch T. Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility, degradation, and toxicity. Environ. Sci. Technol. 2015, 49 (1), 16–32. 10.1021/es503724k. [DOI] [PubMed] [Google Scholar]
- Ferrar K. J.; Michanowicz D. R.; Christen C. L.; Mulcahy N.; Malone S. L.; Sharma R. K. Assessment of effluent contaminants from three facilities discharging marcellus shale wastewater to surface waters in pennsylvania. Environ. Sci. Technol. 2013, 47 (7), 3472–3481. 10.1021/es301411q. [DOI] [PubMed] [Google Scholar]
- Arthur J. D.; Langhus B. G.; Patel C.. Technical Summary of Oil and Gas Produced Water Treatment Technologies; All Consulting, 2005. [Google Scholar]
- Fakhru’l-Razi A.; Pendashteh A.; Abdullah L. C.; Biak D. R. A.; Madaeni S. S.; Abidin Z. Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170 (2–3), 530–551. 10.1016/j.jhazmat.2009.05.044. [DOI] [PubMed] [Google Scholar]
- Alzahrani S.; Mohammad A. W. Challenges and trends in membrane technology implementation for produced water treatment: A review. Journal of Water Process Engineering 2014, 4 (C), 107–133. 10.1016/j.jwpe.2014.09.007. [DOI] [Google Scholar]
- Hayes T. D.; Halldorson B.; Horner P. H.; Ewing J. L. R.; Werline J. R.; Severin B. F. Mechanical Vapor Recompression for the Treatment of Shale-Gas Flowback Water. Oil Gas Facil. 2014, 3 (4), 54–62. 10.2118/170247-PA. [DOI] [Google Scholar]
- Boschee P. Handling Produced Water from Hydraulic Fracturing. Oil Gas Facil. 2012, 1 (1), 23–26. 10.2118/0212-0022-OGF. [DOI] [Google Scholar]
- Abousnina R. M.; Nghiem L. D.; Bundschuh J. Comparison between oily and coal seam gas produced water with respect to quantity, characteristics and treatment technologies: a review. Desalin. Water Treat. 2015, 54 (7), 1793–1808. 10.1080/19443994.2014.893541. [DOI] [Google Scholar]
- Acharya H. R.; Henderson C.; Matis H.; Kommepalli H.; Moore B.; Wang H.. Cost Effective Recovery of Low-TDS Frac Flowback Water for Re-Use; GE Global Research: 2011. [Google Scholar]
- Drewes J. E.An Integrated Framework for Treatment and Management of Produced Water.; RPSEA final report #07122–12: 2011.
- Saba B. Potential Treatment Options for Hydraulic Fracturing Return Fluids: A Review. ChemBioEng Rev. 2014, 1 (6), 273–279. 10.1002/cben.201400003. [DOI] [Google Scholar]
- Bayati F.; Shayegan J.; Noorjahan A. Treatment of oilfield produced water by dissolved air precipitation/solvent sublation. J. Pet. Sci. Eng. 2011, 80 (1), 26–31. 10.1016/j.petrol.2011.10.001. [DOI] [Google Scholar]
- Parker W. J.; Monteith H. D. Stripping of VOC’s from dissolved air flotation. Environ. Prog. 1996, 15 (2), 73–81. 10.1002/ep.670150210. [DOI] [Google Scholar]
- Aromatics in Produced Water: Occurrence, Fate & Effects, and Treatment; International Association of Oil and Gas Producers, Report No. I.20/324, January 2002. [Google Scholar]
- Younker J. M.; Walsh M. E. Bench-scale investigation of an integrated adsorption-coagulation-dissolved air flotation process for produced water treatment. J. Environ. Chem. Eng. 2014, 2 (1), 692–697. 10.1016/j.jece.2013.11.009. [DOI] [Google Scholar]
- Steliga T.; Jakubowicz P.; Kapusta P. Changes in toxicity during treatment of wastewater from oil plant contaminated with petroleum hydrocarbons. J. Chem. Technol. Biotechnol. 2015, 90 (8), 1408–1418. 10.1002/jctb.4442. [DOI] [Google Scholar]
- Asakura H.; Matsuto T. Experimental study of behavior of endocrine-disrupting chemicals in leachate treatment process and evaluation of removal efficiency. Waste Manage. 2009, 29 (6), 1852–1859. 10.1016/j.wasman.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Zhang H.; He P.-J.; Shao L.-M.; Chen Y.; Pang L. Co-removal of phthalic acid esters with dissolved organic matter from landfill leachate by coagulation and flocculation process. Chemosphere 2009, 75 (2), 180–186. 10.1016/j.chemosphere.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Zolfaghari M.; Drogui P.; Seyhi B.; Brar S. K.; Buelna G.; Dubé R. Occurrence, fate and effects of di (2-ethylhexyl) phthalate in wastewater treatment plants: A review. Environ. Pollut. 2014, 194, 281–293. 10.1016/j.envpol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Gopal K.; Tripathy S. S.; Bersillon J. L.; Dubey S. P. Chlorination byproducts, their toxicodynamics and removal from drinking water. J. Hazard. Mater. 2007, 140 (1–2), 1–6. 10.1016/j.jhazmat.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Alexander J. T.; Hai F. I.; Al-aboud T. M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. J. Environ. Manage. 2012, 111, 195–207. 10.1016/j.jenvman.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Kabdaşlı I.; Arslan-Alaton I.; Ölmez-Hancı T.; Tünay O. Electrocoagulation applications for industrial wastewaters: a critical review. Environ. Technol. Rev. 2012, 1 (1), 2–45. 10.1080/21622515.2012.715390. [DOI] [Google Scholar]
- Kabdaşlı I.; Keleş A.; Ölmez-Hancı T.; Tünay O.; Arslan-Alaton I. Treatment of phthalic acid esters by electrocoagulation with stainless steel electrodes using dimethyl phthalate as a model compound. J. Hazard. Mater. 2009, 171 (1–3), 932–940. 10.1016/j.jhazmat.2009.06.093. [DOI] [PubMed] [Google Scholar]
- Nassef E. M. R. Removal of polyaromatic hydrocarbons from waste water by electrocoagulation. J. Pet. Gas Eng. 2014, 5 (3), 32–42. 10.5897/JPGE2014.0192. [DOI] [Google Scholar]
- Smol M.; Włodarczyk-Makuła M.; Mielczarek K.; Bohdziewicz J. Comparison of the retention of selected PAHs from municipal landfill leachate by RO and UF processes. Desalin. Water Treat. 2014, 52 (19–21), 3889–3897. 10.1080/19443994.2014.887451. [DOI] [Google Scholar]
- Agenson K. O.; Oh J.-I.; Urase T. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: controlling parameters of process. J. Membr. Sci. 2003, 225 (1–2), 91–103. 10.1016/j.memsci.2003.08.006. [DOI] [Google Scholar]
- Rodriguez C.; Linge K.; Blair P.; Busetti F.; Devine B.; Van Buynder P.; Weinstein P.; Cook A. Recycled water: Potential health risks from volatile organic compounds and use of 1,4-dichlorobenzene as treatment performance indicator. Water Res. 2012, 46 (1), 93–106. 10.1016/j.watres.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Bodzek M.; Dudziak M.; Luks-Betlej K. Application of membrane techniques to water purification. Removal of phthalates. Desalination 2004, 162 (1–3), 121–128. 10.1016/S0011-9164(04)00035-9. [DOI] [Google Scholar]
- Regnery J.; Coday B. D.; Riley S. M.; Cath T. Y. Solid-phase extraction followed by gas chromatography-mass spectrometry for the quantitative analysis of semi-volatile hydrocarbons in hydraulic fracturing wastewaters. Anal. Methods 2016, 8 (9), 2058–2068. 10.1039/C6AY00169F. [DOI] [Google Scholar]
- Bertheas U.; Majamaa K.; Arzu A.; Pahnke R. Use of DBNPA to control biofouling in RO systems. Desalin. Water Treat. 2009, 3 (1–3), 175–178. 10.5004/dwt.2009.457. [DOI] [Google Scholar]
- Solyom P.Treatment of discarded fire fighting foam premixes. Project 704–011; IVL Svenska Miljöinstitutet AB: 2002.
- Boschee P. Produced and Flowback Water Recycling and Reuse. Oil and Gas Facilities 2014, 3 (1), 17–22. [Google Scholar]
- EPA. Study of the Potential Impacts of Hydraulic Fracturing on Drinking Water Resources. Progress report - EPA/601/R-12/011; 2012.
- Le Roux J.; Nada N.; Khan M. T.; Croué J.-P. Tracing disinfection byproducts in full-scale desalination plants. Desalination 2015, 359, 141–148. 10.1016/j.desal.2014.12.035. [DOI] [Google Scholar]
- Andrade V. T.; Andrade B. G.; Costa B. R. S.; Pereira O. A. Jr; Dezotti M. Toxicity assessment of oil field produced water treated by evaporative processes to produce water to irrigation. Water Sci. Technol. 2010, 62 (3), 693–700. 10.2166/wst.2010.340. [DOI] [PubMed] [Google Scholar]
- Hackney J.; Wiesner M. R., Cost assessment of Produced Water Treatment. Working paper, Duke University, Durham, NC. 1996. [Google Scholar]
- Karapanagioti H. K. Removal of phenanthrene from saltwater solutions using activated carbon. Desalination 2007, 210 (1–3), 274–280. 10.1016/j.desal.2006.05.052. [DOI] [Google Scholar]
- Younker J. M.; Walsh M. E. Impact of salinity and dispersed oil on adsorption of dissolved aromatic hydrocarbons by activated carbon and organoclay. J. Hazard. Mater. 2015, 299, 562–569. 10.1016/j.jhazmat.2015.07.063. [DOI] [PubMed] [Google Scholar]
- Pahari P. K.; Sharma M. M. Recovery of aromatic sulfonic acids and quaternary ammonium compounds from aqueous streams by adsorption. Sep. Technol. 1992, 2 (1), 39–45. 10.1016/0956-9618(92)80005-X. [DOI] [Google Scholar]
- Giusti D. M.; Conway R. A.; Lawson C. T. Activated Carbon Adsorption of Petrochemicals. Journal (Water Pollution Control Federation) 1974, 46 (5), 947–965. [Google Scholar]
- Pavoni B.; Drusian D.; Giacometti A.; Zanette M. Assessment of organic chlorinated compound removal from aqueous matrices by adsorption on activated carbon. Water Res. 2006, 40 (19), 3571–3579. 10.1016/j.watres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Julinová M.; Slavík R. Removal of phthalates from aqueous solution by different adsorbents: A short review. J. Environ. Manage. 2012, 94 (1), 13–24. 10.1016/j.jenvman.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Venkata Mohan S.; Shailaja S.; Rama Krishna M.; Sarma P. N. Adsorptive removal of phthalate ester (Di-ethyl phthalate) from aqueous phase by activated carbon: A kinetic study. J. Hazard. Mater. 2007, 146 (1–2), 278–282. 10.1016/j.jhazmat.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lamichhane S.; Bal Krishna K. C.; Sarukkalige R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. 10.1016/j.chemosphere.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Fang Z. Q.; Huang H. J., Effects of salinity and humic acid on di-n-butyl phthalate adsorption by granular activated carbon. In 4th International Conference on Bioinformatics and Biomedical Engineering, iCBBE 2010, Chengdu, China, 8–20 June 2010.
- Arafat H. A.; Franz M.; Pinto N. G. Effect of salt on the mechanism of adsorption of aromatics on activated carbon. Langmuir 1999, 15 (18), 5997–6003. 10.1021/la9813331. [DOI] [Google Scholar]
- Meijer D. T.; Madin C. Removal of Dissolved and Dispersed Hydrocarbons from Oil and Gas Produced Water with MPPE Technology to Reduce Toxicity and Allow Water Reuse. APPEA Journal 2010, 1–11. [Google Scholar]
- Pars H. M.; Meijer D. T., Removal of dissolved hydrocarbonsfrom production water by Macro Porous Polymer Extraction (MPPE). In SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production., Caracas, Venezuela, 7–10 June 1998.
- Ely J. W.; Horn A.; Cathey R.; Fraim M.; Jakhete S., Game changing technology for treating and recycling frac water. In SPE Annual Technical Conference and Exhibition, Denver, Colorado, USA, 30 October-2 November, 2011.
- Rubio-Clemente A.; Torres-Palma R. A.; Peñuela G. A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total Environ. 2014, 478, 201–225. 10.1016/j.scitotenv.2013.12.126. [DOI] [PubMed] [Google Scholar]
- Trapido M.; Veressinina Y.; Munter R. Ozonation and Advanced Oxidation Processes of Polycyclic Aromatic Hydrocarbons in Aqueous Solutions - A Kinetic Study. Environ. Technol. 1995, 16 (8), 729–740. 10.1080/09593331608616312. [DOI] [Google Scholar]
- Chen B.; Zhang B.; Jing L.; Liu B.; J.S Z.; Ma Y. C. , Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Offshore Produced Water using Advanced Oxidation Processes (AOPs). In 11th IWA Leading Edge Conference on Water and Wastewater Technologies, Abu Dhabi, United Arab Emirates, 26–30 May 2014.
- Hernández-Leal L.; Temmink H.; Zeeman G.; Buisman C. J. N. Removal of micropollutants from aerobically treated grey water via ozone and activated carbon. Water Res. 2011, 45 (9), 2887–2896. 10.1016/j.watres.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Vengris T.; Binkiene R.; Butkiene R.; Ragauskas R.; Stoncius A.; Manusadzianas L. Treatment of water-based wood paint wastewater with Fenton process. Chemija 2012, 23 (4), 263–268. [Google Scholar]
- Kosaka K.; Yamada H.; Tsuno H.; Shimizu Y.; Matsui S. The effects of dissolved organic matter on the decomposition of di-n-butyl phthalate by ozone/hydrogen peroxide process. Water science and technology: a journal of the International Association on Water Pollution Research 2004, 49 (4), 57–62. [PubMed] [Google Scholar]
- Langlais B.; Reckhow D. A.; Brink D. R.. Ozone in Water Treatment: Application and Engineering; CRC Press, 1991. [Google Scholar]
- von Gunten U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37 (7), 1443–1467. 10.1016/S0043-1354(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Walker A. B.; Tsouris C.; DePaoli D. W.; Klasson T. K. Ozonation of Soluble Organics in Aqueous Solutions Using Microbubbles. Ozone: Sci. Eng. 2000, 23, 77–87. 10.1080/01919510108961990. [DOI] [Google Scholar]
- Boncz M. A.; Bruning H.; Rulkens W. H.; Zuilhof H.; Sudhölter E. J. R. The effect of salts on ozone oxidation processes. Ozone: Sci. Eng. 2005, 27 (4), 287–292. 10.1080/01919510591006382. [DOI] [Google Scholar]
- Sotelo J. L.; Beltrán F. J.; González M.; Domínguez J. Effect of high salt concentrations on ozone decomposition in water. J. Environ. Sci. Health, Part A: Environ. Sci. Eng. 1989, 24 (7), 823–842. 10.1080/10934528909375518. [DOI] [Google Scholar]
- Siedlecka E. M.; Stepnowski P. Decomposition rates of methyl tert-butyl ether and its by-products by the Fenton system in saline wastewaters. Sep. Purif. Technol. 2006, 52 (2), 317–324. 10.1016/j.seppur.2006.05.014. [DOI] [Google Scholar]
- Klasson T. K.; Tsouris C.; Jones S. A.; Dinsmore M. D.; Walker A. B.; DePaoli D. W.; Yiacoumi S.; Vithayaveroj V.; Counce R. M.; Robinson S. M.. Ozone Treatment of Soluble Organics in Produced Water. Petroleum Environmental Research Forum Project 98–04; Oak Ridge National Laboratory, January 2002. [Google Scholar]
- Shafer L., Water recycling and purification in the Pinedale anticline field: results from the anticline disposal project. In SPE Americas E&P Health, Safety, Security, and Environmental Conference, Houston, Texas, USA, 21–23 March 2011.
- Mrowiec B.; Kuglarz M.; Przywara L. Removal of aromatic hydrocarbons (BTX) in anoxic and anaerobic wastewater treatment processes. Desalin. Water Treat. 2013, 51 (7–9), 1577–1583. 10.1080/19443994.2012.715075. [DOI] [Google Scholar]
- Suschka J.; Mrowiec B.; Kuszmider G. Volatile organic compounds (VOC) at some sewage treatment plants in Poland. Water Sci. Technol. 1996, 33, 273–276. 10.1016/0273-1223(96)00485-4. [DOI] [Google Scholar]
- Foght J. Anaerobic biodegradation of aromatic hydrocarbons: Pathways and prospects. Journal of Molecular Microbiology and Biotechnology 2008, 15 (2–3), 93–120. 10.1159/000121324. [DOI] [PubMed] [Google Scholar]
- Manoli E.; Samara C. The removal of Polycyclic Aromatic Hydrocarbons in the wastewater treatment process: Experimental calculations and model predictions. Environ. Pollut. 2008, 151 (3), 477–485. 10.1016/j.envpol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Haritash A. K.; Kaushik C. P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169 (1–3), 1–15. 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Dargnat C.; Teil M. J.; Chevreuil M.; Blanchard M. Phthalate removal throughout wastewater treatment plant. Case study of Marne Aval station (France). Sci. Total Environ. 2009, 407 (4), 1235–1244. 10.1016/j.scitotenv.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Shelton D. R.; Boyd S. A.; Tiedje J. M. Anaerobic biodegradation of phthalic acid esters in sludge. Environ. Sci. Technol. 1984, 18 (2), 93–97. 10.1021/es00120a008. [DOI] [PubMed] [Google Scholar]
- Llop A.; Borrull F.; Pocurull E. Comparison of the removal of phthalates and other organic pollutants from industrial wastewaters in membrane bioreactor and conventional activated sludge treatment plants. Water Sci. Technol. 2016, 60, 2425–2437. 10.2166/wst.2009.314. [DOI] [PubMed] [Google Scholar]
- Yang J.; Tezel U.; Pierson J. A.; Pavlostathis S. G. Biodegradation and Toxicity of Alkyl Benzyl Dimethyl Ammonium Chloride in a Mixed Aerobic Culture. Proceedings of the Water Environment Federation 2007, 2007 (9), 8007–8022. 10.2175/193864707786862198. [DOI] [Google Scholar]
- Ying G.-G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32 (3), 417–431. 10.1016/j.envint.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hernandez Leal L.; Vieno N.; Temmink H.; Zeeman G.; Buisman C. J. N. Occurrence of xenobiotics in gray water and removal in three biological treatment systems. Environ. Sci. Technol. 2010, 44 (17), 6835–6842. 10.1021/es101509e. [DOI] [PubMed] [Google Scholar]
- Butkovskyi A.; Rijnaarts H. H. M.; Zeeman G.; Hernández-Leal L. Fate of Personal Care and Household Products in Source Separated Sanitation. J. Hazard. Mater. 2016, 320, 427–434. 10.1016/j.jhazmat.2016.08.059. [DOI] [PubMed] [Google Scholar]
- Rhiner B. L.Anaerobic and Aerobic Biodegradation of the Oil Dispersant Components 1,2-Propanediol and 2-Butoxyethanol in Seawater. M.S. Thesis, Clemson University, August 2014. [Google Scholar]
- Bruchet A.; Prompsy C.; Fillppi G.; Souall A. A broad spectrum analytical scheme for the screening of endocrine disruptors (EDs), pharmaceuticals and personal care products in wastewaters and natural waters. Water Science and Technology: A Journal of the International Association on Water Pollution Research 2002, 46 (3), 97–104. [PubMed] [Google Scholar]
- Vogel T. M.; Criddle C. S.; McCarty P. L. ES Critical Reviews: Transformations of halogenated aliphatic compounds. Environ. Sci. Technol. 1987, 21 (8), 722–736. 10.1021/es00162a001. [DOI] [PubMed] [Google Scholar]
- Long J.; Stensel H.; Ferguson J.; Strand S.; Ongerth J. Anaerobic and Aerobic Treatment of Chlorinated Aliphatic Compounds. J. Environ. Eng. 1993, 119 (2), 300–320. 10.1061/(ASCE)0733-9372(1993)119:2(300). [DOI] [Google Scholar]
- Belkin S.; Brenner A.; Abeliovich A. Biological treatment of a high salinity chemical industrial wastewater. Water Sci. Technol. 1993, 27 (7–8), 105–112. [Google Scholar]
- Verlicchi P.; Al Aukidy M.; Zambello E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. 10.1016/j.scitotenv.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Tadkaew N.; Hai F. I.; McDonald J. A.; Khan S. J.; Nghiem L. D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45 (8), 2439–2451. 10.1016/j.watres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Mozo I.; Stricot M.; Lesage N.; Spérandio M. Fate of hazardous aromatic substances in membrane bioreactors. Water Res. 2011, 45 (15), 4551–4561. 10.1016/j.watres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Zilverentant A.; Van Nieuwkerk A.; Vance I.; Watlow A.; Rees M. Produced water report: Pilot-scale membrane bioreactor treats produced water. World Oil 2009, 230 (8), 79–82. [Google Scholar]
- Hai F. I.; Tadkaew N.; McDonald J. A.; Khan S. J.; Nghiem L. D. Is halogen content the most important factor in the removal of halogenated trace organics by MBR treatment?. Bioresour. Technol. 2011, 102 (10), 6299–6303. 10.1016/j.biortech.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Pendashteh A. R.; Abdullah L. C.; Fakhru’l-Razi A.; Madaeni S. S.; Zainal Abidin Z.; Awang Biak D. R. Evaluation of membrane bioreactor for hypersaline oily wastewater treatment. Process Saf. Environ. Prot. 2012, 90 (1), 45–55. 10.1016/j.psep.2011.07.006. [DOI] [Google Scholar]
- Kwon S.; Sullivan E. J.; Katz L.; Kinney K.; Chen C.-C.; Bowman R.; Simpson J., Pilot Scale Test of a Produced Water-Treatment System for Initial Removal of Organic Compounds. In SPE Annual Technical Conference and Exhibition, Denver, CO, 21–24 September 2008.
- Brown V. J. Radionuclides in fracking wastewater: managing a toxic blend. Environ. Health Perspect. 2014, 122 (2), A50–55. 10.1289/ehp.122-A50. [DOI] [PMC free article] [PubMed] [Google Scholar]