Abstract
Traditional brachytherapy refers to the placement of radioactive sources on or inside the cancer tissues. Based on the type of sources, brachytherapy can be classified as radionuclide and electronic brachytherapy. Electronic brachytherapy uses miniaturized X-ray sources instead of radionuclides to deliver high doses of radiation. The advantages of electronic brachytherapy include low dose to organs at risk, reduced dose to treating staff, no leakage radiation in off state, less shielding, and no radioactive waste. Most of these systems operate between 50 and 100 kVp and are widely used in the treatment of skin cancer. Intrabeam, Xoft and Papillon systems are also used in the treatment of intra-operative radiotherapy to breast in addition to other treatment sites. The rapid fall-off in the dose due to its low energy is a highly desirable property in brachytherapy and results in a reduced dose to the surrounding normal tissues compared to the Ir-192 source. The Xoft Axxent brachytherapy system uses a 2.25 mm miniaturized X-ray tube and the source almost mimics the high dose rate Ir-192 source in terms of dose rate and it is the only electronic brachytherapy system specifically used in the treatment of cervical cancers. One of the limiting factors that impede the use of electronic brachytherapy for interstitial application is the source dimension. However, it is highly anticipated that the design of miniaturized X-ray tube closer to the dimension of an Ir-192 wire is not too far away, and the new era of electronic brachytherapy has just begun.
Keywords: Brachytherapy, Electronic brachytherapy, Intrabeam, Xoft, Cervical cancer
Core tip: Electronic brachytherapy is a new form of radiotherapy that delivers a very high dose of radiation inside or very close to the cancer tissues. These devices utilize a miniaturized X-ray source to deliver radiation at relatively high dose rates to the target volume. Electronic brachytherapy eliminates some of the accidents related to radionuclide brachytherapy such as loss of sources, radiation leakage in off state, transportation accidents and radioactive waste. It finds wide applications in the treatment of cancers including skin, breast, endometrium, cervix and spinal metastasis. Electronic brachytherapy is a promising technology of the future and could potentially replace radionuclide brachytherapy.
INTRODUCTION
Brachytherapy is a form of radiotherapy treatment technique that delivers a high dose of radiation inside or very close to the cancer tissues. The term brachytherapy was originally derived from the Greek words βραχύς (brachys) and θεραπεία (therapeía) which means “short” and “curing or healing” respectively. The history of brachytherapy dates back to the 1910s; soon after the discovery of radioactivity, Pierre Curie suggested the idea of using radioactive sources for brachytherapy, and it was first utilized in the treatment of lupus and gradually extended to other sites. Traditionally, brachytherapy involves the use of sealed radioactive sources. It is extensively used in the treatment of brain, eye, base of tongue, floor of mouth, tongue, oropharynx, lip, nasopharynx, trachea, esophagus, breast, cervix, endometrium, prostate, rectum, skin, sarcoma and many other treatment sites[1-6]. Brachytherapy can be used alone or in conjunction with conventional external beam radiotherapy.
Based on the type of sources, brachytherapy can now be classified as radionuclide and electronic brachytherapy. The concept of electronic brachytherapy was first envisaged by Alan Sliski of the Photoelectron Corporation[7,8]. He designed a miniaturized, low power X-ray source that can operate in the range of approximately 10 to 90 kV using small currents between 1 nA and 100 μA. The system uses a mini accelerator that generates low energy X-rays at the tip of a needle-like probe. Most of the current electronic brachytherapy systems utilize a miniaturized X-ray source to deliver radiation at relatively high dose rates to the target volume. Miniature X-ray sources have several advantages over the most common radionuclide based brachytherapy. Table 1 highlights some of the strengths and weaknesses of electronic brachytherapy compared to radionuclide based brachytherapy. The low kilovoltage electronic X-ray sources require relatively less shielding as opposed to Co-60, Cs-137 and Ir-192 sources which require heavily shielded bunkers. The highlighting feature of X-ray based sources is the absence of radiation when the source is not in use.
Table 1.
Comparison of radionuclide and electronic brachytherapy sources
| Source | Strengths | Weaknesses |
| Radionuclide | Small source Fixed energy spectrum Easy to predict the output at any point in time using half-life Used in combination with EBRT/alone Rapid dose fall-off Lower dose to organs at risk Proven clinical application Well-established protocols and treatment procedures | Radiation leakage – Off condition Radioactive waste – A big concern Frequent source replacement (depends on half-life) Output correction due to source decay Source transportation related radiation accidents Fixed-dose rate and dosimetric properties Limited treatment sites compared to EBRT |
| Electronic brachytherapy | No radiation leakage in off condition User adjustable energy and current (dose rate) No radioactive waste Source transportation - not an issue Relatively stable output during the life of the X-ray tube Less exposure to staff | Relatively large source size Limited treatment sites compared to radionuclide brachytherapy Minimal experience compared to radionuclide-based brachytherapy |
EBRT: External beam radiation therapy.
RATIONALE FOR ELECTRONIC BRACHYTHERAPY
The International Commission on Radiation Protection published a report in 2005 that reviewed all the radiotherapy accidents associated with high dose rate brachytherapy[9]. The report showed that radionuclide based brachytherapy resulted in 500 accidents including one death along the entire chain of procedures from radioactive source packaging to dose delivery. Some of the accidents were related to loss of sources, radiation leakage and inaccurate calibration of sources. Radiation accidents with radionuclide brachytherapy can also occur due to the use of the wrong isotope, leaking sources, and failure to remove temporary implants[10]. There are occasions when the source may not retract due to a power outage, a kink in the catheter or interlock failure. These conditions may lead to an excessive dose to the treating staff and unwanted doses to the patient while trying to retract the source. It is expected that the introduction of electronic brachytherapy would eliminate most of these accidents associated with radionuclide brachytherapy. Electronic brachytherapy systems do not emit radiation in the off state, which eliminates likely accidents with source packaging, transportation and mishandling of sources related to radionuclide brachytherapy. They can be operated in a standard treatment room with minimal shielding due to low energy and no radiation leakage in the off state. There is also no radioactive waste and hence no concerns with source transportation unlike radionuclides which require a special license for source transportation and radioactive waste disposal.
ELECTRONIC BRACHYTHERAPY MACHINES
Currently, six different types of electronic brachytherapy systems (Figure 1) are available, which includes Intrabeam (Zeiss), Xoft (iCAD), Papillon (Ariane), Photoelectric Therapy (Xstrahl), Esteya (Elekta) and SRT 100 (Sensus Healthcare). Most of these systems operate between 50 and 100 kVp. Esteya delivers 69.5 kV X-ray beam at a dose rate of 2.7 Gy/min at 3 mm and is used to treat non-melanoma skin cancer, including basal cell carcinoma and squamous cell carcinoma[11]. Papillon from Ariane Medical Systems is used to treat breast, rectum, and skin cancers. This X-ray system operates at 30 and 50[12] kVp with a dose rate of > 8 Gy/min and > 20 Gy/min at 20 cm focus to surface distance. The SRT-100 system operates between 50 and 100 kVp and can be used to treat non-melanoma skin cancer including basal cell carcinoma skin and keloids[13]. Intrabeam and Xoft electronic brachytherapy have a wide variety of applicators to treat multiple sites[14-19]. The heart of the intrabeam system is an XS4 miniaturized linear accelerator with an inbuilt internal radiation monitor that monitors the dose delivered to the patients in real time. A mini accelerator section accelerates the electrons emitted by a cathode gun, and the existing electron beam is focussed onto a gold target to generate X-rays. The intrabeam system is employed in the treatment of breast, skin, GI and spinal metastases. This system uses X-rays produced by a gold target, and the Xoft Axxent uses a tungsten target to generate X-rays. The size of a conventional diagnostic/therapeutic X-ray tube typically varies between 30 and 50 cm along the long axis and 20 cm in diameter. As opposed to the standard X-ray tube, the Xoft Axxent brachytherapy X-ray tube is the only system currently available with a diameter of 2.25 mm with a full assembly diameter of 5.4 mm which incorporates an extra space outside the cathode-anode assembly of the X-ray tube for water circulation to extract the heat during X-ray emission. This system can be used to treat cancers of the skin, breast, and endometrium. When compared to other existing electronic brachytherapy systems, the Xoft source almost mimics a Ir-192 source in terms of dose rate. It is the only electronic brachytherapy system closer to a wired radionuclide source such as Ir-192/Co-60 currently available to treat cancer of the cervix. The nominal dose rate of the Axxent HDR X-ray source is 0.6 Gy/min at 3 cm in water. The maximum anode current, at 50 kVp is 300 μA. The Xoft Axxent brachytherapy is a comprehensive system with an inbuilt electrometer and a well-type chamber which enables Physicists to measure and verify the output just before treatment.
Figure 1.

Electronic brachytherapy systems operating. A: The range between 50 and 100 kVp; B: The range between 40 and 60 kVp.
Figure 2 shows the energy spectra measured using an Amptek spectrometer placed close to a 3-4 cm spherical balloon applicator and it also indicates that the maximum energy of the Xoft X-ray source approaching 50 keV. The average energy of the Xoft Axxent X-ray source is between 26 and 35 keV which enables the systems to be used in a CT or diagnostic X-ray bunker for treatment. The dose fall-off with 50 kVp X-rays is quite rapid compared to the Ir-192 source which would help the treating physician to reduce the complications to adjacent normal tissues and may also contribute to dose escalation. Figure 3 shows that the Xoft Axxent X-ray source mimics the dose fall-off characteristics of low energy isotopes with an average energy lying between I-125 and Pd-103 radioactive sources and still maintains the high dose rate property of the Ir-192 source. Figure 4 compares the dose fall-off of the Xoft 50 kV source, 6 MV, 6 MV 60° wedge and 18 MV beams. The dose fall-off measurement was conducted for a bare Xoft X-ray source and the X-ray source placed inside a 3-4 cm balloon applicator in a Welhofer radiation field analyzer. It is evident from the figure that the dose fall-off with electronic brachytherapy is quite rapid compared to megavoltage beams.
Figure 2.
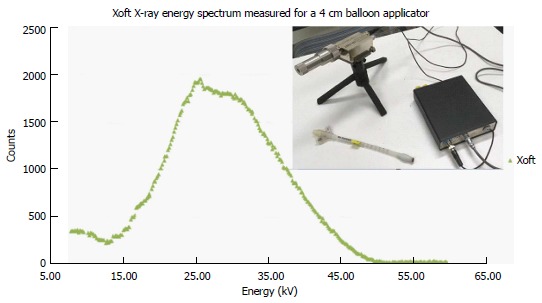
Xoft X-ray energy spectrum measured using an Amptek spectrometer.
Figure 3.
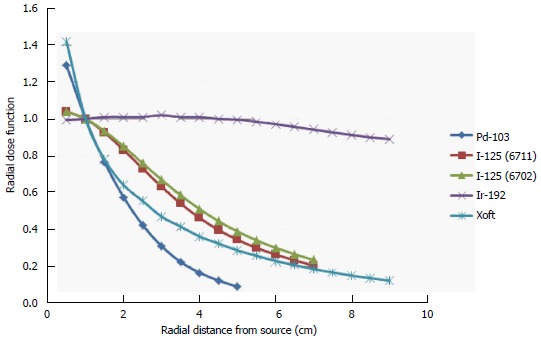
Radial dose function [Pd-103, I-125 (6711), I-125 (6702), Ir-192 and Xoft].
Figure 4.
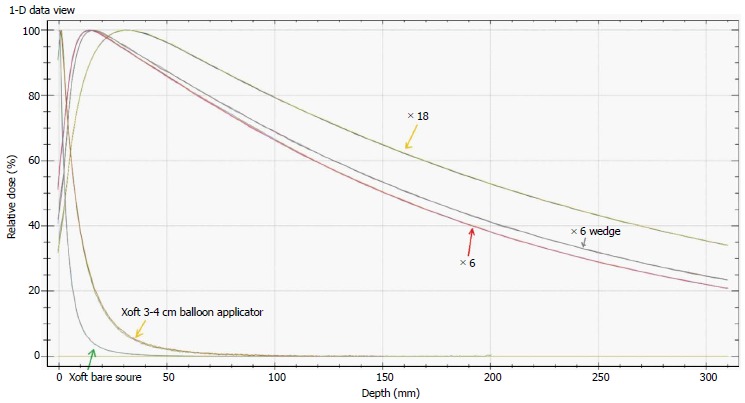
Comparison of 6MV, 6MV wedge, 18 MV, Xoft bare source and Xoft 3-4 cm balloon applicator profiles.
CLINICAL APPLICATIONS AND LIMITATIONS
Electronic brachytherapy is currently used to treat cancers of breast, skin, keloids, spinal metastasis, GI, endometrium, cervix, and rectum. Some of the existing electronic brachytherapy systems such as Esteya and Photoelectric Therapy are limited to small skin cancers due to their specific design. The papillon+, intrabeam, Xoft and SRT-100 electronic brachytherapy systems are also used in the treatment of skin cancers. Most of these systems use a small flattening filter to flatten the beam for the treatment of skin cancers. Several studies have demonstrated that these systems result in lower toxicity and provides excellent cosmesis[18]. Intrabeam uses a special needle applicator with a diameter of 4.4 mm for the treatment of vertebral metastases. The papillon+, intrabeam, and xoft electronic brachytherapy systems produce an isotropic distribution which is highly desirable for breast intraoperative radiotherapy. The TARGIT trial is the largest IORT trial to date using electronic brachytherapy in conjunction with breast conservation surgery. This trial studied a total of 3451 patients wherein 1721 subjects were randomized to TARGIT and 1730 to external beam radiotherapy. The 5-year results of the local control and overall survival from the TARGIT-A randomized trial published in Lancet 2014 concluded that a risk-adapted approach should be considered for patients treated with electronic brachytherapy[20]. In 2010, the first multi-center study to evaluate the safety and device performance of Xoft Electonic Brachytherapy was published by Mehta et al[21]. This study enrolled a total of 65 patients between March 2007 and March 2008 and had completely resected invasive ductal carcinoma or ductal carcinoma insitu with N0, M0. The fractionated regimen of 34 Gy over 10 fractions prescribed 1 cm beyond the balloon surface show similar acute toxicities to other high dose rate approaches for accelerated partial breast irradiation.
According to the World Health Organization, cervical cancer is the second most common cancer in women worldwide and every year more than 270000 women die from cervical cancer[22]. Brachytherapy is one of the most common treatment modalities used in the treatment of cervical cancer. Traditionally, radionuclide-based brachytherapy has been administered alone or in combination with external beam radiotherapy for cervical cancer patients. The majority of the centers in developing countries have a remote afterloading treatment unit for delivering this modality. Most of the radiation accidents associated with these sorts of procedures could be eliminated with the use of electronic brachytherapy. The Xoft Axxent requires relatively fewer resources compared to the standard radionuclide based brachytherapy. Recent studies have shown that Ir-192, Co-60, and Xoft based cervical brachytherapy dosimetry are comparable and could result in reduced dose to the bladder and rectum[23]. Figure 5 shows a simulated intracavitary treatment plan planned using the Xoft X-ray source for a predefined dwell positions. The rapid dose fall-off of 50 kV compared to Ir-192 is one of the main reasons for this reduced dose to the bladder and rectum. Recently, Xoft introduced a Henschke type applicator that could enable the delivery of radiation to cervical cancers. The cervical Henschke type applicators are made of a titanium material with 0°, 15°, 30° and 45° tandem angles and 2.0, 2.5 and 3 cm ovoid diameters. The standard flexible source channel used for cancers other than cervical is 25 cm and exclusively for cervical brachytherapy the source channel is 50 cm (Figure 6). Xoft also provides a set of vaginal applicators for the treatment of endometrial cancers. The existing miniaturized X-ray tube has few limitations in its longevity and source size. With radionuclide-based brachytherapy, the specific activity of the source determines the size of the source. Radionuclide sources such as Iridium-192 have a high specific activity which enables the manufacturers to produce small sources with high activity. Due to this, conventional radionuclide-based brachytherapy has wider applications and can deliver interstitial brachytherapy to multiple treatment sites. Head and neck based interstitial brachytherapy such as the base of tongue and floor of mouth require a loop technique where the smaller size source would help to make the sharp bend. The diameter of the Axxent xoft brachytherapy source is 2.25 mm, and when housed inside a cooling tube it increases the effective source diameter to 5 mm. However, this limits its application to interstitial brachytherapy. With a rapid growth in the field of computer technology and biomedical engineering could one day lead to the design of a stable sub-millimetre X-ray tube. Such a miniaturized X-ray tube would enable the expansion of electronic brachytherapy to most sites currently treated by Ir-192 sources.
Figure 5.
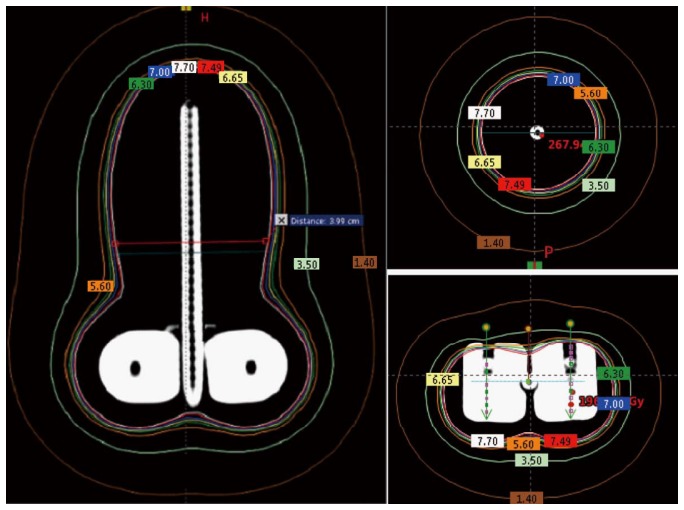
Simulation of a Tandem and Ovoid applicators planned in a Brachyvision software for a predefined Xoft X-ray source dwell positions.
Figure 6.
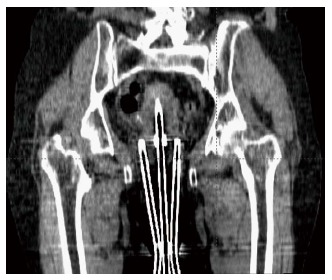
Coronal view of the Xoft Henschke applicator.
CONCLUSION
The ever-growing technological advancements have led to the development of a miniaturized X-ray tube and this has been explored for various treatment sites. Electronic brachytherapy is a promising technology that has more potential to replace the existing radionuclide-based brachytherapy procedures. One of the limiting factors that impede the use of electronic brachytherapy for interstitial application is the source dimension. However, it is highly anticipated that the design of miniaturized X-ray tube closer to the dimension of an Ir-192 wire is not too far away, and the new era of electronic brachytherapy has just begun.
ACKNOWLEDGMENTS
The author would like to thank Associate Professor Moshi Geso, RMIT University for providing the Amptek X-ray spectrometer. The author would also like to thank Dr. Steven David, Dr. Derrick Wanigaratne and Mr Karl Roozen for helping with this manuscript.
Footnotes
Conflict-of-interest statement: Ramachandran P declares no conflict of interest related to this publication.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Austalia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: September 2, 2016
First decision: September 26, 2016
Article in press: January 18, 2017
P- Reviewer: Chen F, Chow J, Dickler A S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Orton CG, Seyedsadr M, Somnay A. Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys. 1991;21:1425–1434. doi: 10.1016/0360-3016(91)90316-v. [DOI] [PubMed] [Google Scholar]
- 2.Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, van Lanschot JJ, Wijrdeman HK, Mulder CJ, Reinders JG, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364:1497–1504. doi: 10.1016/S0140-6736(04)17272-3. [DOI] [PubMed] [Google Scholar]
- 3.Mohanti BK, Bansal M, Bahadur S, Shukla NK, Deo SV, Prabhakar R, Rath GK. Interstitial brachytherapy with or without external beam irradiation in head and neck cancer: Institute Rotary Cancer Hospital experience. Clin Oncol (R Coll Radiol) 2001;13:345–352. doi: 10.1053/clon.2001.9287. [DOI] [PubMed] [Google Scholar]
- 4.Radek A, Grochal M, Gasiński P, Zieliński K, Kopczyński J, Sobotkowski J, Grzelak M, Łyczak P, Błaszczyk B. Stereotactic biopsy and brachytherapy in the diagnostics and treatment of brain tumors--preliminary report. Neurol Neurochir Pol. 2001;35 Suppl 5:5–11. [PubMed] [Google Scholar]
- 5.King TA, Bolton JS, Kuske RR, Fuhrman GM, Scroggins TG, Jiang XZ. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 6.Ragde H, Grado GL, Nadir BS. Brachytherapy for clinically localized prostate cancer: thirteen-year disease-free survival of 769 consecutive prostate cancer patients treated with permanent implants alone. Arch Esp Urol. 2001;54:739–747. [PubMed] [Google Scholar]
- 7.Peter M, Nomikos PM, Dinsmore MT, Sliski AP. Miniaturized low power x-ray source. United States patent US 5153900. 1992:Oct 6.
- 8.Dinsmore M, Harte KJ, Sliski AP, Smith DO, Nomikos PM, Dalterio MJ, Boom AJ, Leonard WF, Oettinger PE, Yanch JC. A new miniature x-ray source for interstitial radiosurgery: device description. Med Phys. 1996;23:45–52. doi: 10.1118/1.597790. [DOI] [PubMed] [Google Scholar]
- 9.Valentin J. Prevention of high-dose-rate brachytherapy accidents. ICRP Publication 97. Ann ICRP. 2005;35:1–51. doi: 10.1016/j.icrp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Kaulich TW, Becker G, Lamprecht U, Nüsslin F, Bamberg M. Emergency rescue in accidents with HDR afterloading units. Strahlenther Onkol. 1999;175:524–529. doi: 10.1007/s000660050064. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martinez T, Chan JP, Perez-Calatayud J, Ballester F. Dosimetric characteristics of a new unit for electronic skin brachytherapy. J Contemp Brachytherapy. 2014;6:45–53. doi: 10.5114/jcb.2014.40770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myint AS. Novel radiation techniques for rectal cancer. J Gastrointest Oncol. 2014;5:212–217. doi: 10.3978/j.issn.2078-6891.2014.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheu RD, Powers A, Lo YC. Commissioning a 50-100 kV X-ray unit for skin cancer treatment. J Appl Clin Med Phys. 2015;16:5182. doi: 10.1120/jacmp.v16i2.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickler A. Xoft Axxent electronic brachytherapy: a new device for delivering brachytherapy to the breast. Nat Clin Pract Oncol. 2009;6:138–142. doi: 10.1038/ncponc1319. [DOI] [PubMed] [Google Scholar]
- 15.Rong Y, Welsh JS. Surface applicator calibration and commissioning of an electronic brachytherapy system for nonmelanoma skin cancer treatment. Med Phys. 2010;37:5509–5517. doi: 10.1118/1.3489379. [DOI] [PubMed] [Google Scholar]
- 16.Hepel JT, Hiatt JR, Cardarelli GA, Wazer DE. Modeling study for optimization of skin dose for partial breast irradiation using Xoft Axxent electronic brachytherapy applicator. Brachytherapy. 2010;9:81–85. doi: 10.1016/j.brachy.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Richardson S, Garcia-Ramirez J, Lu W, Myerson RJ, Parikh P. Design and dosimetric characteristics of a new endocavitary contact radiotherapy system using an electronic brachytherapy source. Med Phys. 2012;39:6838–6846. doi: 10.1118/1.4757915. [DOI] [PubMed] [Google Scholar]
- 18.Goubert M, Parent L. Dosimetric characterization of INTRABEAM® miniature accelerator flat and surface applicators for dermatologic applications. Phys Med. 2015;31:224–232. doi: 10.1016/j.ejmp.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Williams NR, Pigott KH, Brew-Graves C, Keshtgar MR. Intraoperative radiotherapy for breast cancer. Gland Surg. 2014;3:109–119. doi: 10.3978/j.issn.2227-684X.2014.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, Flyger HL, Massarut S, Alvarado M, Saunders C, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383:603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 21.Mehta VK, Algan O, Griem KL, Dickler A, Haile K, Wazer DE, Stevens RE, Chadha M, Kurtzman S, Modin SD, et al. Experience with an electronic brachytherapy technique for intracavitary accelerated partial breast irradiation. Am J Clin Oncol. 2010;33:327–335. doi: 10.1097/COC.0b013e3181d79d9e. [DOI] [PubMed] [Google Scholar]
- 22.WHO guidance note. Comprehensive cervical cancer prevention and control - a healthier future for girls and women, 2012. Switzerland. [Google Scholar]
- 23.Mobit PN, Packianathan S, He R, Yang CC. Comparison of Axxent-Xoft, (192)Ir and (60)Co high-dose-rate brachytherapy sources for image-guided brachytherapy treatment planning for cervical cancer. Br J Radiol. 2015;88:20150010. doi: 10.1259/bjr.20150010. [DOI] [PMC free article] [PubMed] [Google Scholar]


