Abstract
Interventional radiology (IR) has become an integral part in the management of traumatic injuries. There is an ever-increasing role of IR in traumatic injuries of solid abdominal organs, pelvic and peripheral arteries to control active bleeding by therapeutic embolization or vascular reconstruction using stent grafts. Traditionally, these endovascular treatments have been offered to hemodynamically stable patients. However, in recent times endovascular approach has become preferable to surgery even in hemodynamically unstable patients with injury of surgically difficult-to-access sites. With shifting trends towards non operative management coupled with availability of the current state-of-the-art equipments, hardware and technical expertise, IR has gained an impeccable role in trauma management. However, due to lack of awareness and widespread acceptance, IR continues to remain an ocean of unexplored potentialities.
Keywords: Blunt abdominal trauma, Pelvic trauma, Peripheral arterial injury, Interventional radiology, Embolization
Core tip: Over the past two decades, there has been a paradigm shift in the management of traumatic injuries with inception of the concept of non operative management (NOM), which is followed if there are no peritoneal signs or hollow viscus injury. Interventional radiology (IR) is an extension of NOM which not only saves lives by achieving hemostasis at the site of vascular injury or difficult to access surgical sites but also controls rebleeding following surgery. Time and again it has proven to be a highly effective management option which successfully bridges the conservative management and emergency laparotomy. In current scenario, in vascular injury, IR can obviate emergency laparotomy not only in hemodynamically stable but unstable patients as well, as evidenced by the recent literature. Ignorance and lack of acceptance have limited the utilization of IR significantly, which if practiced judiciously will provide expedient hemodynamic control as well as faster restoration to physiological status.
INTRODUCTION
Trauma continues to be the leading cause of mortality in young population. Uncontrolled exsanguination is the most common cause of the early trauma related mortality, accounting for nearly 30%-40% of such fatalities. At initial hospitalization following trauma, resuscitation is done as per the standard advanced trauma life support guidelines. Patient management as per these guidelines is divided into two categories-primary and secondary survey. Primary survey aims at performing immediate lifesaving interventions. It is defined by the mnemonic ABCDE which include control of Airway, Breathing, Circulation, Disability assessment (neurological) and Exposure of the patient to rule out any injury to unexposed part. In secondary survey, trauma related brief clinical details are obtained and examination is performed. Afterwards, on the basis of clinical status (hemodynamic stability), mechanism of injury and the presence of hemoperitoneum, patients are triaged into surgical and nonsurgical candidates. Surgical candidates are immediately shifted to the operation-theatre without any further investigation, for exploration. On the contrary, hemodynamically stable non-surgical candidates are further subjected to cross-sectional imaging to evaluate the extent and severity of injuries, besides localizing the site of active bleeding, if any[1,2].
Recent times have witnessed an increasing role of IR in the management of acutely injured polytrauma patients. Similar to the shifting trends towards minimally invasive procedures as in other surgical disciplines, therapeutic embolization by endovascular route is the next benchmark in the non operative management (NOM) of hemodynamically stable patients which considerably decreases the morbidity and duration of hospitalization apart from imminently saving an endangered life[1].
Currently, interventional radiology (IR) procedures are targeted at managing vascular injuries in a hemodynamically stable non-surgical patient. Whereas, in hemodynamically unstable patients, surgery is the mainstay of treatment. However, exception to this rule is the bleeding at certain surgically difficult-to-access sites which are preferably managed by endovascular interventions irrespective of the hemodynamic status. Nonetheless, when and how to intervene by endovascular route depends on a multitude of parameters including clinical stability, type and location of injury. Although, IR procedures may not reduce overall mortality, there is definite immediate survival benefit, especially in traumatic vascular injuries[3].
On the basis of mechanism of injury, traumatic injuries may be classified into blunt or penetrating. This has implications in management as penetrating injuries are more commonly associated with hollow visceral organs and mesenteric injury as compared to blunt trauma, and hence, they more often undergo surgery. Contrast-enhanced CT (CECT) is imperative in all those cases of penetrating trauma where depth of wound is critical (based on surgeon’s discretion), provided the patient is hemodynamically stable. Nonetheless, the indications and technique of radiological interventions remain same regardless of the mechanism of injury[1-3].
The purpose of this article is to review the principles and techniques of IR used in the treatment of arterial injuries of solid organs, pelvic fractures and extremities along with an abridged recommended protocol practiced at the author’s institute. Also, an overview of the scope of endovascular interventions as well as non-vascular interventions is briefed.
CURRENT STATUS OF INTERVENTIONAL RADIOLOGIST IN A TRAUMA TEAM
Managing traumatic injury is a multidisciplinary approach with the interventional radiologist increasingly becoming an integral part of the trauma team. Importance of IR lies in the fact that it has now emerged as a separate sub-specialty of radiology. Tools and techniques of IR include image guidance to approach the bleeding site through endovascular route and clog the bleeder by a host of embolizing agents such as coil, gelfoam or particles. Apart from this, IR also has a definite role in the management of peripheral vascular injuries by reconstruction of the vessel through stent graft. The old adage “time is money” holds true in managing critically injured patients. Hence, a dedicated IR team is imperative round the clock to perform these non-invasive interventions, thereby, minimizing the patient’s morbidity besides saving lives[1,2].
In traumatic injuries, IR procedures may be useful as the sole definitive treatment or as a temporary adjunct prior to surgery.
Definitive indications
Most common indication of IR in trauma is endovascular embolization in vascular injury of solid abdominal organs. Besides, endovascular route is preferred in injuries at anatomically complex sites like pelvis and maxillofacial regions, even if the patient is hemodynamically unstable. Re-bleeding following surgery is preferably managed through endovascular route as there may be anatomical distortion and coagulopathy which can complicate re-laparotomy. Further, interventions like transcatheter-embolization or stent placements have shown promising results in peripheral vascular injuries by controlling the ongoing bleeding as well as maintaining the vascular continuity; hence, preventing limb ischemia. Nowadays, if the facility is available, endovascular interventions are favored over surgery wherever feasible as there is less morbidity and mortality associated with the former[1,4].
Temporary indications
Endovascular interventions can also be used as a temporary adjunct to definitive surgery to prevent massive exsanguination. For example, Balloon occlusion of the internal iliac arteries or abdominal aorta/ its branches may be done in cases of life-threatening hemorrhage prior to emergency laparotomy. Prophylactic embolization may be done in high grade (≥ 4) solid visceral organ injuries (liver, spleen) even without any vascular injury as these are more likely to fail with NOM[1-4].
Principles, tools and techniques of IR in the setting of trauma[1,2,4]
General principles of IR remain the same as in elective procedures, however, in trauma, aim is to save life by rapidly controlling exsanguinating hemorrhage. Hence, many a times balance needs to be struck between precision vs suboptimal embolization. The chief mechanism of action of the embolic agents include reduction in perfusion pressure which subsequently reduces or halts the ongoing bleeding. Furthermore, embolization should be as close to the site of vascular injury as possible. However, drawbacks of too proximal embolization include recruitment of collaterals leading to re-bleeding whereas distal embolization will result in infarct.
SPECIAL CONSIDERATIONS FOR ENDOVASCULAR INTERVENTIONS IN TRAUMA[1,2,4]
In traumatic injuries, ongoing hemorrhage and hypovolemia frequently leads to arterial spasm. Hence, catheterization should be as super-selective as possible as this may aid in detection of injuries which may be occult on initial non-selective angiograms.
Technique of the embolization, i.e., selective vs non-selective depends on the site and size of vessel injured, e.g., bleeding from an end-artery may be seen in certain organs like liver, kidney, spleen, and sacrificing it by embolization whether temporary or permanent is acceptable. In order to minimize the loss of functioning parenchyma and tissue necrosis, embolizing agent should be delivered as proximal to the target site as possible. Reaching the peripheral most terminal branch super-selectively which gradually narrows in calibre, requires the use of micro-catheters which can tolerate small pieces of gel-foam, coils or particles. However, in hemodynamically unstable patients, if there is difficulty in reaching the site of bleeding more selectively, procedure should be expedited by settling for non-selective proximal embolization with an intent to save life over an organ[1,4].
At certain sites with rich anastomotic network, like the buttock or pelvis, the goal is to occlude the arteries of small calibre while preserving the distal anastomoses. These small arteries are filled with small pieces of gelfoam followed by occlusion of the proximal branch by coil. In another modification of this technique, if catheterization distal to the site of vascular injury (e.g., pseudoaneurysm) is achieved, then coils are deployed from distal to proximal, spanning the injured site, also called “sandwich technique”. This technique avoids the risk of procedure failure that would result from retrograde blood flow by collateral circulation leading to vascular leakage. Due to the similar risks of rebleeding due to collaterals in pelvic trauma, contralateral internal iliac artery angiography and embolization should be done.
If there is a rent in large calibre arteries like aorta or iliac artery, it is repaired by covered stent grafts whereas bleeding from smaller vessels is dealt with material embolization. Similarly, restoration of the injured non-expandable conduit vessels of extremities require either surgery or stent graft. Other embolizing agents (except for non-axial branches, vide infra) are not used as there is risk of ischemia due to paucity of collateral circulation[2,4].
It is worthwhile to mention that the most important determinant for the successful control of bleeding by embolizing agents is an intact coagulation cascade which helps in clot formation. Hence, early endovascular intervention is desirable before hypothermia, acidosis or coagulopathy sets in. Before concluding a negative angiogram, possibility of variant anatomy needs consideration. Of the numerous variants, following are the commonest[5]: (1) celiac axis angiogram: Dorsal pancreatic artery arising from celiac trunk (approximately 10%), not to be mistaken for contrast extravasation; (2) superior mesenteric artery angiogram: Replaced accessory right hepatic artery (approximately 12%); and (3) pelvic angiogram: Origin of obturator artery from inferior epigastric or common femoral artery requiring catheterization of external iliac artery.
ESSENTIALS OF RADIOLOGICAL INTERVENTIONS[1-4]
Pre-procedure preparation
Ongoing resuscitation measures (airway maintenance, fluid, medications and blood transfusion) is imperative during all aspects of trauma care even during angiographic intervention. Review of available imaging such as CT scan if already performed plays an important role in the detection, location and description of the visceral and vascular injuries (e.g., the extent of organ injuries, presence of active contrast extravasation, pseudoaneurysm, dissection or arterio-venous fistula).
Laboratory studies are not prerequisite for trauma angiography and the procedure should not be delayed for results of coagulation profile. Management plan should be discussed with the trauma surgeon and also with the family. Informed consent must always be obtained.
Procedure
Initial steps of angiographic intervention for trauma are similar regardless of the location of injury. Arterial access is usually obtained via common femoral artery and vascular sheath is placed (6F for adults and 5 F for pediatric patients) after ascertaining the puncture site by fluoroscopy. In cases of severe hemodynamic shock, the femoral pulses may not be palpable, and in such cases ultrasound guidance may be used to gain vascular access. If pre-procedural imaging findings are known, then angiography should be targeted immediately to the site of injury to achieve haemostasis. In cases where patient has been shifted directly to IR suite without any prior imaging, then diagnostic angiography should be extensive enough to include all the potential sites of injury (solid abdominal viscera, mesenteric and pelvic vasculature). Angiographic findings suggestive of vascular injury include contrast extravasation, arterial occlusion, arteriovenous fistula, pseudoaneurysm and diffuse abnormal blush. Super-selective catheterization (e.g., proper hepatic artery, gastroduodenal artery, splenic artery, renal artery, superior mesenteric artery, anterior and posterior division of internal iliac artery) is imperative not only to identify the potential site of injury but also helps in targeted embolization. It is prudent to perform multiple angiographic views as just one angiographic view or run can miss significant findings. Based on the angiographic findings and site of injury, appropriate embolizing material is used (vide infra).
Occasionally, nonselective gelfoam embolization of the internal iliac arteries without angiographic evidence of vascular injury especially of bilateral internal iliac arteries as in pelvic trauma may help in controlling venous bleeds, however its potential benefit is controversial. Post-embolization or post stent placement angiography is critical to confirm hemostasis prior to removal of the access sheath.
Special situations[2,3]
False negative diagnostic angiography: Discrepancies between CT and angiography leading to negative diagnostic angiography may result from intermittent vasospasm, spontaneous occlusion, non-arterial bleeder (venous or capillary), failure to achieve selective catheterization which may miss vascular injuries especially if ongoing bleeding is slow/from a terminal branch or if there is a variant arterial anatomy (vide supra). Occasionally, there may not be any flow through the ruptured vessel at the time of examination which is also referred as “cut-off” sign on angiography.
Pelvic trauma: In severe pelvic trauma, usually branches of bilateral internal iliac arteries are injured. In such cases, both the internal iliac arteries can be accessed through a single arterial puncture with the help of specially designed uterine artery catheter.
Teaching point: Not all arterial injuries require prompt management. Bleeding from small arterial branches (1-2 mm) are usually self-limiting, provided the patient is normothermic with an intact coagulation cascade.
Embolizing agents[4,6-10]
Selection of embolization agent depends on the type and site of injury, selectivity of catheterization, level of permanence of procedure (temporary vs permanent), coagulation profile (Figure 1) amiliarity and comfort level of interventional radiologist performing the procedure (Tables 1 and 2).
Figure 1.
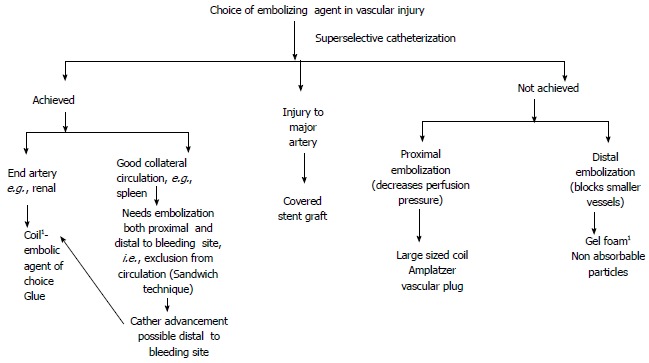
Choice of embolizing agent in vascular injury. 1Workhorse of IR in trauma. IR: Interventional radiology.
Table 1.
Embolizing agents used in traumatic injuries
| Embolizing agent | Indications | Advantages | Demerits |
| Permanent | |||
| Coil (covered with thrombogenic fibers) (Size of coil should be 20%-30% more than the target vessel size) | Active contrast extravasation Pseudoaneurysm | Rapid and effective control of bleeding Agent of choice when site of bleeding can be approached superselectively Relatively cheap (standard coils) | Reduced effectiveness in coagulopathy which hampers effective thrombosis Limited utility when target site can not be selectively approached |
| Non-absorbable particles (e.g., Polyvinyl alcohol) | Injury to terminal vessels | Permanent control of bleeding Adjunct to gelfoam | Tendency to clump and aggregate at the catheter site leading to proximal embolization, catheter block Non targeted embolization due to small size Tissue necrosis No added benefit over gelfoam, incurs additional cost |
| Liquid embolic agent [e.g., glue(N-butyl cyanoacrylate)] | As an alternative to coil especially in rebleeding | Rapid control of bleeding in hemodynamically unstable patients | Expertise for controlled delivery at target site Propensity for non targeted distal embolization leading to infarct or necrosis Rarely glue embolization of pulmonary circulation |
| Amplatzer vascular plug (AGA Medical Corporation, Plymouth, MN, United States) Available in various sizes | Large caliber vessel or large AVF (large size plug) | Single device (mesh shaped metal coil): Deployed with much greater accuracy and replaces the need of multiple coils | Costly Less beneficial in cases of distal vascular injury with good collateralization as it is deployed in proximal larger branch |
| Temporary | |||
| Gelatin sponge (CuraMedical, Assendelft, the Netherlands) Either in the form of pledgets (cut from gelfoam sheet) or slurry (non- ionic iodinated contrast mixed with gelfoam) | Cornerstone of IR in trauma: Controls majority of haemorrhage | Rapid, effective temporary occlusion of bleeding site Easily available and cheap Can be easily refashioned to the size of target artery | Non-targeted embolization to proximal branches can occur in case of rapid injection Resorbed after 3 wk: Potential risk of delayed haemorrhage Reduced effectiveness in impaired hemostasis Small risk of infection due to entrapped air during preparation |
| Autologous clot | Nonselective and rapid control of haemorrhage | Easy availability No cost | Clot dissolution in cases of coagulopathy and hemodilution |
| Starch microparticles | Usually complete reperfusion after 60 min, less tissue damage | Temporary occlusion is usually complete Uniform distribution | Allergic, non-target embolization |
Table 2.
| Embolizing agent | Reason |
| Powdered form gelatin sponge | Obliteration of small caliber vessels: May lead to infarction or abscess Large caliber vessels: Risk of rebleeding due to potential collateralization from neighboring undamaged parenchyma |
| Non absorbable particles of small size (e.g., polyvinyl alcohol), liquid embolic agents (glue) | Difficult to handle: Striking balance between selective embolization vs optimizing end point of embolization an issue Non-targeted embolization: Irreversible unwanted tissue damage |
| Alcohol | Extensive tissue necrosis Difficult to the handle while delivering at the target site |
According to the mechanism of delivery coils can be categorized into two types - pushable or detachable coils. Once deployed, pushable coils cannot be repositioned unlike detachable colis. Method of deployment of detachable coils can be mechanical, electrolytic or hydrolytic. Amongst these, Guglielmi detachable coil which is detached electrolytically is most widely used. Due to the benefits of controlled and precise delivery at the target sits, these coils are most commonly used in cerebral aneurysms. However, they are not commonly used in trauma due to the longer time needed for their delivery. Besides, they are expensive and require more expertise[8-10].
Embolizing agents which can be used in coagulopathy
Choice of embolizing material in traumatic injuries is a special concern due to frequent prevalence of coagulopathy in these patients as compared to the general population. As mentioned above, most of the commonly used embolizing agents like gelfoam or coils need an intact coagulation mechanism for clot formation. On the contrary, mechanism of clot formation in liquid embolic agents is independent of the coagulation profile. However, the major limitation precluding their widespread use is tissue infarction which is especially more with absolute alcohol and sclerosants. Nevertheless, few other liquid embolic agents like N-butyl cyanoacrylate or ethylene vinyl alcohol co-polymer (onyx) with better safety profile may be used in coagulopathy. Nevertheless, these agents are costly and require expertise for controlled administration at target site so as to limit the complications.
Another alternative in coagulopathy is hydrogel coil which is a platinum coil coated with an expandable polymer. After polymerization, there is an increase in the volume of coil by up to nine-fold. This property of expansion ensures more complete occlusion which is especially beneficial in large aneurysms. However, cost and possible blockage in incompatible delivery system are its major disadvantages[5,8,9].
Teaching point: Specific indications and options for embolization are based on location and type of injury
It is of utmost importance that the ultimate goal of radiological interventions as well as the choice embolizing material is to treat the patient and not just the imaging findings.
Endovascular management in various traumatic injuries
Liver injury: Liver is the second most commonly injured organ in the blunt abdominal trauma following spleen. In majority of these cases, clinically significant hemorrhage occurs due to the injury to hepatic artery. However, venous injury (central hepatic or portal vein) is even more dreadful which mandates surgical repair irrespective of the hemodynamic status.
After initial resuscitation, hemodynamically stable patients initially undergo NOM which may include endovascular interventions, if needed. Hepatic artery embolization (HAE) in this subset is indicated if there is injury to the hepatic artery injury or may be done prophylactically in high grade liver injuries (Figures 2, 3 and 4). Surgery is not the preliminary treatment of choice in these cases as during surgery only main hepatic artery is ligated with potential chances of re-bleeding due to collateralization. On the contrary, in endovascular interventions, site-specific distal embolization can be performed which obviates collateralization[11]. If superselective catheterization is not feasible or if bleeding is from the multiple sites, then non-selective gelfoam embolization can be done without any undue prolongation of the procedural time (Figure 5). If hemodynamic instability persists despite resuscitative efforts, then the patients are straightway taken to the operating room for rapid “damage control surgery” (DCS) with extensive peri-hepatic packing and temporary abdominal closure[12-14].
Figure 2.
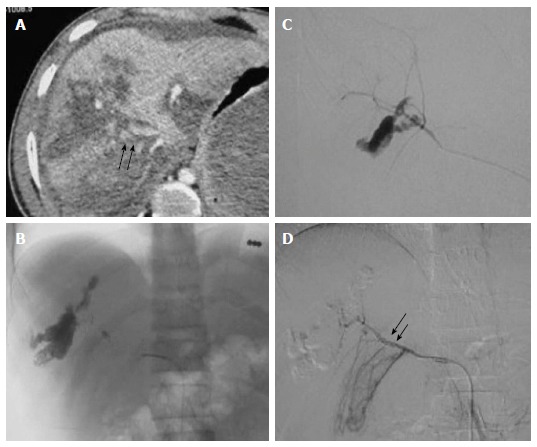
High grade liver injury with active contrast extravasation. Case of blunt trauma abdomen, FAST positive, hemodynamically stable (A) CECT abdomen showed extensive laceration, intraparenchymal hematoma consistent with Grade V liver injury alongwith diffuse active contrast extravasation (arrow) within the lacerated segment VIII (arrow) of liver with resultant hemoperitoneum (B) Selective hepatic artery angiogram revealed active contrast extravasation from anterior division of right hepatic artery with increased conspicuity in (C) superselective right hepatic artery angiogram which was subsequently embolized by microcoils (D) Post embolization angiogram revealed faint opacification of branches of anterior division of RHA (arrow) with subsidence of extravasation. FAST: Focused assessment with sonography in trauma; RHA: Right hepatic artery; CECT: Contrast enhanced computed tomography.
Figure 3.
Gun shot injury with hepatic artery pseudoaneurysm. A 38-year male with gunshot injury presented after a week with falling hematocrit. A: CECT showed a contrast filled outpouching in segment VIII of liver paralleling the attenuation of adjacent hepatic artery branch s/o PsA (arrow) with adjacent subscapular hematoma (arrowhead) subsequently DSA was done; B: Initial hepatic angiogram showed opacification of only branches of left lobe of liver,no opacification of right hepatic artery -s/o variant anatomy; C: SMA angiogram showed replaced right hepatic artery with PsA arising from the terminal end of anterior division (arrow). Due to smaller caliber and spasm, neck of the PsA could not be negotiated, hence, proximal embolization with microcoils was performed; D: Post embolization angiogram showed faint opacification of branches of anterior division of RHA alongwith exclusion of PsA. CECT: Contrast enhanced computed tomography; RHA: Right hepatic artery; PsA: Pseudoaneurysm; SMA: Superior mesentery artery; DSA: Digital subtraction angiography.
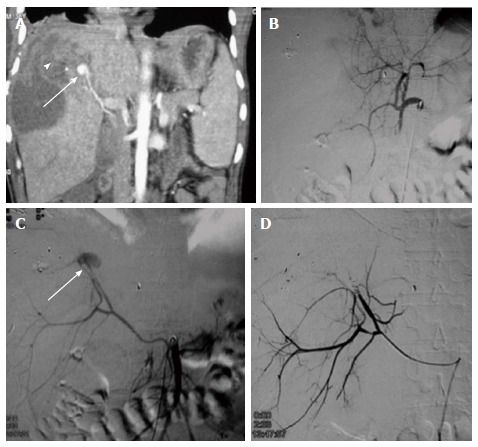
Figure 4.
Prophylactic embolization of right hepatic artery in high grade liver injury. A: CECT abdomen of a 35 years old male, c/o BTA showed extensive deep lacerations and intraparenchymal contusions in the right lobe of liver s/o Grade IV injury. Majority of these lacerations were reaching upto the hepatic surface (arrow) with possible capsular breach. Mild hemoperitoneum was present in pelvis; no active contrast extravasation or pseudoaneurysm; B: In view of high grade injury involving right lobe, prophylactic gelfoam embolization of right hepatic artery was done; C: Post embolization,there was non-opacification of RHA. CECT: Contrast enhanced computed tomography; BTA: Blunt trauma abdomen; RHA: Right hepatic artery.
Figure 5.
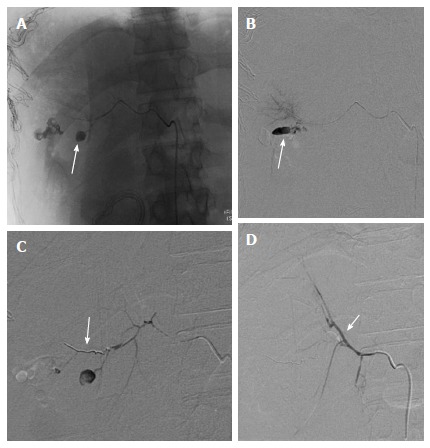
Re-bleed after perihepatic packing. A 38-year-old male with BTA, hemodynamically unstable and taken directly to OR for perihepatic packing. DSA was contemplated in view of persistent hypotension after perihepatic packing. A and B: Selective right hepatic angiogram showed PsA arising from its posterior division (arrow). Subsequently, guidewire followed by micro catheter manipulation was done across its neck; C: Microcoil was deployed across the neck of PsA from distal to proximal followed by gelfoam embolization; D: Post embolization angiographic run showed faint opacification of posterior branch due to sluggish flow. Note brilliant opacification of anterior branch of RHA (arrow) due to reflux of contrast. OR: Operating room; RHA: Right hepatic artery; BTA: Blunt trauma abdomen; DSA: Digital subtraction angiography.
It has been observed that despite refinement of the surgical skills, there is risk of intraparenchymal re-bleeding, especially with high grade injuries. In such scenarios, a re-laparotomy is not preferred due to the ensuing acidosis and metabolic derangements coupled with difficulty in accessing the site of vascular injury which may be either remotely located or anatomically distorted by the previous surgery[11]. In such demanding situations, HAE has emerged as a complementary tool in managing complex hepatic injuries with favorable outcomes. Even in hemodynamically unstable patients, recent studies have shown that HAE has an edge over the traditional management due to the uniqueness of angioembolization to reach the targeted site; thus providing a better control at the site of vascular injury[6,15].
Complications of HAE: It is an essentially safe procedure with only few complications observed. These include delayed hemorrhage, infarction/ necrosis, abscess, bilioma or biliary fistula. Risk of complications are more in high grade liver injuries with extensive vascular and biliary disruption[15,16].
Technical success: HAE is successful in approximately 80%-100% of cases. Risk of technical failure is higher in those cases who have initially undergone laparotomy[17]. Nevertheless, even in such cases, results of HAE is excellent with significant reduction in morbidity and mortality as compared to re-laparotomy. In one series, therapeutic angioembolization was performed in 21 of the 24 patients after DCS and none of these cases had delayed hemorrhage[13]. In an another major Norwegian study comprising of 114 patients with liver injury, it was observed that with the inclusion of endovascular management, there was significant reduction in the laparotomy rate from 58% to 34% and complication rate by 40% (including abscess, biloma, and bile leak), although overall survival rate remained stable at 89%-90%[18].
Other studies have reported significant reduction in mortality when higher grades of liver injury (Grade IV-V) were managed by multidisciplinary approach; especially, with the availability of expertise of angioembolization at the need of hour[11,19]. In a retrospective series by Lin et al[20] consisting of 16 cases of ongoing haemorrhage, DCS was immediately followed by transarterial embolization (TAE) which undoubtedly had the survival benefit, saving lives in about 50% cases. The authors further propounded that the duration of DCS should be as short as possible and to be closely followed by TAE without any undue delay.
At our level Ι trauma center, HAE is done in hepatic artery injury or prophylactically in high grade liver injury, if the patient is hemodynamically stable (Figure 6). Further, it is also done if the patient continues to remain hemodynamically unstable despite DCS. This protocol has been adopted from the inception of our center (2007) and it has lowered the need for laparotomy and complications like abscess, bilioma, and biliary leak.
Figure 6.
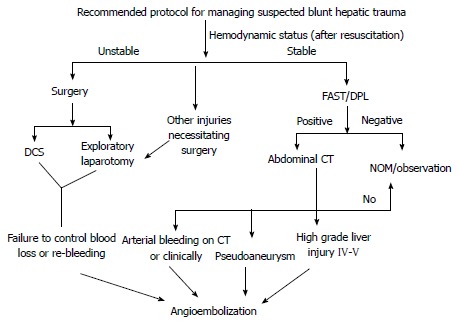
Recommended protocol for managing suspected blunt hepatic trauma. CT: Computed tomography; DCS: Damage control surgery; FAST: Focused assessment with sonography in trauma; DPL: Diagnostic peritoneal lavage.
Splenic injury
Spleen is the most commonly injured solid organ in blunt abdominal trauma. Splenectomy, which once used to be performed in all splenic injuries, is not the preliminary choice in every case due to the concern for the long term immunity. Nowadays, it is restricted to high grade injury with unsalvageable spleen or failure of endovascular interventions to control the bleeding. Management protocol for splenic injury is essentially similar to that of liver injury. In hemodynamically stable patients, splenic artery embolization (SAE) is indicated in arterial injury or prophylactically in high grade splenic injuries. This approach offers the advantage of preserving the functioning splenic parenchyma unlike surgery[1,6,21,22].
In SAE, initially celiac axis angiogram is done followed by selective splenic artery angiogram. There are two embolization options available - proximal or distal, the choice of which is guided by the angiographic findings. Proximal embolization is non-selective which sufficiently reduces the inflow and pulp pressure. Hence, it is preferred in intrasplenic hemorrhage. Additionally, collateral circulation from the small pancreatic branches is preserved; thus, lowering the risk of splenic infarction. In this technique, size of the coil size should be approximately 20%-25% larger than the diameter of the splenic artery to avoid its proximal or distal migration. It is positioned just distal to the origin of dorsal pancreatic artery and proximal to arteria pancreatica magna (anatomical landmark is lateral to the left transverse process of L2 vertebra) (Figure 7).
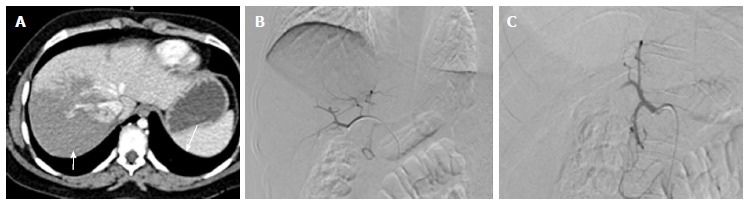
Figure 7.
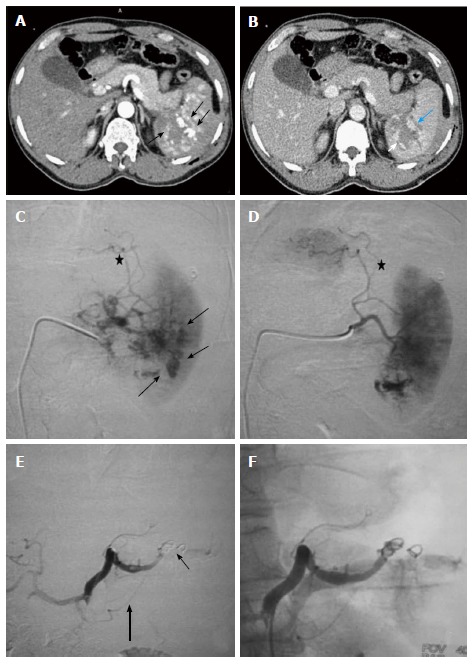
Grade IV splenic injury with active contrast extravasation in a 29-year-old male with blunt trauma abdomen, hemodynamically stable and FAST positive. Arterial phase image (A) showing blobs of contrast (arrow) marginating the deep splenic laceration. In portovenous phase (B), lacerations are better appreciated, few of which are involving splenic hilum. Also, blobs of contrast in the arterial images has slight increased in size (arrow), nonetheless, remain localised s/o focal active contrast extravasation. DSA was done (C and D), selective splenic artery angiogram showed multiple areas of active contrast extravasation from trabecular arteries of lower pole (arrow) with disruption of parenchymal continuity due to laceration (asterisk). Proximal embolization of splenic artery was done with coil followed by gelfoam instillation (E). Note coils (thin arrow) are deployed distal to the origin of dorsal pancreatic artery (thick arrow). Post embolization angiographic run (F) showed proximal contrast stasis with no opacification distal to embolization site. DSA: Digital subtraction angiography; FAST: Focused assessment with sonography in trauma.
Distal or selective embolization, on the other hand, is done in extrasplenic haemorrhage or if there is localized site of injury like in pseudoaneurysm or AV fistula. In this, with the help of co-axial microcatheter system, site specific distal embolization is performed to achieve maximum hemostatic control (Figure 8). Embolization is done by the placement of smaller sized coils, gelfoam pledgets or combination of both[6,23].
Figure 8.
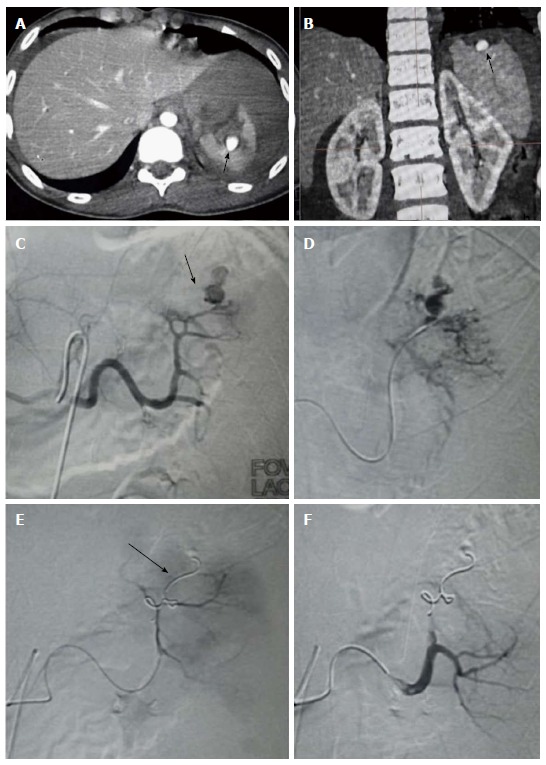
Splenic artery pseudoaneurysm. Case of 39-year-old female with BTA, FAST positive, hemodynamically stable. CECT revealed (A and B) Extensive lacerations involving spleen with presence of contrast filled intrasplenic outpouching paralleling the aortic attenuation s/o pseudoaneurysm. Splenic artery angiogram showed (C) PsA (arrow) arising from the upper polar branch which on delayed phases showed contrast spillage s/o leaking PsA (D). Subsequently, upper pole branch was selectively catheterised by microcatheter followed by deployment of microcoils (arrow) across the neck of PsA (distal embolization) (E). Post embolization angiography (F) showed no flow distal to coil with exclusion of PsA and good contrast opacification of lower pole branch. CECT: Contrast enhanced computed tomography; BTA: Blunt trauma abdomen; PsA: Pseudoaneurysm; FAST: Focused assessment with sonography in trauma.
Proximal splenic artery embolization in traumatic injuries has a success rate of 90%-95% with low incidence of splenic infarction. Distal embolization is also associated with similar success rate for hemostasis but there is higher risk for splenic infarction. Overall procedural time and radiation exposure is more in proximal than distal embolization. Choosing one technique over the other is solely left to the discretion of interventional radiologist and at times combination of both may be required (Figure 9)[1,21-23].
Figure 9.
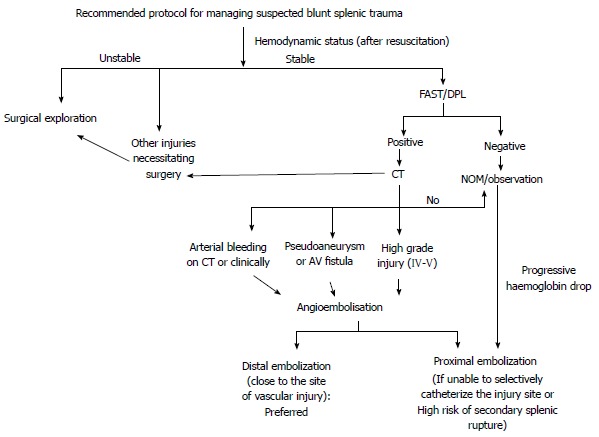
Recommended protocol for managing suspected blunt splenic trauma. CT: Computed tomography; FAST: Focused assessment with sonography in trauma; DPL: Diagnostic peritoneal lavage; NOM: Non operative management.
Complication of SAE: Results of SAE are excellent with faster recuperation as compared to surgery. Although infrequent, there is risk of certain complications like recurrent hemorrhage, splenic abscess, post embolization syndrome, coil migration or symptomatic infarct necessitating splenectomy[1,6,15,21,24].
Technical success rate: Reported success rate of SAE in various series ranges from 73%-100% which has markedly increased the success rate of NOM to over 90%. Several studies suggest that in haemodynamically stable patients, survival rates of angioembolization and splenectomy are comparable[1,23]. In a study by Gaarder et al[25], it was found that performing angiography with embolization (if needed) in all the cases of high grade splenic injury increased the success rates of NOM from 75%-96% as delayed bleeding was the most important reason for the failure of NOM. Also, there was increase in splenic salvage rate with fewer complications than the historical controls when angiography was empirically performed in high grade splenic injuries.
Renal injury
Injuries involving the renal arteries usually occur in conjunction with other solid visceral injuries. A vast majority of blunt renal injuries are minor which usually get resolved NOM. Nephrectomy is reserved only if the organ is non-salvageable like shattered kidney or vascular pedicle avulsion. However, in special circumstances like solitary functioning kidney, nephron sparing surgery is the aim rather than nephrectomy which may render the subsequent need for renal replacement therapy.
Nowadays, most of the endovascular injuries (active bleeding, pseudoaneurysm or arteriovenous fistula) are preferably managed by endovascular interventions. These minimally invasive procedures offer the advantage of rapid control of ongoing hemorrhage besides obviating the surgery and the associated risks[26]. Selection of embolization technique is based on the site and nature of vascular injury (Table 3). The technique of angioembolization in renovascular injury is essentially similar to other organs. An important highlight of the renal circulation is that it is an end arterial supply with paucity of collateral circulation unlike liver or spleen. The corollary to the fact is that, there is high risk of tissue infarction which limits the leverage of non-selective embolization in renal vasculature,which may be performed in liver or spleen in special circumstances. Hence, as a rule, embolization in renovascular injury should be as selective as possible in order to maximize the preservation of viable and perfused renal tissue. Proximal renal artery embolization, on the other hand, is primarily reserved for diffuse renal injury with little or no salvageable renal parenchyma. It is done with an intent to control the life threatening exsanguination besides avoiding surgery (Figures 10, 11 and 12)[1,26,27].
Table 3.
Endovascular interventions in renal artery injury[27]
| Vascular injury | ||
| Site and type of injury | Endovascular management | Comments |
| Main renal artery-dissection, laceration, contrast extravasation | Stent graft | Preserves flow across the site of injury if negotiated safely and successfully |
| Main renal artery occlusion | Intra-arterial thrombolysis using urokinase or tissue plasminogen activator | Salvages kidney if administered within 6 h of injury |
| Intra-renal haemorrhage or pseudoaneurysm | Superselective embolization via microcatheter system and microcoils | Targeted embolization: Preserves functioning renal parenchyma |
| Arterio-venous or arterio-calyceal fistula | Embolization just proximal to fistulous site | Targeted embolization: Preserves functioning renal parenchyma |
Figure 10.
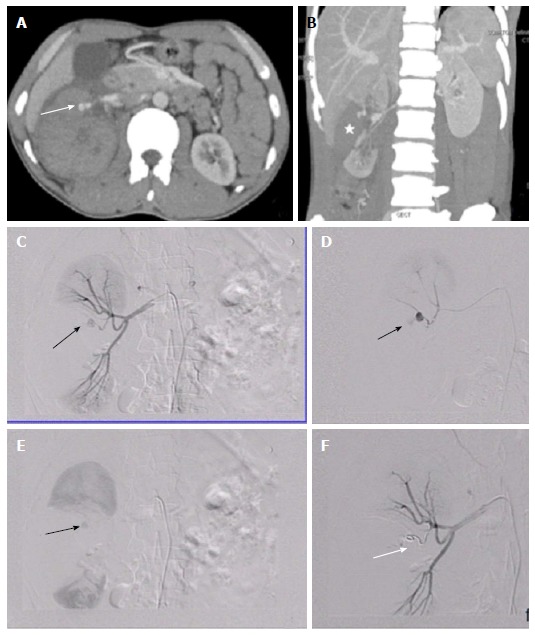
Renovascular injury: 35 years old male with blunt trauma abdomen. A, B: CECT abdomen showed deep lacerations involving the interpole of right kidney extending upto the renal hilum (asterisk). A pseudoaneurysm (arrow) was seen arising from upper polar branch alongwith presence of perinephric hematoma; C, D: DSA with catheterisation of right main renal artery confirmed the presence of pseudoaneurysm (arrow); E: In view of end arterial supply, involved branch was selectively accessed and catheter was subsequently manipulated across the neck of PsA with the help of microwire followed by deployment of coil; F: Post embolization angiography showed non opacification distal to the coil (arrow). CECT: Contrast enhanced computed tomography; PsA: Pseudoaneurysm; DSA: Digital subtraction angiography.
Figure 11.
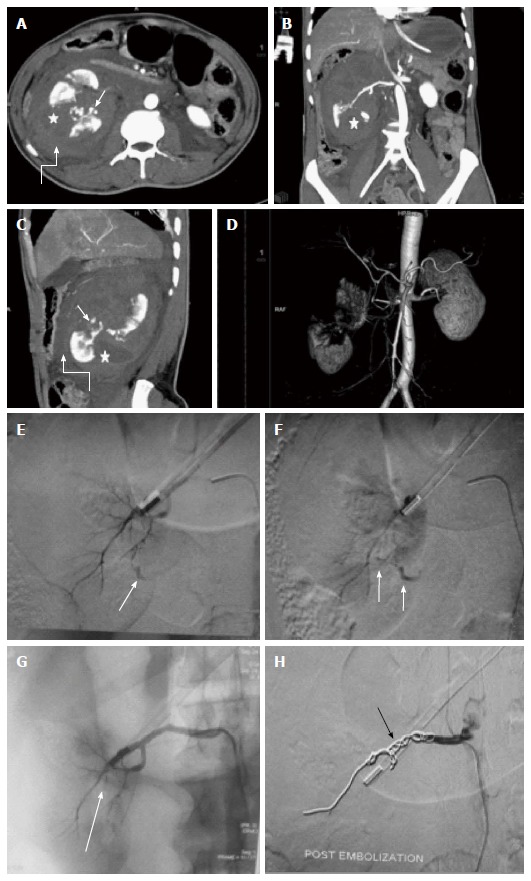
Main renal artery injury. A-D: CECT in a 22 years old male following road traffic accident revealed multiple deep lacerations (asterisk) involving entire thickness of right kidney resulting in shattered kidney. Few areas of active contrast extravasation (thin arrow) were noted at the edge of laceration alongwith presence of extensive pernephric hematoma (curved arrow). Although, main renal artery was showing good luminal opacification, however, less than 25 % remaining functioning renal parenchyma precluded salvagability. Due to high post operative morbidity, angiography was resorted over nephrectomy; E, F: Selective catheterisation of main renal artery showed multiple areas of active contrast extravasation (arrow); G: Subsequently, in view of potentially life threatening haemorrhage and non-salvagable kidney, coil embolization (arrow) of main renal artery was contemplated; H: Post embolization angiography showed opacification of only proximal stump of renal artery. Following conservative management, patient was then discharged 8 d later. CECT: Contrast enhanced computed tomography.
Figure 12.
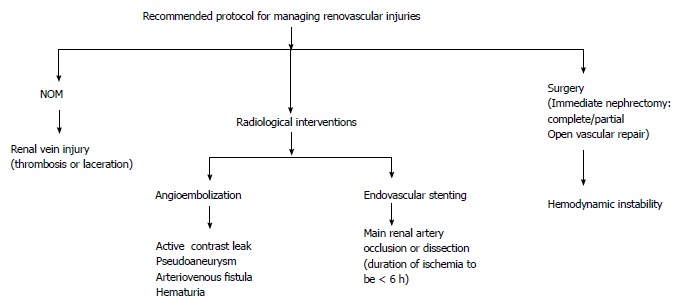
Recommended protocol for managing renovascular injuries. NOM: Non operative management.
Complications of renal artery embolization
Although there is limited data available on the long term success rate of angioembolization; as per the literature it is an extremely well tolerated procedure. Complications which may be occasionally encountered include renal infarction, renal abscess or hypertension. Nevertheless, renal function is largely preserved[28].
Technical success: Endovascular approach has a high success rate in managing renovascular injury. As per the literature, the procedure (including repeat angioembolization) is successful in > 95% of cases in achieving hemostasis and preserving renal function, besides obviating nephrectomy[29-31].
In a study by Hotaling et al[29], of the 77 patients undergoing angioembolization for renovascular injuries, 68 patients further required some additional treatment. Technical success rate was especially lower with higher grade renal injuries (IV and V). However, result of the repeat angioembolization were quite dramatic, with an overall success rate of 92%. Similar results of low success rates in initial angioembolization were observed in studies by Van der Wilden et al[30] and Menaker et al[31], highlighting the importance of carefully scrutinizing such patients for signs of recurrent hemorrhage. There are limited reports of thrombolysis in posttraumatic renal artery occlusion.
A high initial failure rate of endovascular interventions in renal injuries has been attributed variously to the high fluid and transfusion requirements, older age group (> 55 years), concomitant injury to other abdominal organs and metabolic derangements (especially acidosis)[32,33].
Prospects of stent-grafting in renovascular injuries[27]
There is limited data available on the long term effects of stent graft placement in renal artery injuries. However, few reports suggest a higher propensity for refractory hypertension and renal atrophy in traumatic injuries as compared to the general population. Although the procedure remains similar irrespective of the causative etiology of renal artery occlusion; the low technical success rate in traumatic injuries may be due to below mentioned factors.
In traumatic injuries, duration of renal ischemia is the most critical determinant of the technical success of the endovascular stenting as well as restoration of the renal vitality. For optimal results, total duration of ischemia should be < 3 h in complete and < 6 h in incomplete arterial occlusion. Further, to prevent stent occlusion, anticoagulation should be administered immediately after stent placement. However, in trauma due to ongoing bleeding or potential risk of re-bleeding, anticoagulation is not recommended, which increases the likelihood of stent occlusion. Another important determinant is the extent of collaterization and patency of renal vein which increases the tolerance to the ischemic event; thereby, increasing the success rate of stenting.
Pelvic fracture
Management of severe pelvic injuries continue to remain an enigma for trauma surgeons. Mortality in pelvic injury ranges from 2%-23% and nearly 54% of the early deaths are attributed to the ongoing hemorrhage. As hemodynamic instability adversely influence the prognosis of these severely injured patients, prompt and effective management is warranted. Pelvis stabilization with binders or external fixators is the most vital step in the preliminary management of pelvic injuries which serves as an adjunct to IR to limit the bleeding until embolization can be performed. This acts by reducing the pelvic volume as well as approximation of the fracture ends which prevents further bleeding and leads to hematoma formation which provides tamponade to the bleeding site, especially, if it is from the end of the fracture or venous injury. Pelvic fractures are often associated with other abdominal injuries; hence, CT is imperative before planning further management[3,6,33-38].
As compared to other sites, bleeding in pelvic injury may be of multifactorial etiology like venous, arterial or osseous. Amongst these, venous bleeding is the most common and is due to the disruption of presacral venous plexus which leads to the pelvic retroperitoneal hemorrhage (PRH). Arterial injury may result from the shearing pelvic injury, avulsion/transection of artery from the sharp bony fragment or iatrogenic while managing pelvic fractures. Internal pudendal and superior gluteal arteries are the most commonly injured arteries. Clinically, arterial injury may be suggested by hemodynamic instability requiring massive blood transfusions. In such cases, there is a nearly 43%-78% incidence of positive angiographic findings. Musculoskeletal injury is another cause of pelvic hemorrhage due to the fracture of vascular cancellous bone or intramuscular hematoma. As mentioned above, venous and osseous bleeding usually get controlled by pelvic stabilization. On the contrary, arterial bleeding due to the high pressure circulation, usually needs to intervened[3,6,36,37].
Pelvic fracture related retroperitoneal hemorrhage may be managed either by surgery or endovascular embolization. Surgical management consists of intra-operative fixation with or without pre-peritoneal packing. Urgent laparotomy is indicated in persistent hypotension, gross hemoperitoneum or polytrauma with other indication of surgical exploration[6]. However, there are several limitations in pelvic surgery which increases the risk of complications. Amongst these, complex anatomy of the pelvis is the most important determinant which may hinder the accessibility to the bleeding site. Due to difficult access, there is increased intra-operative risk of destabilization of already formed clot which may be disturb its tamponade effect at the site of bleeding. Furthermore, arterial bleeding is usually due to multiple small peripheral branches which may be difficult to access or identify during surgery. Even, bilateral internal iliac ligation may prove futile in these cases due to the rich collateral circulation in pelvis[6,39,40].
Angiographic embolization (Figures 13, 14 and 15) in pelvic injury is indicated in major pelvic fracture with signs of ongoing bleeding despite ruling out other non-pelvic sources of blood loss. These include persistent hypotension even after administration of fluid and/or transfusion requirements exceeding 4 units of blood/24 h or > 6 units/4 h. It is also done in inadequately controlled hemorrhage after surgical exploration or if there is large expanding hematoma during laparotomy. Endovascular embolization is also indicated if there are CT findings suggestive of pelvic vascular injury which may be active contrast extravasation, pseudoaneurysm or pelvic hematoma exceeding 600 cc in volume. CT angiography is highly sensitive in diagnosing such injuries with a negative predictive value of 99.6%. Accordingly, catheter angiography is usually not performed if CT angiography does not reveal any findings suggestive of vascular injury. Embolization may also be a temporary adjunct before the definitive surgery[6,36,37,39,40].
Figure 13.
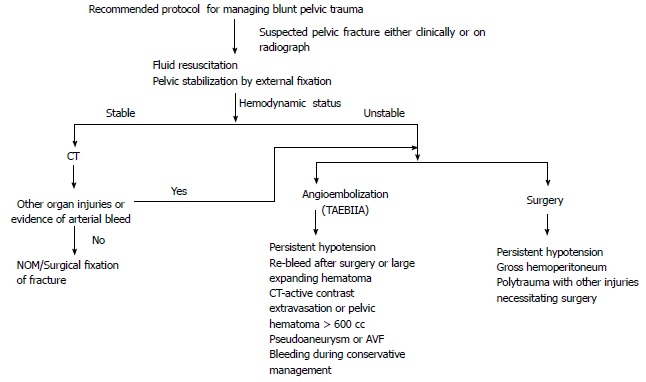
Recommended protocol for managing blunt pelvic trauma. NOM: Non operative management; TAEBIIA: Transarterial embolization of bilateral iliac artery; CT: Computed tomography; AVF: Arterio-venous fistula.
Figure 14.
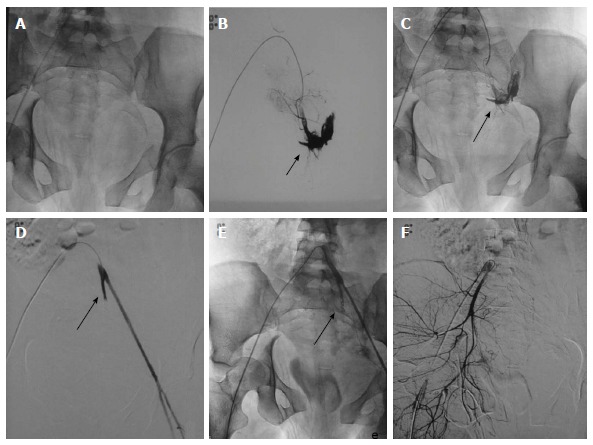
Pelvic fractures with injury of left internal iliac artery. DSA of 24-year-old hemodynamically unstable patient with extensive pelvic fractures. A-C: Selective catheterization of left common iliac artery showed active contrast extravasation from left internal iliac artery subjacent to the disrupted left sacroiliac articulation which was subsequently embolized with coil; D, E: Angiography post embolization showed non-opacification of internal iliac artery distal to coil (arrow) with complete disappearance of contrast extravasation. Left external iliac artery showed good opacification; F: Subsequently, right common iliac artery was cannulated which revealed no vascular injury. DSA: Digital subtraction angiography.
Figure 15.
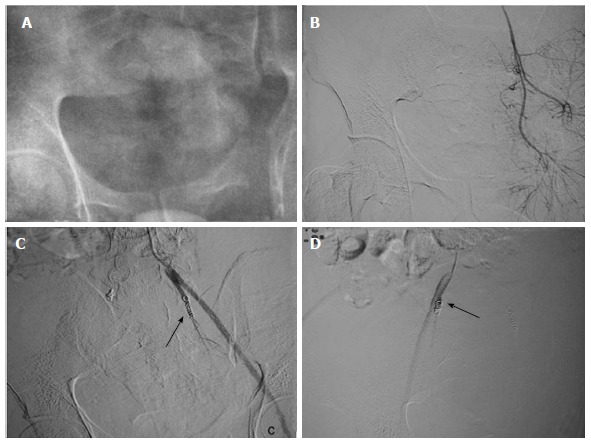
Transarterial embolization of bilateral internal iliac artery: Another case of severe pelvic injury with persistent hypotension. A-D: Bilateral internal iliac arteries were selectively cannulated, however, no vascular injury present. Prophylactic coil embolization of bilateral internal iliac artery was done (arrow). Few hours later patient became normotensive.
Pelvic angioembolization may be selective if the site of vascular injury may be negotiated precisely. However, non-selective embolization may be done if selective catheterization is not achieved or prophylactically if angiography does not reveal any evidence of vascular injury. If non-selective, then both the internal iliac arteries are embolized as there may be chances of re-bleeding due to extensive collateral circulation in pelvis. For the similar reason, embolization should be as peripheral as possible for effectively controlling the bleeding. Other causes of re-bleeding include anomalous or accessory branches of the external iliac and inferior mesenteric artery. Choice of embolizing agent is dependent on the selectiveness of catheterization achieved as well coagulation profile. In nonselective embolization of bilateral internal iliac artery, bleeding is most effectively controlled by flow-directed particulate embolization using Gelfoam slurry. It is easily available, inexpensive, and temporary measure which leads to mechanical occlusion. It is prepared by diluting Gelfoam shavings with iodinated non-ionic contrast through a three-way stop-cock between two 10-cc syringes Since this embolization approach is non-targeted, it results in occlusion of multiple vessels in a traumatized vascular bed (as compared to multiple selective catheterizations). On the contrary, coil is preferred when the bleeding vessel can be selectively approached. It achieves rapid and permanent vascular occlusion. For effective clot formation, coagulation profile must be intact. It should not be used if transfusion requirements ≥ 8 packed cell units or hypothermia both of which can induce coagulopathy[6,37,39,40].
There are several advantages of angioembolization over surgery. Angioembolization rapidly controls of the bleeding without disturbing the tamponade effect of hematoma (Figures 13, 14 and 15). Irrespective of the pelvic anatomy, embolization may access even the remote site of vascular injury with maintained tissue barriers unlike surgery. Besides, the procedure can be done under local anaesthesia as opposed to general anaesthesia needed for surgery[36,37,39,40].
As mentioned above, pelvic hemorrhage is one of the exceptions to the general rules of radiological interventions in traumatic injuries, as it is always a preferred option over surgery irrespective of the hemodynamic status of the patient. However, optimal therapeutic approach remains controversial which largely depends on the expertise and availability of subspecialty physicians and support staff[6,36,37,39,40].
Complications of pelvic embolization include failure to control the bleeding which may be due to rapid bleeding due to extensive injuries, failure to achieve selective catheterization or prolonged delay since the time of injury to angiographic intervention. Further, re-bleeding may occur in 5.8%-7.5% of cases requiring repetition of the procedure. Rarely, there may be ischemic necrosis of the rectum or gluteal muscles, peripheral nerve damage with paralysis or paresthesia, colonic or ureteric injury or sexual dysfunction[39-41].
Technical success: Angioembolization has become the cornerstone in management of the pelvic injuries with a reported success rate of about 85%-90% in controlling pelvic hemorrhage[6]. A positive angiogram is reported in 28% to 100% of studies of traumatic PRH. Time from the onset hemorrhage to shifting to IR suite is a crucial determinant as reported in a study by Agolini et al[42] in which authors found a significant reduction in the mortality when this duration was < 3 h. In another study by Velmahos et al[43], it was reported that of the 30 patients, angiographic embolization could control haemorrhage and achieve hemodynamic stability in 27 patients (90%) which additionally increased to 97% after repeat angiography. Temporary arterial embolization of the bilateral internal iliac arteries (TAEBIIA) is a safe, rapid and extremely safe method to control PRH. Theoretically TAEBIIA can cause pelvic ischemia, although has never been reported till date. Nonetheless, the reported mortality rate remains high at 5% to 35% which is usually due to concomitant organ injuries or multiple organ failure[40].
Peripheral vascular injuries
Peripheral vascular injuries (PVI) require meticulous triage to identify hard (distal pulselessness, expanding or pulsatile hematoma/bleeding, thrill or bruit and critical limb ischemia) or soft signs (pallor, cold limbs, non-expanding hematoma, neurological deficit or unexplained hypotension). Presence of hard signs heralds further surgical or endovascular management[4,6].
PVI can be broadly classified into limb threatening (active contrast extravasation, laceration, pseudoaneurysm, AV fistula, transection or thrombosis) or non-limb threatening (attenuation, spasm, small AV fistula or pseudoaneurysm in non-critical branches). All these injuries can further be typified into ischemic or hemorrhagic based on clinical presentation which has therapeutic implications. Patients with ischemic type of peripheral vascular injuries are usually managed by surgical exploration whereas hemorrhagic type of injuries are managed either by surgical or by endovascular approach, which depends on hemodynamic status, amount and rapidity of bleeding, availability of local resources and technical expertise[41,42].
IR is an adjunct to surgical management of peripheral vascular injuries. If the patient is hemodynamically stable, endovascular treatment plays an important role in managing the hemorrhagic type of peripheral vascular injuries. In PVI, endovascular interventions include embolization and vascular reconstruction (Figures 16, 17, and 18). Choice of procedure depends on the type (end vs main branch) and size (small, medium sized or large) of injured vessel as well as nature of vascular injury (pseudoaneurysm, arteriovenous fistula, active leak) (Figures 19 and 20)[42,43].
Figure 16.
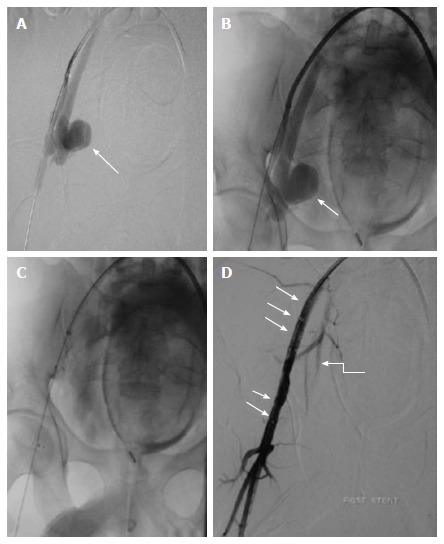
Stent grafting in pseudoaneurysm. Case of gun shot injury, CECT showed presence of PsA from right internal iliac artery (not shown). A, B: Arterial access was obtained through the left common femoral artery and a long arterial sheath was placed. Angiogram showed a large pseudoaneurysm (arrow) arising from the proximal portion of anterior division of right internal iliac artery with no contrast opacification distally (except for a short stump); C, D: Subsequently, a covered stent graft (arrow) was placed in right external artery. Post procedural angiography showed preservation of flow across the right external iliac artery and branches of right internal iliac artery (curved arrow). CECT: Contrast enhanced computed tomography; PsA: Pseudoaneurysm.
Figure 17.
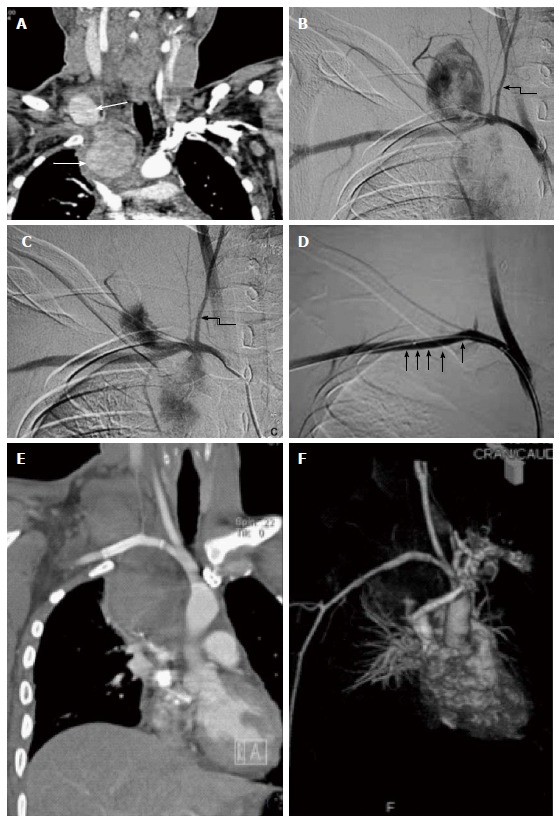
Peripheral vascular injury. A 56-year-old male with multiple rib fractures and neck swelling following road traffic accident. A: CT angiography showed two distinct pseudoaneurysms arising from the proximal right subclavian artery, one cervical and other intrathoracic (arrow). However, there was good opacification of distal subclavian artery with no disruption in continuity, ruling out transection (curved arrow); B, C: DSA corroborated CT findings and revealed two large pseudoaneurysms arising from the proximal right subclavian artery, lateral to the origin of thyrocervical branch (arrow); D: Subsequently, guidewire was manipulated across the PsA and covered stent graft (arrow) was deployed, excluding the origin of right common carotid artery. Post stenting angiography showed no opacification of pseudoaneurysm with good flow in distal subclavian artery; E, F: Follow up CT angiography done 2 wk later revealed both the pseudoaneurysm to be thrombosed. The stent (arrow) was optimally placed stent (arrow) with preservation of distal flow across it. PsA: Pseudoaneurysm; DSA: Digital subtraction angiography.
Figure 18.
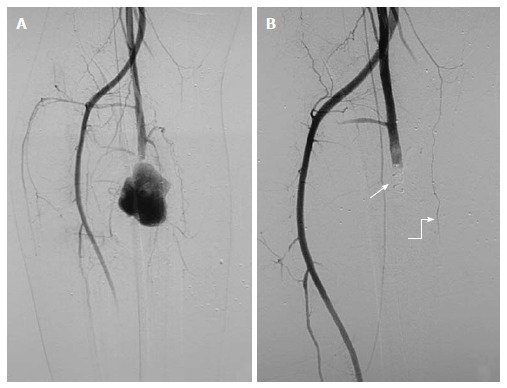
Pseudoaneurysm from right profunda femoris artery: 21-year-old male with stab injury. A: DSA showed a large PsA arising from right profunda femoris artery with no distal flow, likely vascular transection with pseudoaneurysm of proximal end; B: As guidewire could not be negotiated across the pseudoaneurysm due to possible discontinuity, proximal coil embolization (arrow) was done. Post embolization angiography showed complete exclusion of pseudoaneurysm. Note the presence of collaterals (curved arrow) along the course of profunda femoris artery. PsA: Pseudoaneurysm; DSA: Digital subtraction angiography.
Figure 19.
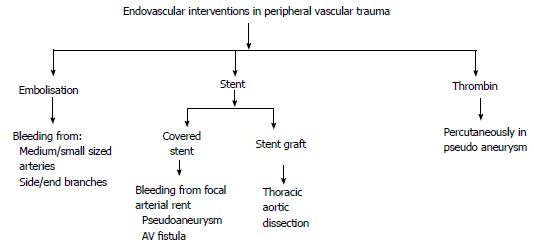
Endovascular interventions in peripheral vascular trauma. AV: Arterio-venous.
Figure 20.
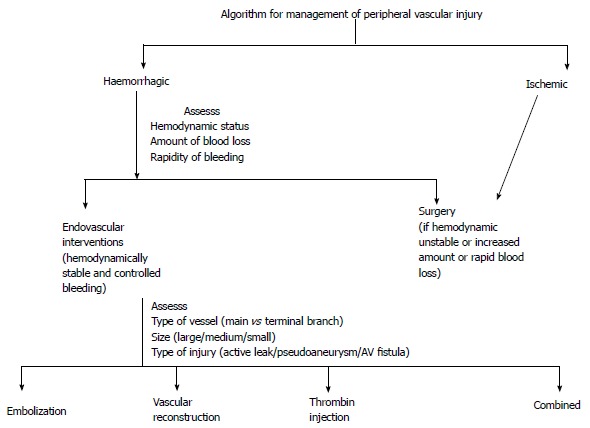
Algorithm for management of peripheral vascular injury.
Endovascular treatment has certain advantages over conventional surgical techniques like reduced operative time, estimated blood loss and complication rates. At times in situations where definitive endovascular treatment is not possible, simple technique such as temporary balloon occlusion can control ongoing hemorrhage before proceeding for definitive surgical repair. If indicated, endovascular interventions are treatment of choice as there is significant reduction in morbidity and mortality as compared to surgery. Further in IR procedures, general anaesthesia is usually not required, hence can be performed even in patients with head injury[4].
Contraindications of endovascular interventions in peripheral vascular trauma (Table 4)[44,45]
Table 4.
Permanent embolization in peripheral vascular injury[4]
| Indication | Contraindication |
| Non vital branches | Major vessels like axillary, brachial, superficial femoral and popliteal arteries due to risk of critical limb ischemia |
| Proximal non-axial branches, e.g., profunda brachii and profunda femoris |
Only absolute contraindication for endovascular interventions is inability to negotiate the vascular injury with guidewire unless embolization is aimed. Endovascular approach is not preferred for stenting at anatomically mobile sites like common femoral, popliteal or axillary arteries, especially if surgical access is not difficult.
Bowel and mesentery injury
Bowel and mesenteric injuries rank next to the liver and spleen in incidence in the blunt trauma abdomen. There is no role of endovascular IR in bowel injury which essentially undergo surgical repair. On the other hand, isolated mesenteric vascular injuries without any associated bowel injury may be managed endovascularly. Such injuries include active bleeding or pseudoaneurysm and are preferably managed surgically. The caveat in endovascular management of the mesenteric injuries is the potential risk of bowel ischemia or infarction due to high risk of non-selective or non-target embolization. Moreover, these complications are more if there is injury to peripheral branches which are extremely difficult for selective catheterization due to small calibre or angulated course. Accordingly, choice of embolizing agent may vary depending on the final position of the catheter’s tip, type and site of vascular injury, and the preference of the operator. Various embolic agents which can be used include microcoils, glue and gelfoam[46].
Injury to aorta and its branches
Aortic injuries are usually incurred in high velocity thoraco-abdominal injuries. Spectrum of injury to aorta and its branches include aortic transection, dissection, pseudoaneurysm and minimal aortic injuries. Majority of these injuries prove detrimental due to fatal exsanguination. Common sites of aortic injury in the descending order are aortic isthmus, root of the ascending aorta and descending aorta at the level of diaphragm due to relative immobility at these sites. These injuries are usually diagnosed on CECT/CT angiography in most of the cases. Endovascular management is usually reserved for the dissection and pseudoaneurysm in the hemodynamically stable patients. Endovascular management options include placement of the stent to maintain the luminal integrity. Additionally, other embolizing agent like coil, glue etc may be used for packing of the sac of pseudoaneurysm. Nowadays, endovascular management of aortic injuries is preferred over surgery wherever feasible due to less mortality and morbidity in the former[47,48].
Face and neck injuries
Cervico-facial injuries whether blunt or penetrating may potentially injure the carotid and/or vertebral arteries. Besides causing exsanguination, there is risk of compromise to the central nervous system blood supply. These major vascular injuries may be managed conservatively, surgically or by endovascular interventions. This in turn depends on multiple factors which include hemodynamic and neurological status, any additional injury which mandates surgery, site of injury and extent of collateral circulation.
In a hemodynamically stable patient, CT angiography may be done to characterize the type and site of vascular injury. CT also helps in evaluation of injury to other structures. Nowadays, surgery in cerebrovascular injuries is preferred only in those cases which are hemodynamically unstable, other surgical indication or if endovascular embolization is risky which may be either due to non-selective catheterization or inadequate collateralization. Cerebrovascular injuries are now increasingly indicated in such injuries as this approach is minimally invasive, rapid and access even the complex anatomical sites. Initially diagnostic angiography is done to localize/ characterize the site and type of vascular and adequacy of the collateral circulation. Choice of embolizing agent depends on the calibre of the vessel, collateral network and selectiveness of catheterization. If vascular patency can be sacrificed without the risk of ischemia then gel foam, coils or NBCA may be used. If luminal conduit needs to be maintained as in injury to large or medium calibre vessels, then endovascular stenting may be done[49].
Non vascular interventions
Non vascular interventions are frequently used in the management of the sequelae of traumatic injuries either alone or as an interim to surgery. Percutaneous drainage is done for the collections or hematoma under imaging guidance. Moreover, percutaneous hepatobiliary (Percutaneous transhepatic biliary drainage) and renal (Percutaneous nephrostomy) interventions may be done in obstruction or biliary/urinary leak.
CIRSE guidelines for quality improvement in endovascular treatment of traumatic injuries
Cardiovascular and interventional radiological society of Europe (CIRSE, 2012) has laid down the guidelines for imaging and radiological interventions in the traumatic injuries. These are evidence based guidelines which mention the indications and contraindications of IR in various injuries. It is intended to maximize the safety of radiological interventions by providing levels of evidence and recommendations[26].
Recent trends: IR in hemodynamically unstable patients
Until now interventional radiological procedures were reserved for hemodynamically stable patients as an adjunct to NOM or surgery. However, recently concept of time conscious trauma management protocol ‘‘Prompt and Rapid Endovascular Strategies in Traumatic Occasions’’ (PRESTO) has been introduced which conceptualizes initiating the role of interventional radiologist right from the time of hospital entry. Akin to DCS which aims at lifesaving measures, specifically tailored interventional radiological procedure as per this protocol has been termed as “Damage control IR”. The idea is to save a potentially life threatening exsanguination even if it is achieved at the cost of organ sacrifice. As per this protocol, as soon as patient arrives, IR team is informed and during primary survey itself femoral arterial sheath is placed, which potentiates and supplements ongoing resuscitative efforts by providing a quick access route for resuscitative endovascular balloon occlusion of the aorta, thus maintaining the normal pressure. This approach buys time for shifting the patient for surgery/IR suite or CT scan and provides access for subsequent endovascular interventions.
If the patient can be shifted, then pan CT is performed also termed as FACT (focused assessment with CT) which includes head, chest, abdomen and pelvis scan and rapid assessment of acquired images by a special reading mode which is programmed to detect preselected severe injuries within 3 min. If the patient is taken directly to IR suite, then diagnostic angiography is performed with allotted time of approximately 10 min for each major vessel. In cases if CT is done, then targeted angiography is done aiming at site of vascular injury. Procedure should not be prolonged for want of selective embolization and rapidly winded up by doing proximal embolization. Regarding embolizing agents, glue (N-Cyanobutylacrylate) is preferred as its mechanism of action is not hampered by coagulopathy. Hydrocoil and onyx are other alternative embolizing agents in coagulopathy and are comparatively easier to handle. With unprecedented success of IR in NOM for hemodynamically stable patients, it can be forecasted that if strictly followed then PRESTO may not only reduce the early trauma related mortality but will also redefine the status of IR marking the beginning of new era in trauma management[50].
CONCLUSION
Recent times have witnessed a paradigm shift in the management of traumatic injuries with trend towards NOM. Integration of IR procedures is an extension of such approach requiring minimally invasive interventions to achieve hemostasis. Uniqueness of endovascular techniques lies in approaching site of vascular injury without altering the normal tissue barrier or tamponade effect of hematoma coupled with expeditiously controlling life threatening hemorrhage by embolization. Not only has it helped in reducing mortality but a considerable reduction in morbidity with faster recuperation. Along with the availability of new generation CT scanners which permits faster image acquisition and hence providing an overview of extent of injuries, surgical expertise is now sought mainly for managing penetrating injuries, multiple bleeding sites or in case of hemodynamically instability.
Footnotes
Conflict-of-interest statement: The authors have nothing to disclose.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: India
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: September 28, 2016
First decision: November 2, 2016
Article in press: January 18, 2017
P- Reviewer: Andersen PE, Kambadakone A, Tarazov PG, Vogl TJ S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
References
- 1.Wallis A, Kelly MD, Jones L. Angiography and embolisation for solid abdominal organ injury in adults - a current perspective. World J Emerg Surg. 2010;5:18. doi: 10.1186/1749-7922-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopera JE. Embolization in trauma: principles and techniques. Semin Intervent Radiol. 2010;27:14–28. doi: 10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna P, Phan H, Hardy AH, Nolan T, Dong P. Multidisciplinary management of blunt pelvic trauma. Semin Intervent Radiol. 2012;29:187–191. doi: 10.1055/s-0032-1326927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer JR, Ray CE. Transcatheter arterial embolization in the trauma patient: a review. Semin Intervent Radiol. 2004;21:11–22. doi: 10.1055/s-2004-831401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore HM, List A, Holden A, Osborne TM. Therapeutic embolization for acute haemorrhage in the abdomen and pelvis. Australas Radiol. 2000;44:161–168. doi: 10.1046/j.1440-1673.2000.00000.x. [DOI] [PubMed] [Google Scholar]
- 6.Gould JE, Vedantham S. The role of interventional radiology in trauma. Semin Intervent Radiol. 2006;23:270–278. doi: 10.1055/s-2006-948766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffer EK, Borsa JJ, Bloch RD, Fontaine AB. Endovascular techniques in the damage control setting. Radiographics. 1999;19:1340–1348. doi: 10.1148/radiographics.19.5.g99se051340. [DOI] [PubMed] [Google Scholar]
- 8.Medsinge A, Zajko A, Orons P, Amesur N, Santos E. A case-based approach to common embolization agents used in vascular interventional radiology. AJR Am J Roentgenol. 2014;203:699–708. doi: 10.2214/AJR.14.12480. [DOI] [PubMed] [Google Scholar]
- 9.Lubarsky M, Ray CE, Funaki B. Embolization agents-which one should be used when? Part 1: large-vessel embolization. Semin Intervent Radiol. 2009;26:352–357. doi: 10.1055/s-0029-1242206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubarsky M, Ray C, Funaki B. Embolization agents-which one should be used when? Part 2: small-vessel embolization. Semin Intervent Radiol. 2010;27:99–104. doi: 10.1055/s-0030-1247891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doklestić K, Stefanović B, Gregorić P, Ivančević N, Lončar Z, Jovanović B, Bumbaširević V, Jeremić V, Vujadinović ST, Stefanović B, et al. Surgical management of AAST grades III-V hepatic trauma by Damage control surgery with perihepatic packing and Definitive hepatic repair-single centre experience. World J Emerg Surg. 2015;10:34. doi: 10.1186/s13017-015-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prichayudh S, Sirinawin C, Sriussadaporn S, Pak-art R, Kritayakirana K, Samorn P, Sriussadaporn S. Management of liver injuries: predictors for the need of operation and damage control surgery. Injury. 2014;45:1373–1377. doi: 10.1016/j.injury.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Asfar S, Khoursheed M, Al-Saleh M, Alfawaz AA, Farghaly MM, Nur AM. Management of liver trauma in Kuwait. Med Princ Pract. 2014;23:160–166. doi: 10.1159/000358126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira BM. Non-operative management of hepatic trauma and the interventional radiology: an update review. Indian J Surg. 2013;75:339–345. doi: 10.1007/s12262-012-0712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilbao JI, Martínez-Cuesta A, Urtasun F, Cosín O. Complications of embolization. Semin Intervent Radiol. 2006;23:126–142. doi: 10.1055/s-2006-941443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohan B, Bhoday HS, Aslam N, Kaur H, Chhabra S, Sood N, Wander G. Hepatic vascular injury: clinical profile, endovascular management and outcomes. Indian Heart J. 2013;65:59–65. doi: 10.1016/j.ihj.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaarder C, Naess PA, Eken T, Skaga NO, Pillgram-Larsen J, Klow NE, Buanes T. Liver injuries--improved results with a formal protocol including angiography. Injury. 2007;38:1075–1083. doi: 10.1016/j.injury.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Asensio JA, Petrone P, García-Núñez L, Kimbrell B, Kuncir E. Multidisciplinary approach for the management of complex hepatic injuries AAST-OIS grades IV-V: a prospective study. Scand J Surg. 2007;96:214–220. doi: 10.1177/145749690709600306. [DOI] [PubMed] [Google Scholar]
- 19.Asensio JA, Roldán G, Petrone P, Rojo E, Tillou A, Kuncir E, Demetriades D, Velmahos G, Murray J, Shoemaker WC, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. J Trauma. 2003;54:647–653; discussion 653-654. doi: 10.1097/01.TA.0000054647.59217.BB. [DOI] [PubMed] [Google Scholar]
- 20.Lin BC, Wong YC, Lim KE, Fang JF, Hsu YP, Kang SC. Management of ongoing arterial haemorrhage after damage control laparotomy: optimal timing and efficacy of transarterial embolisation. Injury. 2010;41:44–49. doi: 10.1016/j.injury.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Gaba RC, Katz JR, Parvinian A, Reich S, Omene BO, Yap FY, Owens CA, Knuttinen MG, Bui JT. Splenic artery embolization: a single center experience on the safety, efficacy, and clinical outcomes. Diagn Interv Radiol. 2013;19:49–55. doi: 10.4261/1305-3825.DIR.5895-12.1. [DOI] [PubMed] [Google Scholar]
- 22.Bessoud B, Denys A, Calmes JM, Madoff D, Qanadli S, Schnyder P, Doenz F. Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? AJR Am J Roentgenol. 2006;186:779–785. doi: 10.2214/AJR.04.1800. [DOI] [PubMed] [Google Scholar]
- 23.Raikhlin A, Baerlocher MO, Asch MR, Myers A. Imaging and transcatheter arterial embolization for traumatic splenic injuries: review of the literature. Can J Surg. 2008;51:464–472. [PMC free article] [PubMed] [Google Scholar]
- 24.Ekeh AP, Khalaf S, Ilyas S, Kauffman S, Walusimbi M, McCarthy MC. Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg. 2013;205:250–254; discussion 254. doi: 10.1016/j.amjsurg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Gaarder C, Dormagen JB, Eken T, Skaga NO, Klow NE, Pillgram-Larsen J, Buanes T, Naess PA. Nonoperative management of splenic injuries: improved results with angioembolization. J Trauma. 2006;61:192–198. doi: 10.1097/01.ta.0000223466.62589.d9. [DOI] [PubMed] [Google Scholar]
- 26.Chakraverty S, Flood K, Kessel D, McPherson S, Nicholson T, Ray CE, Robertson I, van Delden OM. CIRSE guidelines: quality improvement guidelines for endovascular treatment of traumatic hemorrhage. Cardiovasc Intervent Radiol. 2012;35:472–482. doi: 10.1007/s00270-012-0339-7. [DOI] [PubMed] [Google Scholar]
- 27.Bittenbinder EN, Reed AB. Advances in renal intervention for trauma. Semin Vasc Surg. 2013;26:165–169. doi: 10.1053/j.semvascsurg.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Stewart AF, Brewer ME, Daley BJ, Klein FA, Kim ED. Intermediate-term follow-up of patients treated with percutaneous embolization for grade 5 blunt renal trauma. J Trauma. 2010;69:468–470. doi: 10.1097/TA.0b013e3181e5407a. [DOI] [PubMed] [Google Scholar]
- 29.Hotaling JM, Sorensen MD, Smith TG, Rivara FP, Wessells H, Voelzke BB. Analysis of diagnostic angiography and angioembolization in the acute management of renal trauma uSingh A national data set. J Urol. 2011;185:1316–1320. doi: 10.1016/j.juro.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Wilden GM, Velmahos GC, Joseph DK, Jacobs L, Debusk MG, Adams CA, Gross R, Burkott B, Agarwal S, Maung AA, et al. Successful nonoperative management of the most severe blunt renal injuries: a multicenter study of the research consortium of New England Centers for Trauma. JAMA Surg. 2013;148:924–931. doi: 10.1001/jamasurg.2013.2747. [DOI] [PubMed] [Google Scholar]
- 31.Menaker J, Joseph B, Stein DM, Scalea TM. Angiointervention: high rates of failure following blunt renal injuries. World J Surg. 2011;35:520–527. doi: 10.1007/s00268-010-0927-0. [DOI] [PubMed] [Google Scholar]
- 32.Brewer ME, Strnad BT, Daley BJ, Currier RP, Klein FA, Mobley JD, Kim ED. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol. 2009;181:1737–1741. doi: 10.1016/j.juro.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 33.Starr AJ, Griffin DR, Reinert CM, Frawley WH, Walker J, Whitlock SN, Borer DS, Rao AV, Jones AL. Pelvic ring disruptions: prediction of associated injuries, transfusion requirement, pelvic arteriography, complications, and mortality. J Orthop Trauma. 2002;16:553–561. doi: 10.1097/00005131-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Smith W, Williams A, Agudelo J, Shannon M, Morgan S, Stahel P, Moore E. Early predictors of mortality in hemodynamically unstable pelvis fractures. J Orthop Trauma. 2007;21:31–37. doi: 10.1097/BOT.0b013e31802ea951. [DOI] [PubMed] [Google Scholar]
- 35.Pohlemann T, Bosch U, Gänsslen A, Tscherne H. The Hannover experience in management of pelvic fractures. Clin Orthop Relat Res. 1994;(305):69–80. [PubMed] [Google Scholar]
- 36.Eastridge BJ, Burgess AR. Pedestrian pelvic fractures: 5-year experience of a major urban trauma center. J Trauma. 1997;42:695–700. doi: 10.1097/00005373-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Riemer BL, Butterfield SL, Diamond DL, Young JC, Raves JJ, Cottington E, Kislan K. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35:671–675 discussion 676-677. doi: 10.1097/00005373-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Balogh Z, King KL, Mackay P, McDougall D, Mackenzie S, Evans JA, Lyons T, Deane SA. The epidemiology of pelvic ring fractures: a population-based study. J Trauma. 2007;63:1066–1073; discussion 1072-1073. doi: 10.1097/TA.0b013e3181589fa4. [DOI] [PubMed] [Google Scholar]
- 39.Hoffer EK. Transcatheter embolization in the treatment of hemorrhage in pelvic trauma. Semin Intervent Radiol. 2008;25:281–292. doi: 10.1055/s-0028-1085928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broadwell SR, Ray CE. Transcatheter embolization in pelvic trauma. Semin Intervent Radiol. 2004;21:23–35. doi: 10.1055/s-2004-831402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu CY, Wang YC, Wu SC, Chen RJ, Hsieh CH, Huang HC, Huang JC, Lu CW, Huang YC. Angioembolization provides benefits in patients with concomitant unstable pelvic fracture and unstable hemodynamics. Am J Emerg Med. 2012;30:207–213. doi: 10.1016/j.ajem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Agolini SF, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed JF. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma. 1997;43:395–399. doi: 10.1097/00005373-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Velmahos GC, Chahwan S, Hanks SE, Murray JA, Berne TV, Asensio J, Demetriades D. Angiographic embolization of bilateral internal iliac arteries to control life-threatening hemorrhage after blunt trauma to the pelvis. Am Surg. 2000;66:858–862. [PubMed] [Google Scholar]
- 44.Salazar GM, Walker TG. Evaluation and management of acute vascular trauma. Tech Vasc Interv Radiol. 2009;12:102–116. doi: 10.1053/j.tvir.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Katsanos K, Sabharwal T, Carrell T, Dourado R, Adam A. Peripheral endografts for the treatment of traumatic arterial injuries. Emerg Radiol. 2009;16:175–184. doi: 10.1007/s10140-008-0771-9. [DOI] [PubMed] [Google Scholar]
- 46.Shin JS, Shin JH, Ko HK, Kim JW, Yoon HK. Transcatheter arterial embolization for traumatic mesenteric bleeding: a 15-year, single-center experience. Diagn Interv Radiol. 2016;22:385–389. doi: 10.5152/dir.2016.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steenburg SD, Ravenel JG, Ikonomidis JS, Schönholz C, Reeves S. Acute traumatic aortic injury: imaging evaluation and management. Radiology. 2008;248:748–762. doi: 10.1148/radiol.2483071416. [DOI] [PubMed] [Google Scholar]
- 48.Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, Holcomb JB, Scalea TM, Rasmussen TE. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75:506–511. doi: 10.1097/TA.0b013e31829e5416. [DOI] [PubMed] [Google Scholar]
- 49.Radvany MG, Gailloud P. Endovascular management of neurovascular arterial injuries in the face and neck. Semin Intervent Radiol. 2010;27:44–54. doi: 10.1055/s-0030-1247888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto J, Lohman BD, Morimoto K, Ichinose Y, Hattori T, Taira Y. Damage control interventional radiology (DCIR) in prompt and rapid endovascular strategies in trauma occasions (PRESTO): A new paradigm. Diagn Interv Imaging. 2015;96:687–691. doi: 10.1016/j.diii.2015.06.001. [DOI] [PubMed] [Google Scholar]


