Abstract
Objectives
This study aimed to explore the role of miR-320a in the pathogenesis of osteoarthritis (OA).
Methods
Human cartilage cells (C28/I2) were transfected with miR-320a or antisense oligonucleotides (ASO)-miR-320a, and treated with IL-1β. Subsequently the expression of collagen type II alpha 1 (Col2α1) and aggrecan (ACAN), and the concentrations of sulfated glycosaminoglycans (sGAG) and matrix metallopeptidase 13 (MMP-13), were assessed. Luciferase reporter assay, qRT-PCR, and Western blot were performed to explore whether pre-B-cell leukemia Homeobox 3 (PBX3) was a target of miR-320a. Furthermore, cells were co-transfected with miR-320a and PBX3 expressing vector, or cells were transfected with miR-320a and treated with a nuclear factor kappa B (NF-κB) antagonist MG132. The changes in Col2α1 and ACAN expression, and in sGAG and MMP-13 concentrations, were measured again. Statistical comparisons were made between two groups by using the two-tailed paired t-test.
Results
Expression of miR-320a was elevated in OA cartilage tissues and chondrocytes, and in IL-1β-stimulated C28/I2 cells (p < 0.05 or p < 0.01). MiR-320a overexpression enhanced IL-1β-induced down-regulation of Col2α1 and ACAN and sGAG, and increased the IL-1β-induced overexpression of MMP-13 (p < 0.01). PBX3 was a direct target of miR-320a. PBX3 and MG132 co-transfection attenuated the effects of miR-320a on the expression of Col2α1, ACAN, sGAG and MMP-13(p < 0.01).
Conclusion
Overexpression of miR-320a might enhance IL-1β-induced cartilage degradation factors. These effects might be via targeting PBX3 and regulating NF-κB.
Cite this article: Y. Jin, X. Chen, Z. Y. Gao, K. Liu, Y. Hou, J. Zheng. The role of miR-320a and IL-1β in human chondrocyte degradation. Bone Joint Res 2017;6:–203. DOI: 10.1302/2046-3758.64.BJR-2016-0224.R1.
Keywords: Osteoarthritis (OA), microRNA-320a, PBX3, Extracellular matrix, NF-κB
Article focus
This study assessed the influence of miR-320a on human chondrocytes.
Key messages
miR-320a is elevated in arthritic cartilage tissues and chondrocytes
miR-320a overexpression enhances IL-1β-induced matrix degradation by targeting PBX3
miR-320a overexpression enhances matrix degradation by regulating NF-κB.
Strengths and limitations
Strengths: These findings indicate that miR-320 may be a marker of OA.
Limitations: More work is needed to confirm the role of miR-320a in the pathogenesis of OA.
Introduction
Osteoarthritis (OA) is a type of joint disease, characterised by articular cartilage degeneration, subchondral bone abnormalities, and non-specific synovial inflammation.1,2 The most common symptoms of OA are pain, joint stiffness, crepitation on motion and limitation of joint motion, and OA leads to a significantly reduced quality of life.3 Main risk factors of OA include age, mechanical injury, obesity, joint infection and osteochondrosis.4 In the last several decades, multiple studies have devoted efforts to the treatment methods exploration for OA. However, its treatment is still relatively limited.5 Thus, it is necessary to determine possible new therapeutic targets to improve the outcomes of this disease.
microRNAs (miRNAs) are small non-coding RNAs consisting of 19~22 nucleotide base pairs.6 miRNAs regulate the expression of target genes by base pairing to complementary sites in the 3’-untranslated region (3’-UTR) of target mRNAs, and lead to translational repression or the degradation of the mRNAs.7 A large number of previous studies have reported that various miRNAs are implicated in the pathogenesis of OA by mediation of the expressions of their targets. Iliopoulos et al monitored the expressions of 365 miRNAs in articular cartilage obtained from OA patients, and found that 16 miRNAs were differentially expressed during OA.8 Furthermore, miR-148a has been found to act as a potentially disease-modifying compound in OA, as miR-148a overexpression promotes hyaline cartilage production.9 miR-33a has been identified as a target for ameliorating the OA phenotype by regulating cholesterol synthesis and cholesterol efflux-related genes.10
miR-320a belongs to the miR-320 family and plays a pivotal role in many diseases, such as Waldenstrom macroglobulinaemia (WM), cerebral ischaemia and various cancers.11-14 However, the role of miR-320a in the pathogenesis of OA has not yet been revealed. In this study, the expression of miR-320a and its target gene was investigated in OA chondrocytes. Furthermore, the effects of dysregulation of miR-320a and its target gene on interleukin 1 beta (IL-1β)-induced matrix degradation were determined in vitro. This study might provide us with a basic understanding of miR-320a on OA.
Materials and Methods
Specimen selection and chondrocyte isolation
OA articular cartilage samples were harvested from 15 OA patients, aged 56 to 74 years, who underwent joint replacement or joint surgery. Normal articular cartilage was collected from 15 individuals, aged 33 to 55 years, who underwent amputation resulting from trauma, with no history of any form of secondary OA or inflammatory joint diseases. OA patients were diagnosed according to the American College of Rheumatology (ACR) criteria.15 This study was approved by our local ethics committee, and written informed consent was obtained from all subjects for the use of their articular cartilage samples for research.
Cartilage specimens were cut into 1 mm to 2 mm3 pieces. For detection of miR-320a level, cartilage pieces were milled in liquid nitrogen, and total RNA were extracted using the Rneasy Fibrous Tissue Mini Kit (Qiagen, Valencia, California) according to the manufacturer’s instructions. For chondrocyte culture, cartilage pieces were predigested with 1 mg/mL trypsin (Sigma-Aldrich, St Louis, Missouri) for 30 minutes and followed by digestion with 1 mg/mL collagenase (Sigma) for 16 hours at 37°C with gentle shaking. Afterwards, the suspension was filtered through a 100 mm nylon cell strainer (BD Falcon, BD Biosciences, San Jose, California) and cells were collected by centrifugation.
Cell culture
Chondrocytes were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/F-12 medium (Gibco, Invitrogen Life Technologies, Carlsbad, California) supplemented with 10% foetal bovine serum (FBS; Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco).15 The immortalised human juvenile chondrocyte cell line C28/I2 was obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. C28/I2 cells were grown in DMEM (Gibco) supplemented with 10% FBS (Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco).16 All cells were incubated in a humidified incubator with 5% CO2 at 37°C.
Cell transfection and treatment. Cells were plated onto 60 mm dishes and after cells were grown to 80% confluence, miR-320a, antisense oligonucleotides (ASO) of miR-320a or their negative controls (Shanghai Genechem Co., Shanghai, China) were transfected into the cells.The transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. After 48 hours of transfection, cells were serum starved for 12 hours and then treated with 5 ng/mL of IL-1β (PeproTech, Rocky Hill, New Jersey) or 5 μM MG132 (Sigma-Aldrich), an antagonist of nuclear factor kappa B (NF-κB) for 24 hours. Cells were then collected for the forthcoming analyses.
Luciferase reporter assays. The 3’-UTR of Pre-B-Cell Leukemia Homeobox 3 (PBX3) was amplified by PCR and placed in the pMIR-Report vector (Ambion, Austin, Texas). These vectors were co-transfected with miR-320a, ASO-miR-320a or their negative controls into cells using Lipofectamine 2000. After 48 hours of transfection, luciferase assays were carried out using the Dual-Luciferase Reporter Assay System (Promega, Madison, Wisconsin).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA of chondrocytes were isolated by using TRIzol reagent (Invitrogen), and total RNA of cartilage tissue were extracted as described above. Synthetic of cDNA was performed using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). RT-PCR was performed in the ABI PRISM 7500 Real-time PCR System (Applied Biosystems, Foster City, California) and by using FastStart Universal SYBR Green Master (ROX) (Roche), according to the manufacturer’s instructions. Data were normalised to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or U6 snRNA expression, and were analysed using the 2-∆∆Ct method.
Western blotting
Cellular protein was extracted with a lysis buffer (Beyotime, Shanghai, China), and equal amounts of protein samples were mixed with a sample buffer (Beyotime) and boiled for five minutes. Protein samples were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bed-ford, Massachusetts). After blocking within 5% skim milk for one hour, the membranes were incubated with primary antibodies for PBX3 or GAPDH (dilution: 1:1,000; Santa Cruz Biotechnology, Santa Cruz, California) overnight at 4°C. Afterwards, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for one hour at room temperature. The blots were visualised by Super Signal Femto (Pierce, Rockford, Illinois) according to the manufacturer’s instructions.
Sulfated glycosaminoglycans (sGAG) analysis
In order to quantitatively assess sGAG content released due to breakdown of the cartilage extracellular matrix. C28/I2 cells were digested with 300 μg/mL papain solution (Sigma) at 65°C for four hours. Afterwards, the lysates were removed by centrifugation and the sGAG content was measured by using the Blyscan sGAG Assay Kit (Biocolor, Newton Abbey, UK) according to the manufacturer’s instructions. Chondroitin 4-sulfate was used as a standard.17
Enzyme-linked immunosorbent assay (ELISA). The level of matrix metallopeptidase 13 (MMP-13) secreted by C28/I2 cells in the culture supernatants was quantified using an MMP-13 ELISA kit (CUSABIO, Wuhan, China) according to the manufacturer’s instructions. The MMP-13 concentration was normalized to the concentrations of the standard controls provided by the manufacturer.
Statistical analysis
All data are expressed as means and standard deviations (sd) from at least three independent analyses. Statistical comparisons were made between two groups by using the two-tailed paired t-test and GraphPad Prism 5 software (GraphPad, San Diego, California). A value of p < 0.05 was considered significant.
Results
miR-320a was elevated during OA
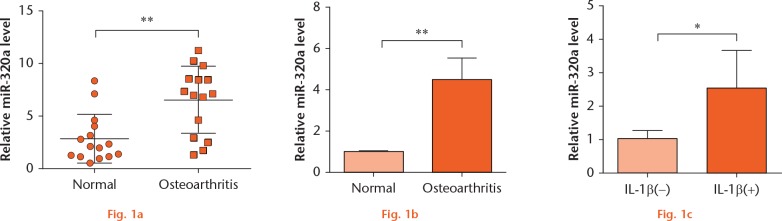
To explore the role of miR-320a in the pathogenesis of OA, the expression of miR-320a in OA cartilage tissues and OA chondrocytes, as well as in IL-1β-stimulated C28/I2 cells, was assessed by RT-PCR. As results show in Figures 1a to 1c, the expression of miR-320a was significantly elevated in OA chondrocytes, and in IL-1β-stimulated C28/I2 cells, when compared with that in normal chondrocytes and C28/I2 cells without IL-1β stimulation, respectively (p < 0.05 or p < 0.01). These results indicated that miR-320a might play a pivotal role in the pathogenesis of OA, or that miR-320a might serve as a biomarker during OA.
miR-320a was elevated in OA. (a) Expression of miR-320a in normal (n = 15) and OA cartilage cells (n = 15) was measured by RT-PCR. (b) The level of miR-320a in normal and OA chondrocytes was measured by RT-PCR. (c) C28/I2 cells were stimulated with IL-1β for 24 hours, and then the level of miR-320a was determined by RT-PCR. *, p < 0.05; **, p < 0.01. Statistical analysis was performed using two-tailed paired t-test.
miR-320a overexpression enhanced IL-1β-induced matrix degradation factors
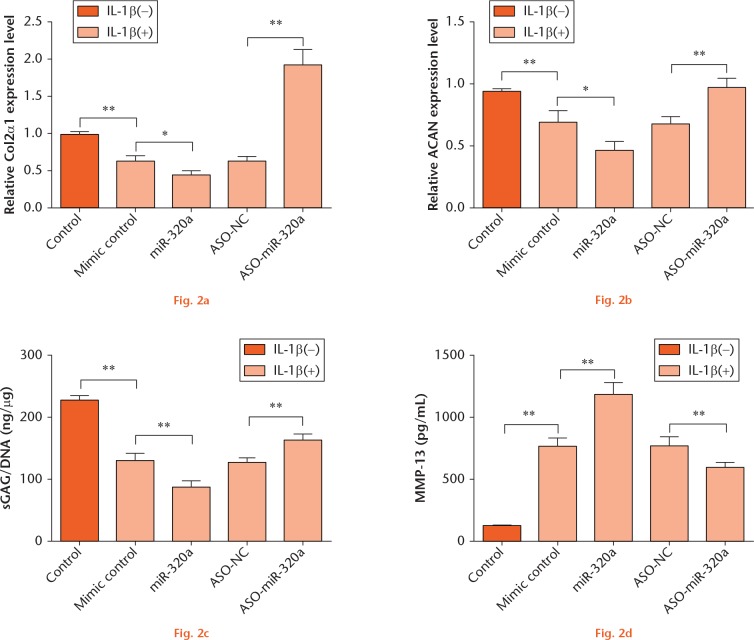
To explore the effects of miR-320a on IL-1β-induced matrix degradation factors, C28/I2 cells were transfected either with miR-320a or ASO-miR-320a and then stimulated with IL-1β. RT-PCR results showed that IL-1β significantly downregulated the expression of Col2α1 and ACAN (p < 0.01) (Figs 2a and 2b). miR-320a overexpression significantly enhanced the downregulative effects of IL-1β on Col2α1 and ACAN expression (p < 0.05), while miR-320a suppression displayed the opposite results (p < 0.01). Moreover, sGAG content and MMP-13 expression were determined. The results in Figures 2c and 2d showed that IL-1β led to lower concentrations of sGAG (p < 0.01) and higher concentrations of MMP-13 (p < 0.01) when compared with the control group. miR-320a overexpression enhanced the concentration of sGAG and the increase of MMP-13 induced by IL-1β (p < 0.01). As expected, miR-320a suppression displayed contrary results (p < 0.01). Thus, we speculated that miR-320a overexpression might aggravate IL-1β-induced cartilage matrix degradation through controlling the levels of Col2α1 and ACAN and the concentrations of sGAG and MMP-13.
miR-320a overexpression enhanced IL-1β-induced matrix degradation factors. C28/I2 cells were transfected either with miR-320a or ASO-miR-320a and then stimulated with IL-1β. mRNA level expressions of (a) Col2α1 and (b) ACAN were determined by RT-PCR. The concentrations of (c) sGAG and (d) MMP-13 were measured using the Blyscan sGAG Assay Kit and MMP-13 ELISA kit. *, p < 0.05; **, p < 0.01. Statistical analysis was performed using two-tailed paired t-test.
PBX3 was a directed target of miR-320a
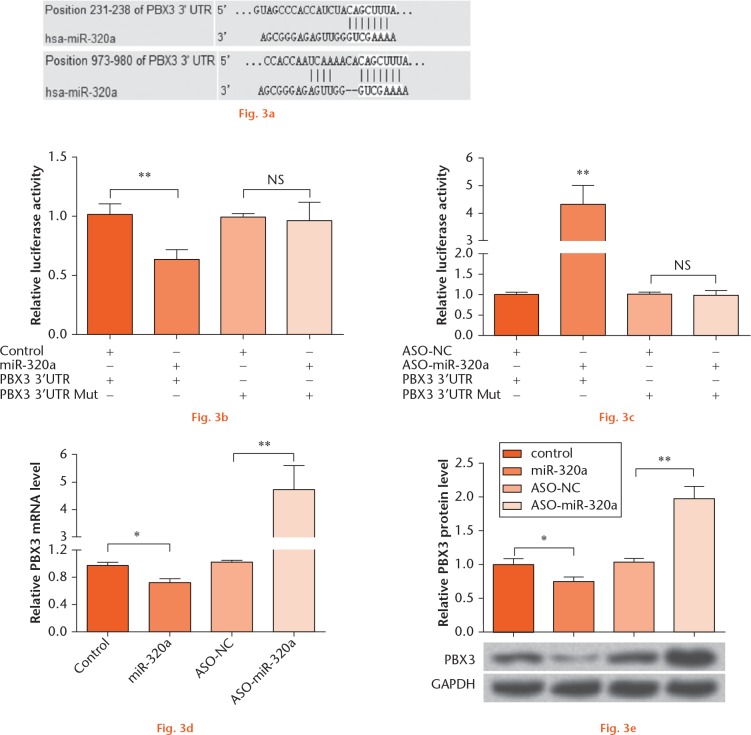
In order to elucidate how miR-320a impacted IL-1β-induced cartilage matrix degradation, the microRNA.org database was used18 to predict the target genes of miR-320a. As the analysis results showed in Figure 3a, PBX3 is a candidate target gene of miR-320a. To confirm this prediction and explore whether PBX3 was a directed target of miR-320a, a double-luciferase assay was performed (Figs 3b and 3c). We found that the PBX3 3’-UTR luciferase expression was significantly decreased by miR-320a overexpression (p < 0.01), while it was increased by miR-320a suppression (p < 0.01). Furthermore, in order to investigate how miR-320a targeted PBX3, cells were transfected either with miR-320a or ASO-miR-320a, and then the mRNA and protein levels of PBX3 were monitored (Figs 3d and 3e). We found that both the mRNA and protein levels of PBX3 were downregulated by miR-320a overexpression (p < 0.05), but upregulated by miR-320a suppression (p < 0.01). Taken together, PBX3 might be a direct target of miR-320a, and was negatively regulated by miR-320a.
PBX3 was a directed target of miR-320a. (a) microRNA.org database was used to predict the target genes of miR-320a. (b) miR-320a or (c) ASO-miR-320a was co-transfected with the pMIR-Report vector containing full length PBX3 3’-UTR or a mutated seed site within pMIR-PBX3 into C28/I2 cells. Subsequently, a dual-luciferase reporter assay was performed. miR-320a or ASO-miR-320a was transfected into C28/I2 cells, and the (d) mRNA and (e) protein level expression of PBX3 were detected by RT-PCR and Western blot analysis, respectively. NS, no significance; *, p < 0.05; **, p < 0.01. Statistical analysis was performed using two-tailed paired t-test.
miR-320a overexpression enhanced IL-1β-induced matrix degradation by targeting PBX3
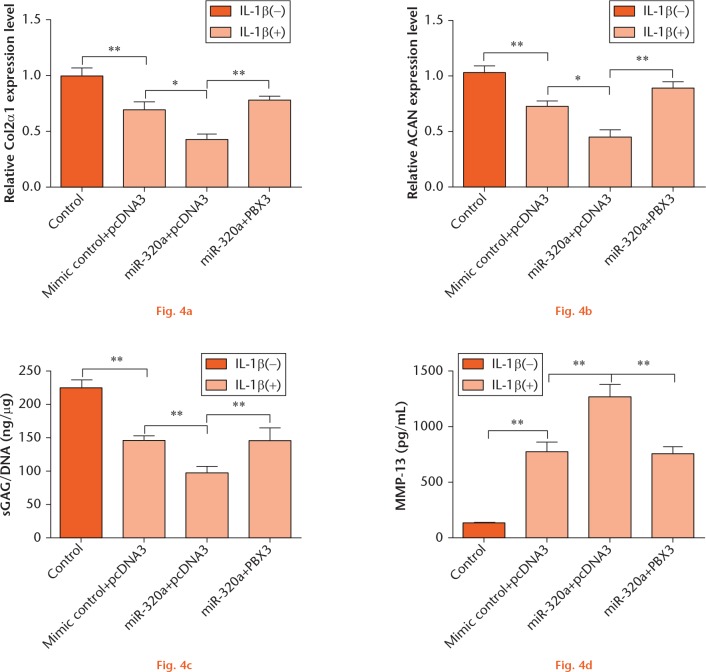
In order to further explore the correlation between miR-320a and PBX3 in IL-1β-induced matrix degradation factors, C28/I2 cells were transfected with miR-320a and/or PBX3 vector and then stimulated with IL-1β. The changes in Col2α1 and ACAN expressions, as well as in sGAG and MMP-13 production, were evaluated. We found that PBX3 significantly attenuated the regulatory effects of miR-320a overexpression on the expression of Col2α1 and ACAN and the production of sGAG and MMP-13 (p < 0.01) (Figs 4a to 4d). Therefore, we deduced that PBX3 might be involved in the regulation of miR-320a overexpression on IL-1β-induced matrix degradation factors.
miR-320a overexpression enhanced IL-1β-induced matrix degradation by targeting PBX3. C28/I2 cells were transfected with miR-320a and/or PBX3 vector and then stimulated with IL-1β. mRNA level expressions of (a) Col2α1 and (b) ACAN were determined by RT-PCR. The concentrations of (c) sGAG and (d) MMP-13 were measured by Blyscan sGAG Assay Kit and MMP-13 ELISA kit, respectively. *, p < 0.05; **, p < 0.01. Statistical analysis was performed using two-tailed paired t-test.
miR-320a overexpression enhanced IL-1β-induced matrix degradation by regulating nuclear factor kappa B (NF-κB)
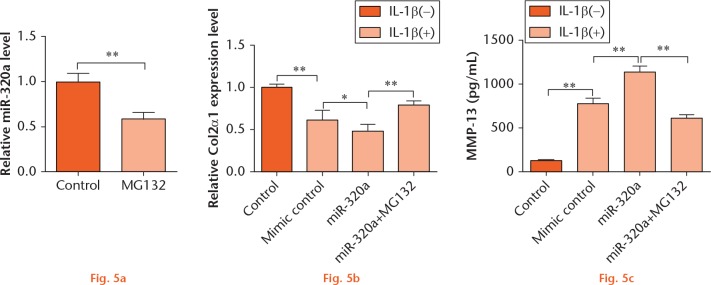
To explore the underlying mechanism of miR-320a on IL-1β-induced cartilage matrix degradation further, C28/I2 cells were treated with MG132, a NF-κB pathway inhibitor, and/or transfected with miR-320a, and then the expression level of Col2α1 and the concentration of MMP-13 were measured. As results showed in Figure 5a, the level of miR-320a was negatively regulated by MG132 (p < 0.01). In addition, MG132 significantly attenuated the regulatory effects of miR-320a on the expression of Col2α1 and the concentration of MMP-13 (Figs 5b and 5c; p < 0.01). These findings suggested that miR-320a overexpression impacted IL-1β-induced matrix degradation factors might be through NF-κB signaling pathway.
miR-320a overexpression enhanced IL-1β-induced matrix degradation by regulating NF-κB. (a) C28/I2 cells were treated with MG132 for 24 hours, and the expression of miR-320a was monitored by RT-PCR. C28/I2 cells were transfected with miR-320a and/or treated with MG132, and the expression of (b) Col2α1 and the concentration of MMP-13 (c) were measured by RT-PCR and MMP-13 ELISA kit, respectively. *, p < 0.05; **, p < 0.01. Statistical analysis was performed using two-tailed paired t-test.
Discussion
OA is a type of joint disease characterised by articular cartilage degeneration. Recent studies have reported that various miRNAs are implicated in the pathogenesis of OA. However, the role of miR-320a in OA has not yet been revealed. Here, we reported that miR-320a was upregulated during OA, implying that miR-320a might be a potential regulator in the pathogenesis of OA. PBX3 was a direct target of miR-320a, and was negatively regulated by miR-320a. Of note, PBX3 and NF-κB signaling pathway might be involved in miR-320a-mediated IL-1β-induced matrix degradation factors in OA chondrocytes.
Extracellular matrix degradation is a main characteristic of OA, and the major components of extracellular matrix are Col2 and proteoglycans.19 During OA, the synthesis of ACAN, Col2 and sGAG is reduced, while production of MMP-13 is enhanced.20 IL-1β is well known as a major catabolic inducer and contributes to the cartilage extracellular matrix degradation by inducing the production of MMP-13, and decreasing the synthesis of proteoglycan and collagens.15 To date, multiple miRNAs have been found to be implicated in the processes of IL-1β-triggered cartilage breakdown. Akhtar et al revealed that miR-27b overexpression inhibited the IL-1β-induced expression of MMP-13 protein in chondrocytes.21 Park et al found that miR-558 could influence cartilage homeostasis by targeting cytochrome c oxidase subunit 2 (COX-2) and regulating IL-1β-stimulated catabolic effects in human chondrocytes.22 In the current study, we provided the first insight into the effects of miR-320a on IL-1β-induced matrix degradation.
In order to further explore the possible mechanism by which miR-320a affected IL-1β-induced matrix degradation, the target of miR-320a was predicted by using the microRNA.org database and verified in vitro. We found that PBX3 was a direct target of miR-320a, and PBX3 was negatively regulated by miR-320a. In addition, PBX3 remarkably attenuated the enhancive effects of miR-320a on IL-1β-induced matrix degradation by controlling the production of Col2, proteoglycans, sGAG and MMP-13. PBX3 is one member of a group of PBX transcription factors belonging to the TALE (three amino acid loop extension) homeobox gene family.23 By using gene expression analysis, upregulation of PBX3 has been found in OA rats.24 However, the detailed functions of PBX3 on OA have not been fully identified. Furthermore, PBX3 has been identified as a direct target of many miRNAs, such as miR-181a/miR-181b, miR-200b, miR-222 and miR-424.25,26 Lu et al have identified PBX3 act as a direct downstream target of miR-320a, and reintroduction of PBX3 abrogated the effects of miR -320a on the cell growth and apoptosis of multiple myeloma cells.27 Our findings were partly consistent with the study of Lu et al, that overexpression of miR-320a promoted IL-1β-induced matrix degradation factors by targeting PBX3 directly.
NF-κB is a pivotal regulator in inflammation and the immune response, and the NF-κB pathway is abnormally activated in OA.28-30 Abnormal activation of NF-κB provokes the loss of the growth-arrested state of articular chondrocytes, and accompanied by the production of procatabolic mediators, including aggrecanases and MMPs that induce cartilage degradation.30,31 MG132 is an antagonist of NF-κB, and MG132 always used to block NF-κB activation by suppressing the phosphorylation or degradation of an inhibitory subunit of NF-κB (IκBα).32 In this study, MG132 was used to block NF-κB activation, and we found that the expression of miR-320a was downregulated by MG132, indicating that miR-320a expression was NF-κB-dependent. Moreover, MG132 recovered the effects of miR-320a on IL-1β-induced matrix degradation, suggesting that miR-320a might mediate NF-κB and contribute to the escalating severity of the OA.
In summary, this study uncovered the role of miR-320a in OA. miR-320a was elevated during OA, and overexpression of miR-320a promoted IL-1β-induced matrix degradation factors, at least in part by targeting PBX3 and regulating NF-κB. These findings underscored that miR-320a might be a marker for OA. Nevertheless, more work is still needed to confirm the role of miR-320a in the pathogenesis of OA.
Footnotes
Author Contribution: Y. Jin: Study design, Data collection and analysis, Manuscript preparation.
X. Chen: Study design, Data collection and analysis, Manuscript preparation.
Z. Y. Gao: Data collection and analysis, Manuscript preparation.
K. Liu: Data collection and analysis, Manuscript preparation.
Y. Hou: Data collection, Manuscript preparation.
J. Zheng: Study design, Manuscript preparation, Final approval of paper
ICMJE Conflicts of Interest: None declared.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1. Yimam M, Lee YC, Jiao P, et al. UP1306, a Botanical Composition with Analgesic and Anti-inflammatory Effect. Pharmacognosy Res 2016;8:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang PL, Liu J, Xu L, et al. Synovial Fluid Macrophage Migration Inhibitory Factor Levels Correlate with Severity of Self-Reported Pain in Knee Osteoarthritis Patients. Med Sci Monit 2016;22:2182-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panahi Y, Alishiri GH, Bayat N, et al. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: a randomized controlled trial. EXCLI J 2016;15:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmona JU, Ríos DL, López C, et al. In vitro effects of platelet-rich gel supernatants on histology and chondrocyte apoptosis scores, hyaluronan release and gene expression of equine cartilage explants challenged with lipopolysaccharide. BMC Vet Res 2016;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev 2006;58:226-242. [DOI] [PubMed] [Google Scholar]
- 6. Chan YC, Khanna S, Roy S, Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem 2011;286:2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10:704-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One 2008;3:e3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vonk LA, Kragten AH, Dhert WJ, et al. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthritis Cartilage 2014;22:145-153. [DOI] [PubMed] [Google Scholar]
- 10. Kostopoulou F, Malizos KN, Papathanasiou I, Tsezou A. MicroRNA-33a regulates cholesterol synthesis and cholesterol efflux-related genes in osteoarthritic chondrocytes. Arthritis Res Ther 2015;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubiczkova Besse L, Sedlarikova L, Kryukov F, et al. Combination of serum microRNA-320a and microRNA-320b as a marker for Waldenström macroglobulinemia. Am J Hematol 2015;90:E51-E52. [DOI] [PubMed] [Google Scholar]
- 12. Sepramaniam S, Armugam A, Lim KY, et al. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem 2010;285:29223-29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okato A, Goto Y, Kurozumi A, et al. Direct regulation of LAMP1 by tumor-suppressive microRNA-320a in prostate cancer. Int J Oncol 2016;49:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lü M, Ding K, Zhang G, et al. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ. Sci Rep 2015;5:8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang B, Kang X, Xing Y, et al. Effect of microRNA-145 on IL-1β-induced cartilage degradation in human chondrocytes. FEBS Lett 2014;588:2344-2352. [DOI] [PubMed] [Google Scholar]
- 16. Chang Z, Huo L, Wu Y, Zhang P. HIF-1 alpha had pivotal effects on downregulation of miR-210 decreasing viability and inducing apoptosis in hypoxic chondrocytes. Scientific World Journal 2014;2014:876363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seol D, Choe H, Ramakrishnan PS, et al. Organ culture stability of the intervertebral disc: rat versus rabbit. J Orthop Res 2013;31:838-846. [DOI] [PubMed] [Google Scholar]
- 18. Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res; 2008;36(Database issue):D149-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage 2015;23:1057-1070. [DOI] [PubMed] [Google Scholar]
- 20. Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 2001;3:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akhtar N, Rasheed Z, Ramamurthy S, et al. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum 2010;62:1361-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park SJ, Cheon EJ, Kim HA. MicroRNA-558 regulates the expression of cyclooxygenase-2 and IL-1beta-induced catabolic effects in human articular chondrocytes. Osteoarthritis Cartilage 2013;21:981-989. [DOI] [PubMed] [Google Scholar]
- 23. Ho CY, Bar E, Giannini C, et al. MicroRNA profiling in pediatric pilocytic astrocytoma reveals biologically relevant targets, including PBX3, NFIB, and METAP2. Neuro Oncol 2013;15:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rao ZT, Wang SQ, Wang JQ. Exploring the osteoarthritis-related genes by gene expression analysis. Eur Rev Med Pharmacol Sci 2014;18:3056-3062. [PubMed] [Google Scholar]
- 25. Li Z, Huang H, Li Y, et al. Up-regulation of a HOXA-PBX3 homeobox-gene signature following down-regulation of miR-181 is associated with adverse prognosis in patients with cytogenetically abnormal AML. Blood 2012;119:2314-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han H, Du Y, Zhao W, et al. PBX3 is targeted by multiple miRNAs and is essential for liver tumour-initiating cells. Nat Commun 2015;6:8271. [DOI] [PubMed] [Google Scholar]
- 27. Lu Y, Wu D, Wang J, et al. miR-320a regulates cell proliferation and apoptosis in multiple myeloma by targeting pre-B-cell leukemia transcription factor 3. Biochem Biophys Res Commun 2016;473:1315-1320. [DOI] [PubMed] [Google Scholar]
- 28. Liang ZJ, Zhuang H, Wang GX, et al. MiRNA-140 is a negative feedback regulator of MMP-13 in IL-1beta-stimulated human articular chondrocyte C28/I2 cells. Inflamm Res 2012;61:503-509. [DOI] [PubMed] [Google Scholar]
- 29. Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater 2011;21:202-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marcu KB, Otero M, Olivotto E, et al. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets 2010;11:599-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-kappaB/IKKbeta-dependent and kinase-independent effects of IKKalpha in cartilage degradation and chondrocyte differentiation. RMD Open 2015;1(Suppl 1):e000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ni T, Liu Y, Peng Y, et al. Substance P induces inflammatory responses involving NF-κB in genetically diabetic mice skin fibroblasts co-cultured with macrophages. Am J Transl Res 2016;8:2179-2188. [PMC free article] [PubMed] [Google Scholar]