Abstract
The ontogeny of glyoxysomes and leaf peroxisomes has been examined in the cotyledons of germinating watermelon (Citrullus vulgaris) seedlings. Organelles from the cotyledons were extracted by razor blade homogenization and microbodies were separated by sucrose density gradient fractionation. Both kinds of microbodies have the same mean equilibrium density on sucrose gradients.
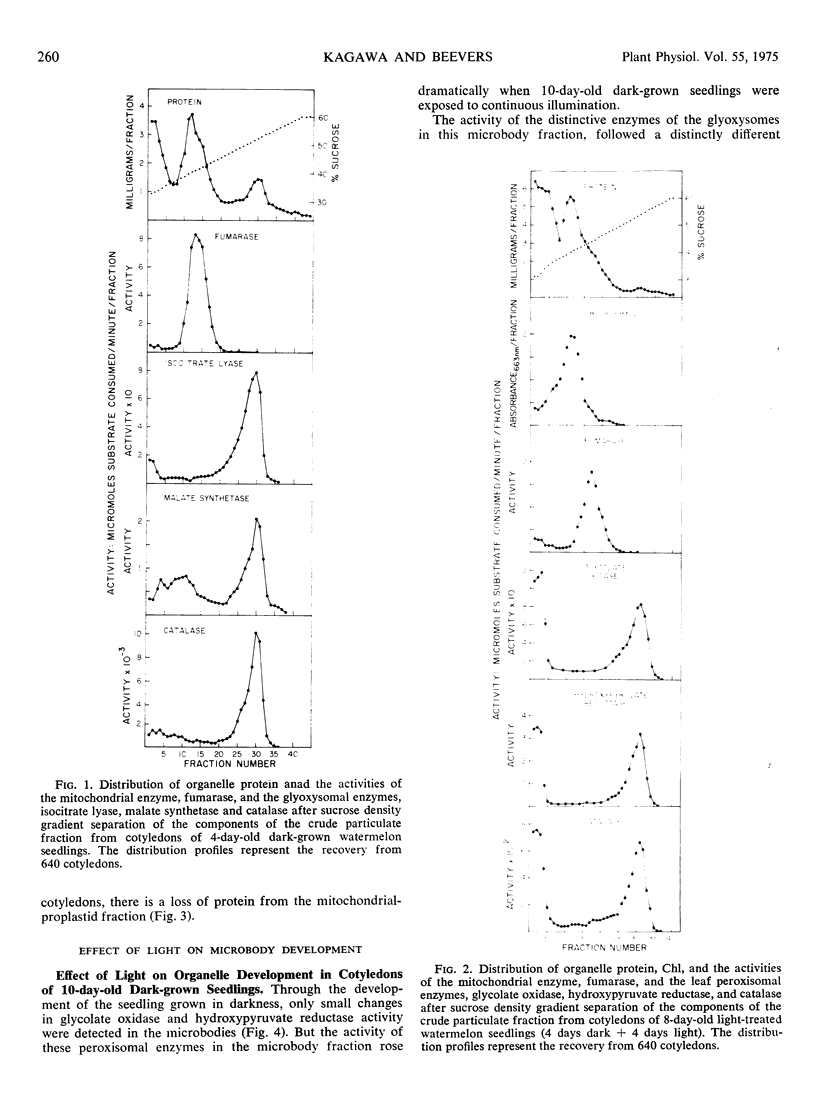
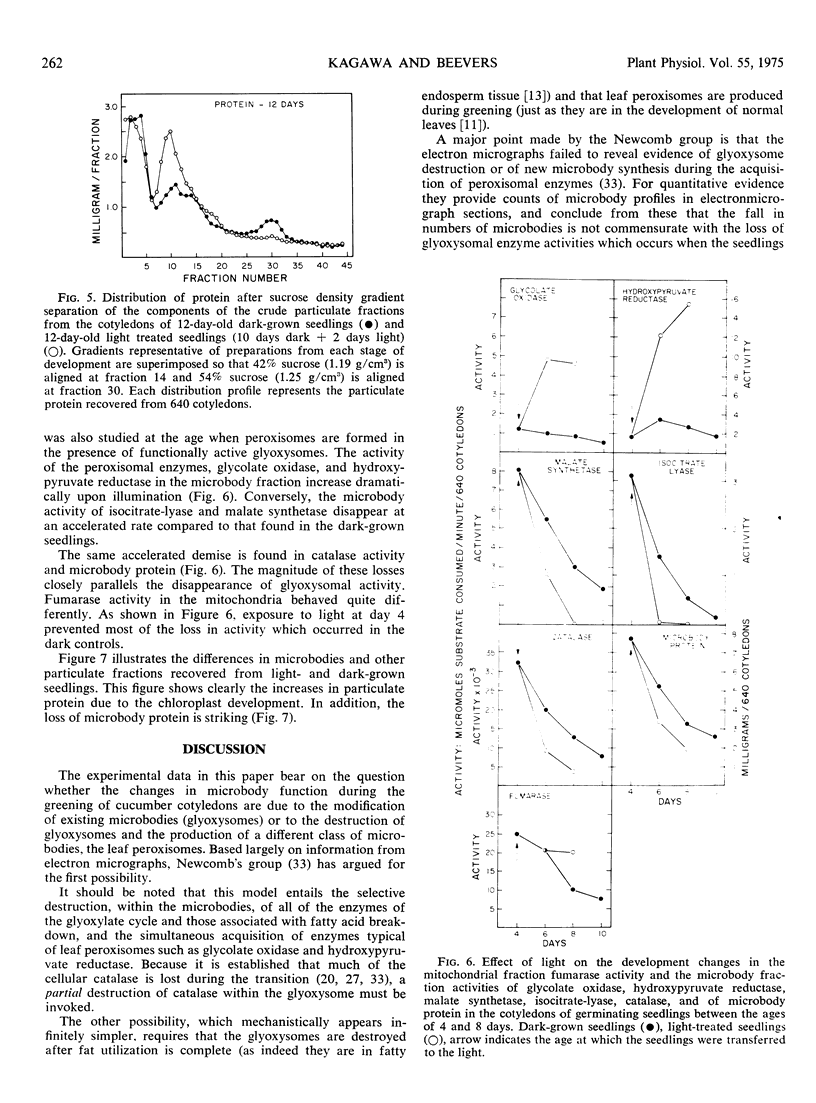
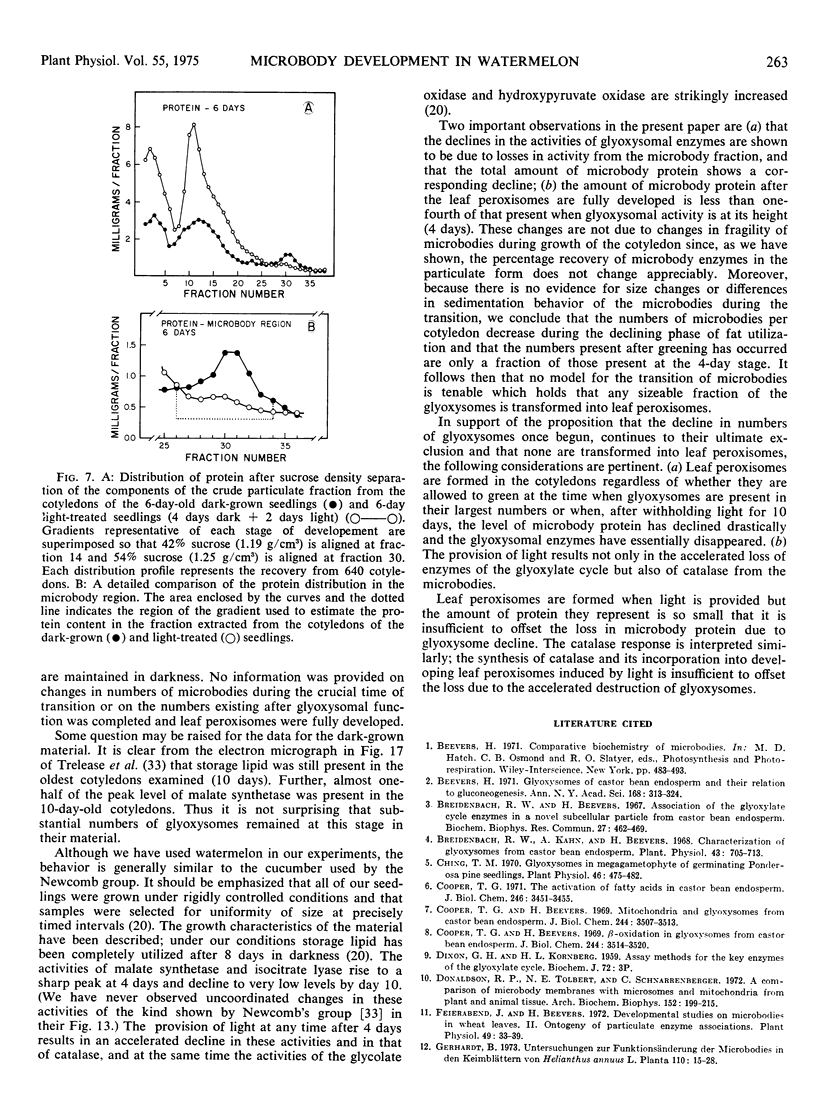
The development of leaf peroxisomes was examined in seedlings transferred to light at 4 days and 10 to 12 days. In seedlings maintained in darkness to the age of 10 to 12 days, glyoxysomal enzymes virtually disappeared, and the losses were paralleled by a corresponding loss in microbody protein. During this period peroxisomal activity was low and changed only slightly. On transfer to light at this stage, the activity of peroxisomal enzymes rose strikingly. The residual glyoxysomal activity disappeared completely, and the developmental pattern of microbody catalase and microbody protein paralleled the light-induced glyoxysomal disappearance.
Similar patterns of microbody development were observed when 4-day-old dark-grown seedlings with maximum glyoxysomal activities were exposed to light. The activity of the peroxisomal enzymes increased and the glyoxysomal enzymes disappeared at a faster rate than in darkness. These changes were again paralleled by the accelerated demise of microbody catalase and microbody protein. Thus under both conditions glyoxysomes were selectively destroyed during peroxisomal development, and the amount of peroxisomes produced was insufficient to offset the loss of glyoxysomal protein. The results do not support the contention that glyoxysomes are transformed to leaf peroxisomes in developing cucurbit cotyledons and favor the view that the two kinds of microbody arise independently of each other.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G. Activation of fatty acids in castor bean endosperm. J Biol Chem. 1971 Jun 10;246(11):3451–3455. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental Studies on Microbodies in Wheat Leaves : II. Ontogeny of Particulate Enzyme Associations. Plant Physiol. 1972 Jan;49(1):33–39. doi: 10.1104/pp.49.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. C., Taylor J. M. Effects of organic acids on ion uptake and retention in barley roots. Plant Physiol. 1970 Oct;46(4):538–542. doi: 10.1104/pp.46.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., McGregor D. I., Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973 Jan;51(1):66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Longo C. P., Longo G. P. The development of glyoxysomes in peanut cotyledons and maize scutella. Plant Physiol. 1970 Mar;45(3):249–254. doi: 10.1104/pp.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theimer R. R., Beevers H. Uricase and allantoinase in glyoxysomes. Plant Physiol. 1971 Feb;47(2):246–251. doi: 10.1104/pp.47.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]


