Abstract
Triple negative breast cancer (TNBC) is the most aggressive and lethal subtype of breast cancer. It is associated with a very poor prognosis and intrinsically resistant to several conventional and targeted chemotherapy agents and has a 5-year survival rate of less than 25%. Because the treatment options for TNBC are very limited and not efficient enough for achieving minimum desired goals, shifting toward a new generation of anti-cancer agents appears to be very critical. Among recent alternative approaches being proposed, small interfering RNA (siRNA) gene therapy can potently suppress Bcl-2 proto-oncogene and p-glycoprotein gene expression, the most important chemotherapy resistance inducers in TNBC. When resensitized, primarily ineffective chemotherapy drugs turn back into valuable sources for further intensive chemotherapy. Regrettably, siRNA’s poor stability, rapid clearance in the circulatory system, and poor cellular uptake mostly hampers the beneficial outcomes of siRNA therapy. Considering these drawbacks, dual siRNA/chemotherapy drug encapsulation in targeted delivery vehicles, especially mesoporous silica nanoparticles (MSNs) appears to be the most reasonable solution. The literature is full of reports of successful treatments of multi-drug-resistant cancer cells by administration of dual drug/siRNA-loaded MSNs. Here we tried to answer the question of whether application of a similar approach with identical delivery devices in TNBC is rational.
Keywords: mesoporous silica nanoparticles, triple-negative breast cancer, chemoresistance, siRNA, siRNA/chemotherapy drug co-delivery
Main Text
As the most frequently diagnosed cancer and second leading cause of death in women worldwide, breast cancer is considered the most troublesome cancer among women today.1 Currently, the development risk of breast cancer in American women is one in eight, and the metastatic form of breast cancer has a 5-year survival rate of less than 25%. Triple-negative breast cancer (TNBC), as a subtype of epithelial breast cancer tumors, is diagnosed by immunohistochemistry negative for estrogen receptor (ER) and progesterone receptor (PR) and lack of human epidermal growth factor receptor overexpression (HER2−); about 10%–14% of all diagnosed breast cancers are TNBC.2 TNBCs mostly belong to the histological ductal type together with a high lymphocytic infiltration and mitotic rate, high tumor grades, and large tumor sizes. At the time of diagnosis, patients often have lymph node involvement and present with visceral metastases.3, 4 Standard chemotherapy regimens form the mainstay of therapy for TNBC, and administration of several valuable targeted therapies fails to achieve beneficial outcomes.5 Furthermore, the highly resistant developing nature of TNBC makes sketching out a definite, highly curative regimen with currently existing conventional and targeted chemotherapeutic agents infeasible. In addition, a matching gene expression profile with histopathological findings demonstrates that TNBC is associated with a high intertumoral heterogeneity.6 Although the majority of TNBCs consist of basal-like breast carcinomas, other subtypes encompassing Claudin-low, mesenchymal stem-like, and luminal androgen receptor also exist. Each subtype highlights specific gene signatures and, consequently, responds differently to an identical chemotherapy regimen.7 In addition to intertumoral heterogeneity, intratumoral heterogeneity also exists, which refers to the differences among cells of a unit tumor, making chemotherapy outcomes more complex. During initiation of chemotherapy, only sensitive cells die, and persistent ones will continue to proliferate and create a new population of resistant cells, resulting in each chemotherapy session becoming less effective (G.V. Echevarria et al., 2016, AACR, abstract). Also, the reactive oxygen species (ROS)-rich highly hypoxic and inflammatory nature of the TNBC microenvironment can further amplify the resistance network at the tumor site.1 So far, six major mechanisms responsible for TNBC resistance development have been proposed, including overexpression of ATP-binding cassette (ABC) transporters; alteration of genes involved in apoptosis, β-tubulin III, ALDH1, and glutathione (GSH)/glutathione S-transferase overexpression; nuclear factor κB (NF-κB) pathway overexpression resulting in tumorigenesis, metastasis, and angiogenesis; and, finally, mutation of enzymes responsible for DNA repair.1, 8
As an alternative therapeutic strategy, non-coding RNA gene therapy can effectively modulate the expression pattern of almost any gene with a known mRNA sequence.9 As a non-coding RNA, small interfering RNAs (siRNAs) have demonstrated excellent therapeutic outcomes in breast cancer therapy in vitro.10 However, the drawbacks associated with this approach have mostly restricted expected therapeutic outcomes in the clinic. Recent progress in nanotechnology and application of nanocarriers for delivering siRNAs can mostly overcome the restrictions associated with systematic siRNA administration. Furthermore, nanocarriers can also efficaciously co-deliver small conventional chemotherapy drugs and siRNAs, improving treatment outcomes in resistant TNBC cells.11 Among the different nanocarriers, various physiochemical characteristics of mesoporous silica nanoparticles (MSNs) have made them a promising vehicle for efficient systematic co-delivery of siRNA/chemotherapy drugs.12 The literature is full of reports demonstrating successful treatment of multi-drug-resistant cells by administering a wide range of engineered MSNs with the capability of co-delivering drug and genes to the tumor site. These promising results encouraged us to further investigate whether application of the same carriers in the case of TNBC brought about similar advantages or not.
Mechanism of Action, Challenges, and Opportunities Associated with siRNA Therapy
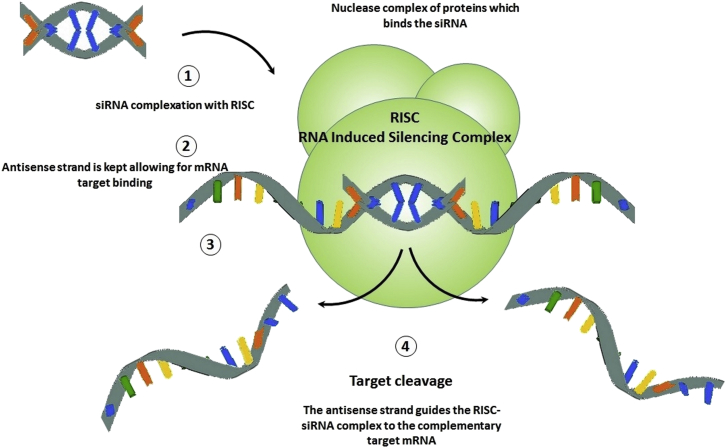
Comparing the efficacy of different chemotherapeutic agents demonstrates that regimens containing agents with inhibitory effects on oncogene expression, such as siRNAs, are more effective compared with ones targeting existing oncoproteins, like monoclonal antibodies, because inhibited oncoproteins can be easily replaced by newly expressed ones during the tumor progression phase.13 siRNAs, as one of the four subtypes of non-coding RNAs, can mediate silencing of target genes through disruption of mRNA activities both efficiently and in a sequence-specific manner through the RNAi process.14 Several intermittents, including dicer enzymes and RNA-induced silencing complexes (RISCs), have been identified to be involved in this process (Figure 1).15, 16 Gene silencing takes place in two main stages, after transcriptional silencing, also referred as posttranscriptional gene silencing (PTGS), and at the transcriptional stage (transcriptional gene silencing [TGS]).15, 17 PTGS take place through two main mechanisms: direct sequence-specific cleavage and translational repression or RNA degradation. If siRNAs and mRNAs possess completely complementary sequences, then sequence-specific cleavage takes place by RISC negotiation. However, limit complementarity to the cognate gene sequence results in RNA degradation through miRNA activity.18 Consequently, prior to administration, large-scale siRNA screening is usually performed to characterize both potential targets and the most effective siRNA sequence. siRNAs are usually 22 nucleotides long, with 3′ dinucleotide overhangs, mimicking dicer cleavage products for accelerating RISC binding. The RNAi pathway begins by unwinding and assembly of siRNAs into the RISC effector complex, which, in turn, results in RNA cleavage, initiation of chromatin modification, and repression of translation. Subsequently, the antisense strand couples with its complementary/target mRNA. Finally, by recognizing the perfect to near-perfect complementary sequence to the guide siRNA present in mRNA, the RISC complex begins mRNA cleavage at a site 10 nt further from the 5′ end of the guide strand, and, subsequently, mRNA degradation takes place through association of both endo- and exonucleases, resulting in corresponding gene silencing.15 Silenced genes can be ones involved in TNBC cell proliferation, apoptosis, survival, and metastasis. Consequently, one of the most important beneficial achievements of siRNA therapy is suppressing further proliferation and metastasis of TNBC cells.19 Several successful examples of breast cancer therapy with siRNAs are reviewed in Table 1.
Figure 1.
RNAi Induced by siRNA
At the beginning of RNA interference process, through the activity of specific nucleases known as Dicers, siRNAs are transferred to specific ribonucleoprotein complexes referred to as RISCs. Transfer of siRNAs results in activation of RISCs, which, in turn, by means of an RNA helicase enzyme, induces unrolling of the double-stranded fragments. The antisense strand, in the next step, guides the RISC-siRNA complex to the complementary target mRNA. Finally, as the complex binds with the complementary mRNA, endonucleases in the RISC complex initiate the degradation process which is then continued by endonucleases in the cytosol, resulting in complete degradation of mRNAs complementary to the siRNA.
Table 1.
A Number of Successful Examples of Breast Cancer Therapy with siRNAs
| siRNA Targeting | Identity of Protein | Function of Protein | Delivered by MSN | Reference |
|---|---|---|---|---|
| EphA2 | a transmembrane receptor tyrosine kinase | a decrease in EphA2 expression both in vitro and in vivo, correlated with reduced migration and experimental metastasis of breast cancer cells | – | 7 |
| AKT1/2 | serine/threonine kinase | cancer progression and metastasis | – | 133 |
| PKN3 | protein kinase N3 | knockdown of PKN3 protein not only blocks metastasis but also impairs primary breast tumor growth and angiogenesis | – | 7 |
| FAK | focal adhesion kinase | cell migration and metastasis | – | 134 |
| VEGF | receptor tyrosine kinase | stimulates endothelial cell proliferation and regulates vascular permeability | Yes | 79 |
| Bcl2 | Bcl2 family protein | predicts response to anthracycline combination | Yes | 135 |
| Mcl1 | Bcl2 family protein | autophagy inhibition | – | 136 |
| Survivin | inhibitor of apoptosis (IAP) family | inhibits caspase activation, leading to negative regulation of apoptosis or programmed cell death | Yes | 101 |
| P-gp | ATP-dependent drug efflux pump | decreases chemotherapy drug concentrations and chemoresistance development | Yes | 12 |
| c-myc | oncogene | resistance development | – | 7 |
| BRCA | tumor suppressor gene | DNA repair | – | 137, 138 |
| EGFR | receptor tyrosine kinase | cell proliferation and differentiation | – | 139 |
| c-KIT | receptor tyrosine kinase | cell survival, proliferation, and differentiation | – | 140 |
Despite numerous beneficial outcomes of cancer therapy with siRNA, several challenges remain to be answered before proceeding further to clinical trials. The short half-life of transient transfected siRNAs in various cell types and their poor stability in the biological milieu, aberrant induction of immune system responses, and exertion of off-target events mostly restrict the application of siRNAs as successful therapeutic agents in systematic cancer therapy.20, 21 Although chemical modifications of these agents’ backbone by glycation and nucleic acid locking have shown some promises in this regard, these modifications have their own difficulties, which have been thoroughly reviewed elsewhere.22, 23 Furthermore, even when at their site of action, siRNAs cannot diffuse through the cell membrane because of their hydrophilic nature and anionic charged backbone. Long double-stranded RNAs (dsRNAs) are also capable of inducing specific innate immune responses with subsequent activation of interferon secretion.24, 25 This mostly results from dsRNAs binding to dsRNA-activated protein kinase (PKR), Toll-like receptors (TLRs), and 2′, 5′-oligoadenylate synthase RNase l system retinoic acid-inducible gene 1 (RIG), all of which belong to receptors activating the first line of defense against viral pathogens.26, 27 Off-target effects are other restricting points observed with siRNA therapy. Despite their specificity, siRNAs also have the potency to regulate a vast variety of transcripts.28, 29 These effects mostly take place because of a crossmatch existence between the seed region of siRNAs (position 2-7) and the sequences at the 3′ UTR of the off-target gene. Because of the fact that backbone modification can minimize off-target effects, rational design of the backbone and modification of the structure are the most important interventions for improving off-target effects.30 Consequently, an appropriate delivery vehicle for siRNAs must primarily seal and protect them from degrading nucleases in the circulatory system and at the site of action and facilitate their transfection to the cell’s cytoplasm.
Opportunities Associated with the Application of Nanovehicles for In Vivo siRNA Delivery
As discussed above, the most fundamental challenge associated with gene-based therapies is the development of effective and concurrently non-toxic transporter vehicles. Viral and non-viral vehicles have both been applied for systemic delivery of siRNAs in the clinic.31, 32 However, several limitations have been found in association with the administration of viruses as vehicles for gene-based therapeutic agents, including the potency for carcinogenesis, limited DNA packaging capability, broad tropism, immunogenicity, and difficulties associated with the production of vectors, including polymeric and lipid-based vehicles.33, 34, 35, 36, 37 Most of these drawbacks can be overcome by utilizing non-viral vehicles, including polymers and lipid-based carriers; however, these are much less effective and efficient at transporting payloads across multiple biological barriers compared with viral vectors.38, 39 Fortunately, this trend is now changing because of the rapid progression in nanotechnology, enabling a better understanding of the nanomaterial capability for gene delivery. Application of nanotechnology for development of nano-delivery systems has also resulted in a significant decrease in the limitations associated with conventional anti-cancer chemotherapy agents, including low solubility and less toxicity to adjacent healthy tissue.40, 41 In the case of siRNA in vivo delivery, both the immunogenicity and nuclease susceptibility of siRNAs can be significantly decreased by encapsulation in nanovehicles. Furthermore, by encapsulation in nanovehicles larger than 20 nm, glomerular filtration of siRNAs can be mostly overcome.42 Based on the enhanced permeation and retention effect at the tumor site, circulating nanoparticles can be easily accumulated at the site of the tumor.40 Through proper ligation of functionalized nanocarriers with receptors on target cells, receptor-mediated endocytosis more effectively delivers nanocarriers to the cytoplasm.43 When endocytosed, materials accumulate in membrane-bound endocytic vesicles that, in the next step, merge with early endosomes and become more acidic as they pass forward to mature endosomes.44 Specific engineering of nanoparticles can make them sensitive to the low-pH environment of endosomes, destabilize the membrane, evade endosomes, and release their cargos in the cytoplasm.45, 46 Finally, in the cytoplasm, siRNAs negotiate with the RISC, resulting in therapeutic effects.47
MSNs as Promising Nanocarriers in Cancer Therapy
Utilizing nanotechnology for engineering target-specific colloidal drug delivery systems has potently succeeded in enhancing therapeutic outcomes and masking siRNAs’ instability during residence time in the circulatory system. Nanoparticles can easily cross biological membranes, including the cytoplasmic membranes of cells, because of their minute size, and, because of their high loading capacity, they release large amounts of loaded cargos inside cells. Furthermore, nanoparticles can be easily surface-functionalized or decorated with a variety of bioactive molecules, enhancing their uptake and accumulation in targeted cells and evading undesired uptake by the reticuloendothelial system. Among the different nanoparticles, recently, bioactive inorganic MSNs have attracted much attention because of their large surface area (>800 m2 g−1), large pore size and volume, definable morphology, and a surface that can be further modified by different cancer-specific moieties.48 MSNs have also been successfully administered as effective gene delivery devices in different cancers.49, 50 Additionally, MSNs can also become finely tailored through pore size modulation and surface functionalization for developing an even more effective controlled release gene delivery device.51 Because cellular uptake of MSNs is mostly depended on their outer surface properties, decoration with different functional groups and surface charge modification appear to be perfect strategies for further improving MSN internalization.52, 53
Mechanisms of MSNs Cellular Uptake
Presented at the site of action, MSNs can easily pass through the cell membrane and enter the cell’s cytoplasm. In general, two mechanisms have been proposed for internalization of external materials to cells; namely, phagocytosis and pinocytosis.50, 54 The pattern of cellular uptake mostly depends on the size of the external material to be engulfed. As small particles (<200–300 nm), MSNs, in most cases, are internalized by endocytosis.55 The main mechanisms involved in endocytosis are clathrin-dependent, caveolin- or clathrin-dependent receptor-mediated, or clathrin- and caveolin-independent endocytosis. These mechanisms consist of several steps, initiating with the cell surface, continuing with invagination and pinching off, and completing with tethering of newly formed vesicles.50, 54, 56 Applying poly-amidoamine (PAMAM) dendrimer-capped MSNs for intracellular drug/gene delivery, Radu et al.50 demonstrated that, in Chinese hamster ovary (CHO) cell lines, PAMAM-capped MSNs localized close to subcellular organelles, including mitochondria and the Golgi apparatus, proving their efficient internalization in the cell. Further studies applying different methods, including flow cytometry and confocal fluorescence microscopy, also confirmed significant fluorescein isothiocyanate (FITC)-labeled MSN uptake by cervical cancer cells.25, 57 MSN endocytosis also demonstrates a concentration-dependent sigmoidal pattern. Studies have also revealed that FITC MSN uptake occurs mostly through the clathrin-dependent endocytosis pathway. Furthermore, internalization of MSNs can also be mostly affected by surface functionalization. Non-functionalized MSNs are either endocytosed by clathrin-dependent endocytosis or micropinocytosis; however, functionalization of MSN mostly results in clathrin-dependent, receptor-mediated endocytosis. Nevertheless, amino- and guanidine-functionalized MSN endocytosis didn’t follow any specific internalization pattern.58
It is a well-established fact than nanoparticle (NP) surface charge and the receptors on cells can affect the internalization pattern of NPs. Synthesizing four different MSN materials with different surface charges controlled by amino or guanidine functionalities and comparing them with bare FITC-labeled MSNs, Chung et al.59 demonstrated an internalization trend based on the surface charge of MSNs. They reported that, as the negative charge of the MSN surface rises, the effective dose at 50% of internalization (ED50) is increased. Other studies have also reported a significant correlation between positive surface charge of MSNs and an increase in internalization to 3T3-L1 cells.60 However, the same trend was not observed with human mesenchymal stem cells (hMSCs), proposing that the surface charge effect can be cell type-dependent, too.61 Further studies also demonstrated that endocytosis may take place through an actin-mediated process.62 Based on the report of Slowing et al.,57 surface functionalization of MSNs with folic acid (FA) significantly increased their internalization through interaction with FA receptor pathways. Vivero-Escoto et al.58 also reported that the uptake of MSNs can be further affected by the shape of NPs.
The Intracellular Fate of Stimulus-Responsive Capped MSNs
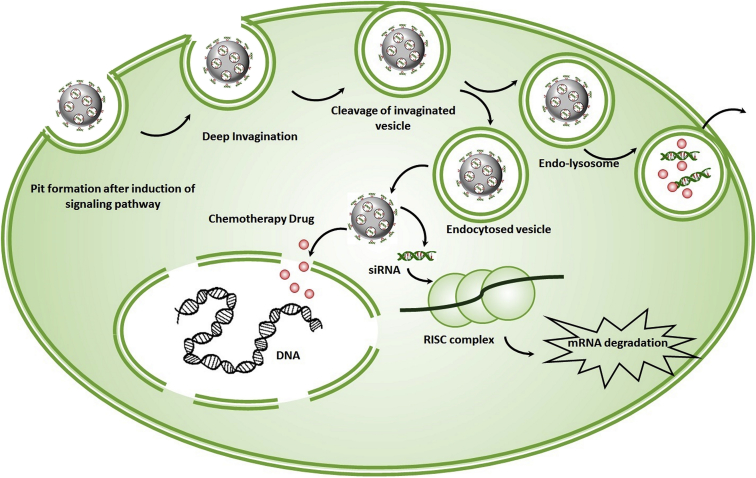
After internalization, MSN-NPs are first transported to primary endosomes, followed by further translocation to sorting endosomes (Figure 2). In the next step, some of the sorting endosomes turn their backs to the cell’s exterior by means of recycling endosomes and the remaining shift into secondary endosomes and become fused with lysosomes. Modified MSNs effectively evade endolysosomes and enter the cytosolic compartment. In the cytoplasm, MSN-loaded cargos are released and initiate the process, leading to beneficial outcomes.56 Based on Slowing et al.,57 negatively charged materials can effectively evade endosomes; however, positively charged particles mostly remain trapped inside endosomes. In addition, Huang et al.61 demonstrated that some of the MSN-internalized materials could be localized further to mitochondria; however, the mechanism remains only partly understood and requires further studies.
Figure 2.
The Fate of MSNs in Cells
After internalization, MSN-NPs are translocated to sorting endosomes. In next step, part of the sorting endosomes turn back to the cell’s exterior, and the remaining shift into secondary endosomes that are then fused with lysosomes. Modified MSNs effectively evade endolysosomes and enter the cytosolic compartment, where their cargos are released, and the RNAi process is begun.
Inside cells, it is necessary for nanoparticles to release their therapeutic cargos either in the cytoplasm or, more specifically, into subcellular organelles. Because the transcription machinery and hereditary material are present in the nucleus, the ultimate destination for various chemotherapy agents is the nucleus. Consequently, conventional therapies mostly focus on specific delivery of agents straight to the nucleus.12, 63, 64, 65, 66, 67 Nevertheless, because of the presence of the RISC in the cytoplasm, the ultimate site of action for siRNAs remains the cytoplasm. Furthermore, mitochondria, considered the energy-providing organelle in eukaryotic cells and the most important modulator of programed cell death and apoptosis, are located in the cytoplasm. Consequently, MSNs with dually loaded siRNA/chemotherapy drugs must release their cargos smartly to achieve an optimal therapeutic outcome.68 Based on this brief preface, agents capable of targeting both mitochondria and the nucleus surely will have significantly more effective role against cancer. For the first time, Luo et al.69 synthesized engineered MCM-41 MSNs that were loaded with the anti-tumor agent topotecan (TPT) and surface-decorated with a mitochondrially targeted therapeutic agent consisting of tri-phenyl phosphonium (TPP) and an antibiotic peptide (KLAKLAK)2, which were linked together with disulfide bonds further coated with a charge reversal polyanion, poly(ethylene glycol)-blocked 2,3-dimethyl maleic anhydride-modified poly (L-lysine) (PEG-PLL)DMA)), through electrostatic interactions. Present at the tumor site, based on an acidic microenvironment, the DMA block is cleaved and results in formation of a cationic NP and further accelerated internalization. Inside cells, because of the presence of GSH, disulfide bonds are cleaved, and pharmacologically effective agents, including TPT and therapeutic peptide (Tpep), are released from MSNs. Each therapeutic agent subsequently translocates to its site of action and demonstrates its therapeutic effects. Based on transmission electron microscopy (TEM) results, Luo et al.69 reported that the mitochondria were significantly damaged, and enhanced cellular uptake in cell lines was also observed.
In another study, Cheng et al.70 synthesized functionalized MSNs for specific delivery of doxorubicin (DOX) in a controlled release manner. To construct this robust drug delivery system, MSNs were encapsulated with DOX, and a tumor-targeting cellular membrane-penetrating peptide (TCPP) was grafted on the MSN and further capped with a mitochondrion-targeting therapeutic peptide (TPP) through disulfide bonds. Based on the results, in the absence or at low concentrations of GSH, only 10% of loaded DOX was released. However, at higher concentrations, 40% of loaded DOX was released in 1 hr, and over 75% of loaded DOX was released in 48 hr. This zero-order release can be explained by the fact that GSH concentrations are significantly high only in cancer cells, guaranteeing an excessive release of cargo in cancer cells. Cheng et al.70 further reported a 3-fold decrease in IC50 compared with free DOX administration.
Stimulus-Responsive Capped MSNs as Potent Non-viral Dual Gene and Drug Carriers
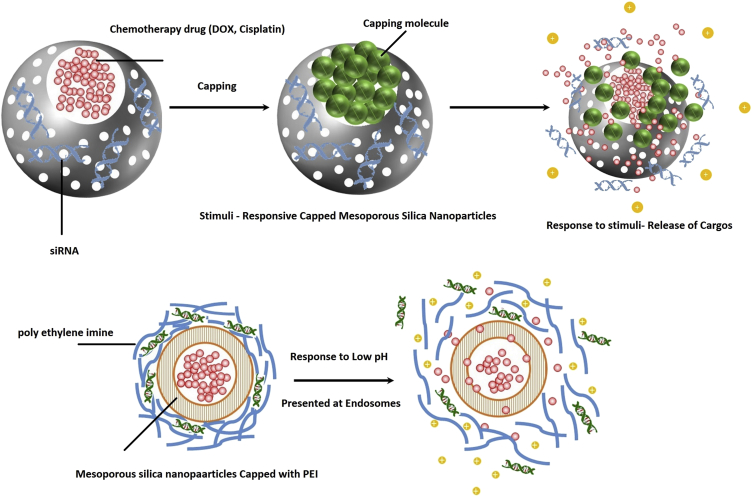
As mentioned earlier, to utilize the specific beneficial effects of siRNAs, the application of nanocarriers seems to be essential. Furthermore, even after internalization to the cells, siRNAs are still prone to degradation before incorporation with the RISC. In the case of MSNs, the narrow pore size distribution results in efficient intracellular protection.49 Furthermore, the ease of surface modification of MSNs can optimize the adsorption and release profile of siRNAs. Nevertheless, direct loading of siRNAs into the core of MSNs is difficult, and siRNAs are mainly adsorbed to the outer surface of nanoparticles that are modified cationically. Xia et al.71 coated MSNs with a layer of polyethylene imide (PEI), a well-known gene transfection agent. Based on their results, the ratio of 10–100 particles /nucleic acid is desired for achieving optimal adsorption and delivery to the site of action (Figure 3).71 Surface functionalization with organophosphate is another approach common in siRNA delivery; however, compared with PEI, lower siRNA adsorption has been reported.49 The benefits of these approaches are limited to in vitro studies. In the case of in vivo administration, because of the presence of nucleases in the plasma, large portions of adsorbed siRNA are rapidly degraded.72 To solve this problem, two methods have been proposed: optimizing the loading conditions and applying MSNs possessing larger diameters or pores. Li et al.73 demonstrated that short salmon DNA could be exclusively adsorbed into the mesopores of MSNs with 2.7-nm length under chaotropic salt conditions. The total particle diameter was reported to be approximately 70 nm. The maximum loading capacity was reported to be 121.6 mg DNA/g MSN, which was significantly more than the amount observed by adsorption under unmodified conditions.73 Application of chaotropic agents is critical because both DNA and MSNs are negatively charged. These conditions significantly decrease the electrostatic repulsions and allow a high loading of DNA to the MSNs. However, passing this threshold, DNA desorption began, and, at temperatures around physiological body temperature, maximum desorption was observed. Furthermore, it was observed that surface modification can effectively lower the speed of DNA release.74 As the length of siRNA increases, the maximum loading capacity significantly decreases, which can be explained by DNA stiffness under normal conditions. PEI adsorption on MSN takes place after drug internalization, and Li et al.75 have shown that, in addition to capping and controlled release of drugs, it can sufficiently protect drugs from degradation.
Figure 3.
Conventional Stimulus-Responsive Capped MSNs
Solberg and Landry76 demonstrated that amino functionalization results in significant enlargement of MSNs. The inner diameter has been shown to be around 20 nm, and the particles were reported to be around 70–300 nm. This can be explained by the fact that amino groups significantly interact with negatively charged DNAs, and the adsorbed plasmid could significantly protect against enzymatic degradation. Kim et al.77 reported that monodispersed MSNs approximately 250 nm in diameter possessing pores of 23 nm could efficiently deliver plasmids responsible for coding luciferase and GFP in vitro. These examples demonstrate the potency of MSNs for gene delivery to the site of action.
Examples of Successful In Vitro siRNA Delivery with Functionalized MSNs
Utilizing a 50-nm PEI-PEG-functionalized MSNs, Meng et al.12 reported a significant increase in cell toxicity by P-glycoprotein (P-gp) co-delivering siRNA/DOX compared with DOX alone in MCF7 multi-drug-resistant (MCF7/MDR) cell lines. In treated cells, immunoblotting demonstrated a more than 50% decrease in expression of P-gp, further confirming the role of P-gp knockdown of applied siRNA. Nevertheless, these amounts were negligible for scrambled siRNA. The maximum suppression in gene expression was observed within the first 48 hr and lasted for more than 96 hr.12 In another study, Meng et al.78 synthetized a group of PEI-modified MSNs and effectively co-delivered DOX and P-gp siRNA into the multi-drug-resistant human cervical carcinoma cell line KBV1 with significantly increased cytotoxicity compared with free DOX or MSN-engineered particles loaded with DOX, further confirming the synergistic effects of siRNA/chemotherapy co-delivery in resistant cancer cell lines.
The usefulness of large-pore MSN (LPMSN) application for in vitro siRNA delivery was clearly demonstrated by Na et al.79 First, they prepared amine-functionalized MSNs with small (2.1-nm) and large (23-nm) mesopores and then adsorbed siRNA molecules on them. Interestingly, after being exposed to RNase for 2 hr, the siRNAs that were adsorbed inside mesopores of LPMSNs remained intact. These siRNA-loaded LPMSNs, which were also conjugated with a PEG moiety and an imaging dye, significantly silenced GFP expression in HeLa cells. They also demonstrated that large PEG-carboxytetramethylrhodamine (TAMRA)-functionalized MSNs that were loaded with siRNAs specific for silencing vascular endothelial growth factor (VEGF) expression could effectively downregulate expression of VEGF and decrease tumor volume compared with injected PBS and naked siRNA targeting VEGF.79
In another study, Hartono et al.80 prepared cubic LPMSNs and further functionalized them with specific degradable poly(2-dimethylaminoethyl acrylate) (PDMAEA) cationic polymer. The specific characteristic of this nanocarrier was that it could gradually become degraded and release nucleic acid in KHOS cell lines.80 The same group also synthetized specific poly-L-lysine (PLL) functionalized LPMSNs loaded with siRNA specific for inhibiting expression of minibrain-related kinase and polo-like kinase 1 in osteosarcoma cell lines. The functionalized particles showed great potential for efficient gene delivery into cancer cells because a significant decrease in the cellular viability of the osteosarcoma cancer cells was observed.81
Examples of Successful In Vivo siRNA Delivery with Functionalized MSNs
Different studies have also verified MSN efficacy in successful delivery of siRNAs to the tumor site in vivo. In the study performed by Meng et al.,12 MCF7/MDR cells were grown as xenografts in nude mice to evaluate whether P-gp siRNA/DOX-loaded MSNs could knock down P-gp expression and suppress tumor growth in vivo. Tumor-bearing mice were subsequently injected with DOX/P-gp-loaded MSNs every 3–6 days up to 30 days with an injected dose of 4 mg/kg and 1.2 mg/kg for DOX and siRNA, respectively. Groups of tumor bearing mice received free DOX, and DOX MSNs were considered the control group. The first finding of the study was that 8% of administered PEI-PEG MSNs accumulated at the tumor site. Second, siRNA/DOX-loaded MSNs demonstrated a significantly higher tumor inhibition rate (80%) compared with DOX-MSNs, free DOX, and drug-loaded particles with scrambled siRNAs (62%, 17%, and 59%, respectively). Interestingly, no tumor-inhibitory activity was observed in mice receiving siRNA-loaded MSNs, further confirming the synergistic effects of siRNA and DOX co-administration.12
In another study, Lin et al.82 synthetized a positively charged, intracellularly cleavable poly(2-dimethylaminoethyl methacrylate)-functionalized MSN (ssCP-MSN) with 10-nm pore size and further analyzed the tumor growth suppression capacity of these particles in a HeLa-lue xenograft murine model. ssCP-MSN/siRNA complexes were tail vein-injected for studying the in vivo tumor growth-inhibitory effects of the particles. Injections were carried out every 3 days with an siRNA dose of approximately 2 mg/kg per injection. Based on the results, naked siRNA targeting PLK1 and saline did not demonstrate any tumor growth inhibition. However, siPLK1-loaded ssCP-MSNs demonstrated a significant tumor growth-inhibitory effect compared with naked siPLK1. This suggests that the concentrations required for silencing the PLK1 gene in tumor cells was achieved only when siRNAs were delivered with ssCP-MSNs.82
Finally, applying a pH-sensitive (PEI-PEG) functionalized MSN loaded with epirubicin (EPI) and BCL2-targeting siRNA, Hanafi-Bojd et al.83 demonstrated that MSNs with 9 mg/kg EPI and 1.2 mg/kg siRNA demonstrated a significantly improved in vivo cytotoxicity compared with EPI or siRNA alone.
The In Vivo Fate of MSN-Based Drug Delivery Systems
Although a fair understanding of the specific correlation between MSNs physiochemical characteristics, mechanisms of action, and rate of cell uptake has helped in the prediction of MSN-based drug delivery system fates in vitro, determination of the in vivo fate of MSN-based drug delivery systems, depending on the existing rules controlling their absorption, biodistribution, and elimination, is still matter of debate. One of the few comprehensive studies dealing with comparative biodistribution of I125-labled spherical and rod-shaped MSNs containing different surface chemistry and porosities was performed by Yu et al.84 Regardless of their shape, following intravenous (i.v.) administration in mice, MSNs significantly accumulated in the liver, spleen, and lung, respectively, and completely cleared within 24 hr post-injection. Meanwhile, the deposition of spherical MSNs surface-modified with amine groups was significantly reduced in the lung, and non-porous silica NP accumulation in the liver was negligible compared with porous NPs. Based on this result, they concluded that MSN surface chemistry and NP porosity are the most important factors determining the biodistribution fate of MSNs in the body. Huang et al.85 examined the effect of PEGylation and shape modification on the biodistribution of fluorescently labeled MSNs. Similarly, studying tissue sections with confocal microscopy demonstrated significant deposition of particles in the liver, spleen, and lungs 2 hr post-injection. Quantifying this amount by utilizing inductively coupled plasma atomic emission spectroscopy (ICP-OES) demonstrated that the deposited particles in these three organs constituted 80% of the total administered doses during the first 2 hr after injection. Interestingly, PEGylated MSNs accumulated more in the lung compared with control particles, which is significantly in contradiction with the study by He et al.,86 who reported that PEGylation decreases MSN lung accumulation. Based on the results from Yu et al.84 and He et al.,86 Huang et al.85 concluded that the answer to this controversy somewhat relies on the morphology of MSNs and that this characteristic is also important in determining the biodistribution of MSNs. Overall, based on these reports, just like other NPs, MSNs mostly accumulate in the liver, lung, and spleen, and, among the governing factors, surface properties are the predominant factors of MSN biodistribution. Many other factors, including surface charge and functionalization and the shape and size of MSNs, may also take part in determining the pharmacokinetic and in vivo fate of MSNs. Additionally, although PEGylation largely increases NP plasma half-life, this rule is not correct for MSNs, and these NPs demonstrate very low biodistribution.
In vivo excretion of MSNs is mostly dependent on silica degradation. Dissolved silica can be absorbed by the body or excreted in the urine in the form of silicic acid or oligomeric silica species.87 MSNs hydrolysis takes place under physiochemical conditions when concentrations are below silica saturation levels.88 Rapid distribution of MSNs suggests that MSNs are mostly dissolved under in vivo conditions.87 As indicated by faster excretion of silica in the urine after injection in mice, it has been demonstrated that large MSNs dissolve faster than smaller ones.86
Cheng et al.89 examined the effect of MSN surface charge on hepatobiliary excretion rate following i.v. administration. They demonstrated that accumulated positively charged MSNs in the liver are rapidly transported into the gastrointestinal tract and mostly excreted in the feces; however, negatively charged MSNs demonstrate high uptake and retention in the liver. According to Cheng et al.,89 positively charged nanoparticles mostly accumulate in hepatocytes because of coupling with apolipoprotein E and immunoglobulin A (IgA). However, negatively charged NPs are taken up by Kupffer cells.89 Cheng et al.90 concluded that because positively charged MSNs are taken up by hepatocytes, they are mostly eliminated via hepatobiliary excretion; however, negatively charged MSNs remain in Kupffer cells and result in hepatotoxicity because no unique elimination pathway has been identified for them. Transportation of positively charged MSNs through hepatobiliary transportation and excretion in the feces without any significant signal in the urine has also been confirmed by fluorescence imaging and ICP-mass spectrometry (MS) studies.90
To examine short and long rod-shaped MSN excretion profiles, Huang et al.85 collected urine and feces samples at different time intervals and determined the Si content via the ICP-OES method. Regardless of being long or short, for all MSNs, Si was detected in NPs 2 hr after injection. Nevertheless, the Si content of long rods was significantly lower compared with short rods. This result is consistent with the observation that MSNs are rapidly biodistributed in the kidneys during the first hr after injection. Nevertheless, after 7 days, all rod MSNs were excreted through the feces. Renal excretion of rod-shaped MSNs is an important finding because it is almost accepted that only particles with a size of under 5 nm can be eliminated by glomerular filtration.91 Consequently, further studies are required to confirm whether this observation results from particle elimination or from excretion of low-molecular-weight silica degradation fragments.85
siRNA and Chemotherapy Drugs: Co-delivery versus Separate Delivery, Simultaneous Release versus Sequential Release
Chemoresistance is a multi-faceted phenomenon generally consisting of two separate categories, first pharmacological resistance, mostly referring to the mechanisms causing insufficient drug delivery to the site of action, including inadequate infusion, tumor microenvironment restrictions, plasma pharmacokinetic, etc., and, second, cellular resistance, mostly referring to resistance in association with cells, which is further subdivided into efflux pump and non-efflux pump resistance. Cellular resistance mechanisms are more important compared with pharmacological ones and mostly consist of ABC transporter overexpression and alterations in pro/anti-apoptotic signaling pathways.1 The application of combination therapies in chemoresistant cells has emerged as a new promising approach because an effective concurrent administration of a gene and chemotherapy drug cocktail is often associated with synergistic effects. Furthermore, this approach can increase the compliance of patients with therapy because of the reduced required doses and decreased drug resistance development.12 As an example, administration of siRNAs specific for the MDR1 gene can effectively result in enhanced intracellular drug concentration.92 In other words, gene silencing results in the development of an “open window of time” through which resistant cells become sensitized to conventional chemotherapy drugs, and, in this period, the chemotherapy agent is much more effective.93 Contemporized pharmacokinetics associated with concomitant drug delivery of double agents to a single population of cells is the other advantage of combination therapies with siRNA and chemotherapy drugs.
Because of their unique scaffold, MSNs can be easily filled with chemotherapy drugs and siRNAs and effectively co-deliver cargos to tumor cells. Steinbacher and Landry94 reported that administration of MSNs loaded concurrently with DOX and siRNA targeting the Bcl-2 oncogene to overcome MDR resulted in a 132-fold increase in DOX cytotoxicity compared with the free form. In another study, application of nanoparticles co-delivering DOX or cisplatin and siRNA targeting either the MRP-1 transporter or Bcl-2 genes and decorated with a specific tumor moiety resulted in an 8-fold decrease in IC50 compared with free drug administered.12 In another attempt, Meng et al.78 conjugated DOX by a specific pH-sensitive linker, hydrazone, to MSNs (MSN-hydrazone-DOX) and further loaded them with siRNAs targeting P-gps, providing a controlled release pattern for DOX and bypassing efflux pump resistance. They observed that this nanovehicle could significantly bypass the efflux pump, effectively released P-gp-silencing siRNA, provided a sustained release profile of DOX, and significantly surmounted cellular resistance.78 In another study, as a benchmark, human uterine sarcoma MES-SA/DOX-resistant tumor (MES-SA/Dx-5) cell lines were utilized to compare the efficacy of DOX/verapamil co-administration and MSNs loaded only with DOX. Interestingly, DOX-loaded MSNs more effectively accumulated in resistant cells compared with the free DOX/verapamil cocktail.95
Although siRNA/chemotherapy drug co-delivery demonstrates significantly enhanced beneficial effects, amplified toxicities sometimes restrict the systematic administration of these agents. Furthermore, in the case of instability or chemical incompatibility, co-administration of drug and siRNA in a single vehicle is not feasible. As an example, Jiang et al.96 demonstrated that co-encapsulation of siRNAs targeting P-gp expression and DOX in liposomes results in the undesired aggregation of liposomes and induction of DOX leakage. In this case, administration of siRNA and DOX in separate vehicles could also effectively enhance the desired anti-cancer effects.97 However, whether the observed effectiveness was as much as the one in the case of co-delivery in single vehicle remains a matter of debate. Furthermore, it must also kept in mind that each vehicle possess unique characteristics that can further affect the results. In other words, administration of a similar drug with identical loaded concentrations in different vehicles may result in different results in similar cells.
From another aspect, it has been proposed that many combinational therapies are effective only when tumor cells are exposed to the therapeutic cargos in a sequential pattern. Several combination therapies with siRNAs and chemotherapy drugs initially induce alterations in specific signaling pathways and, subsequently, sensitize them to anti-neoplastic agents. Consequently, in these cases, sequential release of therapeutic cargos from nanocarriers seems more rational. Generally, it can be stated that, in the case of siRNA/anti-neoplastic agent co-treatment, sequential release demonstrates a more favorable response to therapy. This is mostly due to the lag time required for siRNAs to result in downregulation of the desired protein expression.93 However, designing a delivery system capable of separately controlling the release of individual agents is faced with several complexities and challenges. First of all, a comprehensive understanding of the correlation between the kinetics of release and correlated therapeutic activity in combination therapy is required. For instance, based on the study by Yadav et al.97 exploring the required lag time between siRNA and paclitaxel treatment, it was demonstrated that a 24-hr delay in the administration of paclitaxel after siRNA administration for silencing the P-gp pump was the optimum time for knockdown. However, based on Navarro et al.,98 increased DOX activity after P-gp siRNA administration occurred regardless of lag time. Consequently, a better understanding of the basic kinetics aspects of these therapeutic co-administrations is crucial.
Although combination therapy at first glance seems perfect, the biggest challenge associated with co-delivery of siRNAs and chemotherapy drugs appears to be the selection of an appropriate carrier.99 This is mostly due to the fact that siRNAs are usually negatively charged and possess higher molecular weights compared with the hydrophobic small chemotherapy drugs.100 Consequently, we are dealing with two agents that possess drastically different physiochemical properties. As a result, administration of two separate mechanisms for encapsulation of each therapeutic agent seems to be essential. Small conventional chemotherapeutic agents are usually enclosed in vehicles by means of hydrophobic forces, electrostatic interactions, or conjugation, whereas siRNAs will usually adsorb on carriers by means of electrostatic forces.101 Additionally, several questions need to be addressed before considering a vehicle as an acceptable dual siRNA/chemotherapy carrier in vivo. These include biocompatibility, high loading or encapsulation capacity for chemotherapy drugs, zero premature release, specific accumulation at the site of action, and proper release profile. Furthermore, when at the site of action, the delivery vehicle must be effectively taken up by cancer cells and, when inside the cell, effectively evade endosomal vesicles.
Overall, although sequential administration seems more rational, it must also be considered that decreasing drug administration times as much as possible increases patients’ compliance to therapy. Furthermore, based on the abovementioned advantages of drug co-administration, it seems more rational to focus on designing a delivery vehicle capable of releasing drugs in a sequential manner rather than administering drugs separately at specific intervals. This is, however, coupled with several difficulties, including capping vehicles with different particles as discussed in below or coating with multiple polymers. Perhaps the most feasible answer to this obstacle is co-administration of multiple vehicles, each containing a different desired chemotherapy agent, and further coating them with specific materials that respond to each other in a sequential pattern; for example, administering two MSNs, one loaded with a small conventional chemotherapy drug (e.g., cisplatin in the case of TNBC) further coated with a degradable polymer such as poly-lactide co-glycolic acid (PLGA) and the other loaded with the desired siRNA capped with a pH- or redox-responsive molecule (discussed below). By the time both of these nanocarriers reach the site of action, based on site-specific reactions, capped MSNs begin to release their cargo immediately; however, the ones coated with PLGA remain intact for a certain time. After the lag time, PLGA is degraded and releases cisplatin, consequently demonstrating a sequential release manner. The restrictions and challenges associated with this approach require further studies.
Premature Drug Release versus On-Demand Drug Release: Application of Tumor-Specific Capping Strategies
As nanoparticles circulate in the bloodstream to reach their site of action, because of the perfect sink conditions, a portion of loaded active cargos in nanoparticles undesirably release, which is referred to as “premature drug release,” a major challenge that should be avoided as much as possible.102 To fulfil this purpose in the case of MSNs, they are usually capped by materials sensitive to conditions only observed at the tumor site. In the specific microenvironment, capping molecules start to respond to the new conditions, and, based on the interaction, MSN-locked valves will open, and loaded cargos begin to release. In the case of tumor microenvironments and cancer cells, the most important cappings are the ones responding to changes in pH or redox potential. These types of responses are usually redox-responsive disulfide bonds cleaved under high glutathione concentrations and acid-responsive cleavage because of the low pH in endosomes (Figure 4). Other external stimuli, including light, temperature, and ultrasound, can also result in reconfiguration of capping molecules and in gate opening through obstacle removal. However, because redox and pH stimuli are more important, here we only focus on the characteristics of capping systems sensitive to these stimuli.103
Figure 4.
New-Generation MSNs
For the first time, Lin et al.104, 105, 106 synthetized a redox-responsive capping system for MSNs through coupling cadmium disulfide NPs with MSNs by means of a disulfide linker that was prone to cleave by various types of reducing agents, including DTT, mercaptoethanol (ME), and GSH. In the cytoplasm, because of the high concentrations of GSH, disulfide bonds are easily broken, and initiation of cargo release takes place in the cytoplasm. In similar attempts, MSNs have also been capped through application of Fe3O4 and Au nanoparticles.104, 105, 106
In another attempt, Li et al.107 synthetized two pH-responsive nanovalves constructed from a stalk being covalently bound to the MSM-41 pore entrance, and cyclodextrin was applied as a capping molecule to the organic part of the stalk through hydrophobic-hydrophobic interactions after entrapment of cargo inside pores. Because the pKa of p-anisidine nitrogen is around 6, at physiologic pH, the binding affinity of α-cyclodextrin (α-CD) and the hydrophobic stalk is very high. However, by the time it is present in endosomes, possessing a low pH, p-anisidine nitrogen becomes protonated, and the binding constant significantly decreases and results in dissociation of the α-CD cap from the stalk and initiation of cargo release. Second nanovalves consisted of a 1-methyl-1-H-benzimidazole moiety in the stalk. Again, present in low concentrations in endosomes, the benzimidazole binding affinity to β-cyclodextrin (β-CD) abruptly decreases and results in dissociation of CD. Consequently, both of these valves are fastened at physiological pH; however, they begin to release their cargo at the acidic microenvironment.107
A New Generation of Engineered MSNs for Packing siRNAs inside Mesopores
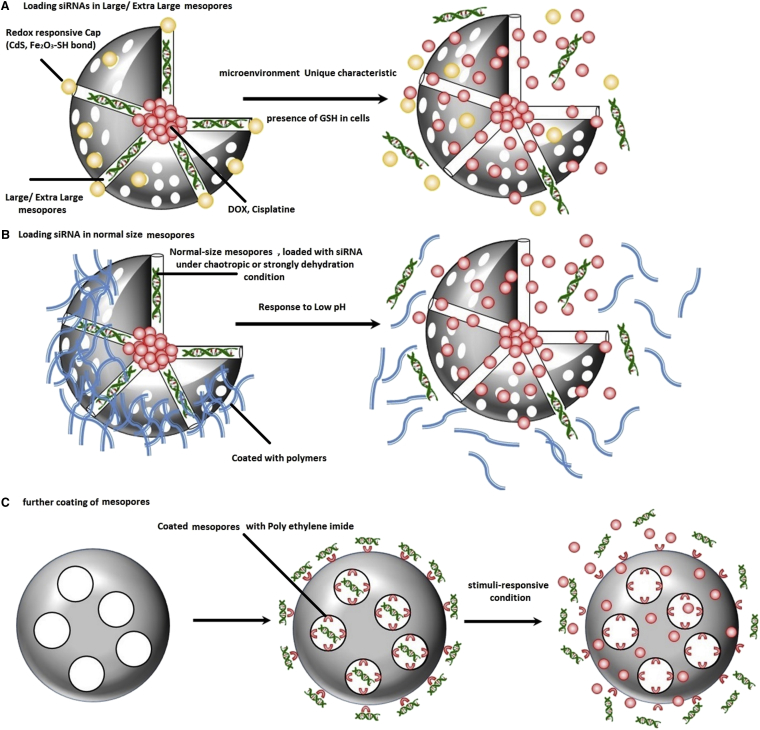
Despite the intensive efforts dedicated to overcoming siRNA delivery defects by developing potent MSN-based delivery systems, because siRNAs are mostly delivered by adsorption on the external surface of MSNs, they are still prone to nucleases present in the plasma and poorly protected from degradation. Consequently, the efficacy of these delivery approaches is not yet acceptable. Additionally, adsorbed siRNAs can significantly reduce the loading capacity of MSNs, and, more importantly, because of the interaction of amino groups present on the external surface of MSNs with siRNAs, further functionalization of MSNs with specific targeting molecules or PEGylation for extended in vivo application of these nanocarriers are mostly restricted.79, 81 As a result, researchers have shifted toward synthesis of more advanced MSNs with the purpose of overcoming these obstacles. One of the most important procedures includes packing of siRNAs within mesopores of MSNs. As a result, several attempts have been focused on synthesizing MSNs possessing larger pores (10–24 nm) and simultaneously modifying them with lots of amino groups to achieve maximum interaction and immobilization of the siRNAs in them. Further prolonging the in vivo circulation time of large mesopore MSNs loaded with siRNA/chemotherapy drug, the external surface of MSNs can be coated with polyethylene glycol (PEGylation) or lipid layers, turning them into excellent dual siRNA/chemotherapy drug carriers for effective cancer therapy.108, 109
Proceeding with this concept, Hartono et al.80 synthesized a large porous silica nanoparticle (LPSNP) and further functionalized it with a self-catalyzing degradable cationic polymer (PDMAEA), demonstrating an independent external degradation pattern. The most important characteristic of this cationic polymer is its high DNA binding capacity and protection capability until nanocarriers can reach the site of action and release loaded cargos in an “on-demand” pattern. They loaded these functionalized LPMSNs with chloroquine for further attribution to NP endosomal escape. They further demonstrated that PDMAEA-LPMSN released oligoDNA that mimicked siRNA within 2 hr, whereas, under similar conditions, PEI-functionalized LPMSNs only released a little of their loaded cargos.80 In another attempt, to improve nucleic acid delivery, including siRNAs, Liu et al.110 synthetized a magnetic silica nanosphere with large nanopores (MSLNPs) that were functionalized by immobilizing cationic PLL. Nuclear and F-actin staining demonstrated significantly high PLL-MSNLP internalization in cells that was reported to mostly result from MSN functionalization with PLL and the presence of large pores on the shell.110
With the purpose of protecting siRNAs from in vivo enzymatic degradation, Na et al.79 developed a specific type of MSNs possessing extra-large mesopores with a diameter of 23 nm by treating small porous MSNs (∼2 nm) with trimethylbenzene and loaded them further with siRNAs targeting VEGF. According to in vivo and in vitro studies, the relative VEGF mRNA levels, by application of these extra-large MSNs, was decreased more than 4-fold compared with naked siVEGF.79 Contrary to previous studies, Li et al.73 reported another method for adsorption of siRNAs in internal mesopores with normal size. By applying chaotropic conditions, they significantly shielded repulsive charges between siRNAs and the silica surface and reported adsorption of up to 27 μg/mg siRNA inside an unfunctionalized MSN with a 3.1-nm pore diameter.73 Finally, Li et al.111 demonstrated that siRNAs can be significantly incorporated into mesopores of magnetic MSNs under strongly dehydrated solution conditions. Afterward, these mesopores can be further capped by PEI to prevent further premature release of cargos.111 Other reported methods in the literature for packing siRNAs in MSN mesopores include exchanging divalent cations such as Mg2+ and Ca2+,76 functionalization by amino-derivative groups,112 and conjugation with specific cationic polymers, including PEI.113
Which Position Is Mostly Conceivable for Engineered MSN Application in TNBC Molecular Therapy?
Patients presenting with residual TNBC after neoadjuvant chemotherapy demonstrate much worse survival rates compared with patients with complete remission, mostly because of the near-future development of chemoresistance. The resistant behavior of TNBC cells mostly originates from the high intratumoral heterogeneity, enabling them to evade chemotherapy by overexpressing specific group of genes, including anti-apoptotic and drug efflux pump-involved genes.114, 115, 116 Because the basis of chemoresistance mostly lies in overexpression of specific genes, siRNA therapy appears to be the most promising strategy for resensitization of chemotherapy-resistant TNBC cells. Further application of chemotherapy agents to resensitize cells brings an end to this stubborn disease.117 Nevertheless, as mentioned earlier, systematic treatment with siRNAs requires an efficient transport vehicle with extended circulatory time and a high loading capacity for siRNAs, and the capability of endosomal evasion is obligatory. Several advanced materials, including polymers, co-polypeptides, cyclodextrins, and charged lipids, can overcome siRNA therapy-associated drawbacks. However, the main question to be addressed is why, among these materials, are mostly MSNs under investigation now? Furthermore, why do recent studies mostly utilize engineered MSNs for TNBC siRNA therapy? Indeed, functionalization and/or optimization of most polyplexes and lipid-based nanocarriers’ characteristics is very difficult because these carriers are mostly composed of cationic polymers or lipids with polycation-to-siRNA molar ratios of as much as 10–20:1, enhancing the risk of toxicity and forcing us to keep administered siRNA amounts as low as possible.118, 119, 120, 121, 122 Furthermore, co-administration of chemotherapy drugs with siRNAs in these delivery carriers is restricted. Contrarily, none of these drawbacks have been associated with MSNs, mostly because of the mesoporous structure, biocompatibility, prolonged stability, ease of synthesis, and functionalization of this carrier. The next question that must be addressed is whether siRNA therapy in TNBC is desired. First of all, it must be considered that therapeutic regimens administered for TNBC therapy are mostly limited, with high failure risk and no further improvement in survival rate. Consequently, development of new strategies for combating TNBC is necessary. Because siRNA therapy in most cancers has shown promising results, similar outcomes in TNBC are possible. Second, the aberrant activity of specific genes has been shown to fuel cell proliferation, survival, and resistance in TNBC. siRNAs are highly potent agents for silencing tumor-propagating and resistance development genes. Third, the resistance of cancer cells has mostly been correlated with several proteins involved in the anti-apoptosis pathway, including BCL2, BAX, and BID. Downregulation of the corresponding genes by siRNAs can enhance the effectiveness of therapy and overcome intrinsic or extrinsic mechanisms. Consequently, siRNA therapy of TNBC can reorient the poor response observed with chemotherapy drugs. Now that the importance of siRNA therapy and the beneficial effects of MSNs have been revealed, the final remaining question is which genes’ expression must be suppressed to observe maximum effectiveness of TNBC therapy. Overexpression of several genes has been shown to be involved in the pathogenesis of TNBC, including the P-gp pump, PI3K, TWIST, PKM2, BCL2, and CDK.1, 123, 124 Studies of different cell lines have shown the effectiveness of siRNAs targeting the expression of these oncogenes.125, 126, 127
Recently, Phannasil et al.127 have demonstrated that application of siRNAs suppressing expression of the glycolytic enzyme pyruvate kinase results in significant inhibition of growth, migration, and invasion of the MDA-MB-231 cell line by suppressing several pathways involved in the production of ATP and angiogenesis. Subsequent starvation of cancer cells will further induce apoptosis127. TWIST 1 is another protein that has been shown to facilitate cancer progression by enhancing epithelial-mesenchymal transition (EMT) and also by promoting the cancer stem cell phenotype, which, in turn, results in a significant increase in cell resistance128, 129.
MDA-MB-435 melanoma cell treatment with TWIST-suppressing siRNA resulted in significantly “reduced tumor burdens.”130 These results can also be repeated in other cell lines, including TNBC. Overexpression of BCL2 among non-pump resistance development mechanisms seems to be the most important one. This gene is significantly overexpressed in TNBC and results in escape from apoptotic pathways.125 Administration of MSNs co-delivering BCL2 siRNA and chemotherapy drugs resulted in a significant decrease in LD50 and resensitization of resistant TNBC cells to chemotherapy drugs. In another study, co-delivery of P-gp siRNAs resulted in significant suppression of P-gp synthesis and, consequently, resensitized KBV1, a specific drug-resistant cancer cell, to DOX.131 Survivin is another important protein mostly involved in inhibiting caspase activation and, consequently, negatively controlling apoptosis. Finally, administration of Survivin-silencing siRNA in TNBC significantly resensitizes resistant cells to DOX.132 Enumerating these examples demonstrates the significant roles of siRNA together with chemotherapy agents in TNBC therapy. As a result, administration of siRNAs incorporated in MSNs can result in significant intensification of TNBC therapy, and further studies would be helpful in developing more and effective co-treatments of TNBC.
Conclusion and Future Perspective
As discussed in this review, currently no definite chemotherapy regimen has been developed for effective TNBC therapy. Both inter- and intratumoral heterogeneity have significantly limited the effectiveness of current chemotherapeutic agents, either conventional or targeted, and motivate the development and maturation of a group of cells with the most resistant nature to chemotherapy drugs. Furthermore, the absence of ER, PR, and HER2 overexpression patterns has resulted in the failure of targeted therapies in TNBC. Consequently, development of new generations of therapies is required. Overall, a brief review of some critical points regarding gene therapy and application of nanotechnology for improving outcomes in TNBC therapy is critical.
First, the development of recent genetic screening approaches has significantly illustrated deeper insights into the genetic nature of TNBC, providing us with the opportunity for further segmenting TNBC patients into smaller groups, identifying rarer subgroups, and developing genotype/phenotype-based chemotherapy regimens for further, more specific selection of therapeutic agents with much more efficacy and fewer observed toxicities and, more importantly, for developing a personalized regimen. Second, identification of several critical gene alterations involved in progression, differentiation, and metastasis of TNBC, including VEGF, Survivin, Bcl-2, Mcl-1, P-gp, and TWIST, has emphasized the importance of siRNA therapy in the case of TNBC. As a new trend in TNBC therapy, effective silencing of these genes offers more fundamental corrections with increased potential for achieving complete remission. Third, TNBCs are only susceptible to a small portion of chemotherapy drugs, mainly because of alterations in the expression of BRCA, ABC transporter, and Bcl2 genes. Resensitizing TNBC by effective silencing of these aberrantly overexpressed genes can offer new therapeutic opportunities by application of effective chemotherapy drugs that were previously intrinsically resistant. Fourth, application of a proper carrier compatible with the oligonucleotide nature of siRNAs that biocompatible, possesses prolonged circulatory time, and demonstrates on-demand release is obligatory for maximizing the effectiveness of siRNA therapy of TNBC. Redox-responsive and pH-responsive capped MSNs loaded with chemotherapy drugs in core siRNAs in mesopores are the most advanced and effective nanocarriers engineered so far. Fifth, coating the surface of MSNs with polymers such as PEG (PEGylation) is another strategy for further improving the pharmacokinetic profile of MSNs in the body. However, PEGylation mostly restricts further surface functionalization of MSNs. Consequently, the optimum formulation in each case can be different. Sixth, pH- and redox-responsive capping are more important because of the acidic and high redox nature of the TNBC microenvironment and cells. Seventh, loading of a specific class of drugs, including chloroquine, which can potentiate NP escape from endosomal vesicles, can significantly improve the outcome of therapy. Overall, administration of more site-specific nanocarriers with the least premature drug release and more on-demand release behavior significantly improves the outcome of gene therapy. Recently, administration of multiple siRNAs possessing the potential of silencing two or more pathways regulated by aberrantly overexpressed genes, such as a combination of c-myc and VEGF or MDM2 and c-myc, theoretically sounds rational. However, different siRNAs representing different lengths and sequences have diverse physiochemical characteristics, significantly raising restricting challenges associated with their packing and delivery by nanovehicles. Additionally, increased immunogenicity and off-target effects further restrict co-administration of two or more siRNAs. In conclusion, administration of siRNAs targeting overexpressed genes responsible for resistance development in TNBC and further co-administration of chemotherapy agents can significantly improve the outcome of therapy.
Conflicts of Interest
There is no conflict of interest.
References
- 1.O’Reilly E.A., Gubbins L., Sharma S., Tully R., Guang M.H.Z., Weiner-Gorzel K., McCaffrey J., Harrison M., Furlong F., Kell M., McCann A. The fate of chemoresistance in triple negative breast cancer (TNBC) BBA Clin. 2015;3:257–275. doi: 10.1016/j.bbacli.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penault-Llorca F., Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann. Oncol. 2012;23(Suppl 6):vi19–vi22. doi: 10.1093/annonc/mds190. [DOI] [PubMed] [Google Scholar]
- 3.Viale G., Rotmensz N., Maisonneuve P., Bottiglieri L., Montagna E., Luini A., Veronesi P., Intra M., Torrisi R., Cardillo A. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res. Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 4.Haffty B.G., Yang Q., Reiss M., Kearney T., Higgins S.A., Weidhaas J., Harris L., Hait W., Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 5.Crown J., O’Shaughnessy J., Gullo G. Emerging targeted therapies in triple-negative breast cancer. Ann. Oncol. 2012;23(Suppl 6):vi56–vi65. doi: 10.1093/annonc/mds196. [DOI] [PubMed] [Google Scholar]
- 6.Montagna E., Maisonneuve P., Rotmensz N., Cancello G., Iorfida M., Balduzzi A., Galimberti V., Veronesi P., Luini A., Pruneri G. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin. Breast Cancer. 2013;13:31–39. doi: 10.1016/j.clbc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann B.D., Bauer J.A., Chen X., Sanders M.E., Chakravarthy A.B., Shyr Y., Pietenpol J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panahi Y., Darvishi B., Ghanei M., Jowzi N., Beiraghdar F., Varnamkhasti B.S. Molecular mechanisms of curcumins suppressing effects on tumorigenesis, angiogenesis and metastasis, focusing on NF-κB pathway. Cytokine Growth Factor Rev. 2016;28:21–29. doi: 10.1016/j.cytogfr.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Roberts T.C., Wood M.J. Therapeutic targeting of non-coding RNAs. Essays Biochem. 2013;54:127–145. doi: 10.1042/bse0540127. [DOI] [PubMed] [Google Scholar]
- 10.Størvold G.L., Andersen T.I., Perou C.M., Frengen E. siRNA: a potential tool for future breast cancer therapy? Crit. Rev. Oncog. 2006;12:127–150. doi: 10.1615/critrevoncog.v12.i1-2.70. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.-M., Yoon T.-J., Cho Y.-S. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. BioMed Res. Int. 2013;2013:782041. doi: 10.1155/2013/782041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H., Mai W.X., Zhang H., Xue M., Xia T., Lin S., Wang X., Zhao Y., Ji Z., Zink J.I., Nel A.E. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young S.W.S., Stenzel M., Yang J.L. Nanoparticle-siRNA: A potential cancer therapy? Crit. Rev. Oncol. Hematol. 2016;98:159–169. doi: 10.1016/j.critrevonc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Judge A.D., Robbins M., Tavakoli I., Levi J., Hu L., Fronda A., Ambegia E., McClintock K., MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J. Clin. Invest. 2009;119:661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogini D.B., Pascoal V.D., Avansini S.H., Vieira A.S., Pereira T.C., Lopes-Cendes I. The new world of RNAs. Genet. Mol. Biol. 2014;37(1, Suppl):285–293. doi: 10.1590/s1415-47572014000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen G.L., Blau H.M. A brief history of RNAi: the silence of the genes. FASEB J. 2006;20:1293–1299. doi: 10.1096/fj.06-6014rev. [DOI] [PubMed] [Google Scholar]
- 17.Castanotto D., Rossi J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil V.S., Zhou R., Rana T.M. Gene regulation by non-coding RNAs. Crit. Rev. Biochem. Mol. Biol. 2014;49:16–32. doi: 10.3109/10409238.2013.844092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artzi N. Trojan siRNA Opens the Door to Chemotherapy. Science Translational Medicine. 2013;5 211ec186. [Google Scholar]
- 20.Fattal E., Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int. J. Pharm. 2008;364:237–248. doi: 10.1016/j.ijpharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Gao S., Dagnaes-Hansen F., Nielsen E.J.B., Wengel J., Besenbacher F., Howard K.A., Kjems J. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol. Ther. 2009;17:1225–1233. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimesh S. Recent patents in siRNA delivery employing nanoparticles as delivery vectors. Recent Pat. DNA Gene Seq. 2012;6:91–97. doi: 10.2174/187221512801327406. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J., Shum K.-T., Burnett J.C., Rossi J.J. Nanoparticle-based delivery of RNAi therapeutics: progress and challenges. Pharmaceuticals (Basel) 2013;6:85–107. doi: 10.3390/ph6010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 25.Sivori S., Falco M., Della Chiesa M., Carlomagno S., Vitale M., Moretta L., Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 27.Goh K.C., deVeer M.J., Williams B.R. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burchard J., Jackson A.L., Malkov V., Needham R.H., Tan Y., Bartz S.R., Dai H., Sachs A.B., Linsley P.S. MicroRNA-like off-target transcript regulation by siRNAs is species specific. RNA. 2009;15:308–315. doi: 10.1261/rna.1326809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson A.L., Bartz S.R., Schelter J., Kobayashi S.V., Burchard J., Mao M., Li B., Cavet G., Linsley P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 30.Guzman-Villanueva D., El-Sherbiny I.M., Herrera-Ruiz D., Vlassov A.V., Smyth H.D. Formulation approaches to short interfering RNA and MicroRNA: challenges and implications. J. Pharm. Sci. 2012;101:4046–4066. doi: 10.1002/jps.23300. [DOI] [PubMed] [Google Scholar]
- 31.Kay M.A. State-of-the-art gene-based therapies: the road ahead. Nat. Rev. Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- 32.Mingozzi F., High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 33.Baum C., Kustikova O., Modlich U., Li Z., Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 34.Bessis N., GarciaCozar F.J., Boissier M.C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 35.Bouard D., Alazard-Dany D., Cosset F.L. Viral vectors: from virology to transgene expression. Br. J. Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 37.Waehler R., Russell S.J., Curiel D.T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mintzer M.A., Simanek E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 39.Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 40.Lee H., Lytton-Jean A.K., Chen Y., Love K.T., Park A.I., Karagiannis E.D., Sehgal A., Querbes W., Zurenko C.S., Jayaraman M. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 2012;7:389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monopoli M.P., Åberg C., Salvati A., Dawson K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 42.Anderson B.R., Muramatsu H., Jha B.K., Silverman R.H., Weissman D., Karikó K. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011;39:9329–9338. doi: 10.1093/nar/gkr586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug. Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 44.Yu B., Zhao X., Lee L.J., Lee R.J. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozema D.B., Lewis D.L., Wakefield D.H., Wong S.C., Klein J.J., Roesch P.L., Bertin S.L., Reppen T.W., Chu Q., Blokhin A.V. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R., Kelly P.M., Åberg C., Mahon E., Dawson K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz D.S., Hutvágner G., Du T., Xu Z., Aronin N., Zamore P.D. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 48.Kecht J., Schlossbauer A., Bein T. Selective functionalization of the outer and inner surfaces in mesoporous silica nanoparticles. Chem. Mater. 2008;20:7207–7214. [Google Scholar]
- 49.Mamaeva V., Sahlgren C., Lindén M. Mesoporous silica nanoparticles in medicine--recent advances. Adv. Drug Deliv. Rev. 2013;65:689–702. doi: 10.1016/j.addr.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Radu D.R., Lai C.-Y., Jeftinija K., Rowe E.W., Jeftinija S., Lin V.S.-Y. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004;126:13216–13217. doi: 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- 51.Slowing I.I., Trewyn B.G., Giri S., Lin V.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007;17:1225–1236. [Google Scholar]
- 52.Saha S., Leung K.F., Nguyen T.D., Stoddart J.F., Zink J.I. Nanovalves. Adv. Funct. Mater. 2007;17:685–693. [Google Scholar]
- 53.You Y.-Z., Kalebaila K.K., Brock S.L. Temperature-controlled uptake and release in PNIPAM-modified porous silica nanoparticles. Chem. Mater. 2008;20:3354–3359. [Google Scholar]
- 54.Mayor S., Pagano R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 55.Prokop A., Davidson J.M. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 2008;97:3518–3590. doi: 10.1002/jps.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maxfield F.R., McGraw T.E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 57.Slowing I., Trewyn B.G., Lin V.S.-Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006;128:14792–14793. doi: 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- 58.Vivero-Escoto J.L., Slowing I.I., Trewyn B.G., Lin V.S.Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 59.Jambhrunkar S., Qu Z., Popat A., Yang J., Noonan O., Acauan L., Ahmad Nor Y., Yu C., Karmakar S. Effect of surface functionality of silica nanoparticles on cellular uptake and cytotoxicity. Mol. Pharm. 2014;11:3642–3655. doi: 10.1021/mp500385n. [DOI] [PubMed] [Google Scholar]
- 60.Chung T.-H., Wu S.-H., Yao M., Lu C.-W., Lin Y.-S., Hung Y., Mou C.Y., Chen Y.C., Huang D.M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Huang D.-M., Hung Y., Ko B.-S., Hsu S.-C., Chen W.-H., Chien C.-L., Tsai C.P., Kuo C.T., Kang J.C., Yang C.S. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. FASEB J. 2005;19:2014–2016. doi: 10.1096/fj.05-4288fje. [DOI] [PubMed] [Google Scholar]
- 62.Gu J., Fan W., Shimojima A., Okubo T. Organic-inorganic mesoporous nanocarriers integrated with biogenic ligands. Small. 2007;3:1740–1744. doi: 10.1002/smll.200700311. [DOI] [PubMed] [Google Scholar]
- 63.Fan H., Hu Q.-D., Xu F.-J., Liang W.-Q., Tang G.-P., Yang W.-T. In vivo treatment of tumors using host-guest conjugated nanoparticles functionalized with doxorubicin and therapeutic gene pTRAIL. Biomaterials. 2012;33:1428–1436. doi: 10.1016/j.biomaterials.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 64.Pavillard V., Kherfellah D., Richard S., Robert J., Montaudon D. Effects of the combination of camptothecin and doxorubicin or etoposide on rat glioma cells and camptothecin-resistant variants. Br. J. Cancer. 2001;85:1077–1083. doi: 10.1054/bjoc.2001.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen Y., Jin E., Zhang B., Murphy C.J., Sui M., Zhao J., Wang J., Tang J., Fan M., Van Kirk E., Murdoch W.J. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J. Am. Chem. Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 66.Wang H., Wu Y., Zhao R., Nie G. Engineering the assemblies of biomaterial nanocarriers for delivery of multiple theranostic agents with enhanced antitumor efficacy. Adv. Mater. 2013;25:1616–1622. doi: 10.1002/adma.201204750. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Gao S., Ye W.-H., Yoon H.S., Yang Y.-Y. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat. Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 68.Verma M., Kagan J., Sidransky D., Srivastava S. Proteomic analysis of cancer-cell mitochondria. Nat. Rev. Cancer. 2003;3:789–795. doi: 10.1038/nrc1192. [DOI] [PubMed] [Google Scholar]
- 69.Luo G.-F., Chen W.-H., Liu Y., Lei Q., Zhuo R.-X., Zhang X.-Z. Multifunctional enveloped mesoporous silica nanoparticles for subcellular co-delivery of drug and therapeutic peptide. Sci. Rep. 2014;4:6064. doi: 10.1038/srep06064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng Y.-J., Zeng X., Cheng D.-B., Xu X.-D., Zhang X.-Z., Zhuo R.-X., He F. Functional mesoporous silica nanoparticles (MSNs) for highly controllable drug release and synergistic therapy. Colloids Surf. B Biointerfaces. 2016;145:217–225. doi: 10.1016/j.colsurfb.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 71.Xia T., Kovochich M., Liong M., Meng H., Kabehie S., George S., Zink J.I., Nel A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Draz M.S., Fang B.A., Zhang P., Hu Z., Gu S., Weng K.C., Gray J.W., Chen F.F. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4:872–892. doi: 10.7150/thno.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X., Zhang J., Gu H. Adsorption and desorption behaviors of DNA with magnetic mesoporous silica nanoparticles. Langmuir. 2011;27:6099–6106. doi: 10.1021/la104653s. [DOI] [PubMed] [Google Scholar]
- 74.He Q., Shi J. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011;21:5845–5855. [Google Scholar]
- 75.Li X., Zhang J., Gu H. Study on the adsorption mechanism of DNA with mesoporous silica nanoparticles in aqueous solution. Langmuir. 2012;28:2827–2834. doi: 10.1021/la204443j. [DOI] [PubMed] [Google Scholar]
- 76.Solberg S.M., Landry C.C. Adsorption of DNA into mesoporous silica. J. Phys. Chem. B. 2006;110:15261–15268. doi: 10.1021/jp061691+. [DOI] [PubMed] [Google Scholar]
- 77.Kim M.-H., Na H.-K., Kim Y.-K., Ryoo S.-R., Cho H.S., Lee K.E., Jeon H., Ryoo R., Min D.H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano. 2011;5:3568–3576. doi: 10.1021/nn103130q. [DOI] [PubMed] [Google Scholar]
- 78.Meng H., Liong M., Xia T., Li Z., Ji Z., Zink J.I., Nel A.E. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Na H.K., Kim M.H., Park K., Ryoo S.R., Lee K.E., Jeon H., Ryoo R., Hyeon C., Min D.H. Efficient functional delivery of siRNA using mesoporous silica nanoparticles with ultralarge pores. Small. 2012;8:1752–1761. doi: 10.1002/smll.201200028. [DOI] [PubMed] [Google Scholar]
- 80.Hartono S.B., Phuoc N.T., Yu M., Jia Z., Monteiro M.J., Qiao S. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B Mater. Biol. Med. 2014;2:718–726. doi: 10.1039/c3tb21015d. [DOI] [PubMed] [Google Scholar]
- 81.Hartono S.B., Gu W., Kleitz F., Liu J., He L., Middelberg A.P., Yu C., Lu G.Q., Qiao S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano. 2012;6:2104–2117. doi: 10.1021/nn2039643. [DOI] [PubMed] [Google Scholar]
- 82.Lin D., Cheng Q., Jiang Q., Huang Y., Yang Z., Han S., Zhao Y., Guo S., Liang Z., Dong A. Intracellular cleavable poly(2-dimethylaminoethyl methacrylate) functionalized mesoporous silica nanoparticles for efficient siRNA delivery in vitro and in vivo. Nanoscale. 2013;5:4291–4301. doi: 10.1039/c3nr00294b. [DOI] [PubMed] [Google Scholar]
- 83.Hanafi-Bojd M.Y., Jaafari M.R., Ramezanian N., Abnous K., Malaekeh-Nikouei B. Co-delivery of epirubicin and siRNA using functionalized mesoporous silica nanoparticles enhances in vitro and in vivo drug efficacy. Curr. Drug Deliv. 2016;13:1176–1182. doi: 10.2174/1567201813666151231094056. [DOI] [PubMed] [Google Scholar]
- 84.Yu T., Hubbard D., Ray A., Ghandehari H. In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J. Control. Release. 2012;163:46–54. doi: 10.1016/j.jconrel.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang X., Li L., Liu T., Hao N., Liu H., Chen D., Tang F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5:5390–5399. doi: 10.1021/nn200365a. [DOI] [PubMed] [Google Scholar]
- 86.He Q., Zhang Z., Gao F., Li Y., Shi J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 2011;7:271–280. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- 87.Rosenholm J.M., Mamaeva V., Sahlgren C., Lindén M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine (Lond.) 2012;7:111–120. doi: 10.2217/nnm.11.166. [DOI] [PubMed] [Google Scholar]
- 88.Lin Y.-S., Abadeer N., Haynes C.L. Stability of small mesoporous silica nanoparticles in biological media. Chem. Commun. (Camb.) 2011;47:532–534. doi: 10.1039/c0cc02923h. [DOI] [PubMed] [Google Scholar]
- 89.Cheng S.-H., Li F.-C., Souris J.S., Yang C.-S., Tseng F.-G., Lee H.-S., Chen C.T., Dong C.Y., Lo L.W. Visualizing dynamics of sub-hepatic distribution of nanoparticles using intravital multiphoton fluorescence microscopy. ACS Nano. 2012;6:4122–4131. doi: 10.1021/nn300558p. [DOI] [PubMed] [Google Scholar]
- 90.Park J.-H., Gu L., von Maltzahn G., Ruoslahti E., Bhatia S.N., Sailor M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond.) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jhaveri A., Deshpande P., Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J. Control. Release. 2014;190:352–370. doi: 10.1016/j.jconrel.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Esquela-Kerscher A., Slack F.J. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 94.Steinbacher J.L., Landry C.C. Adsorption and release of siRNA from porous silica. Langmuir. 2014;30:4396–4405. doi: 10.1021/la402850m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang I.-P., Sun S.-P., Cheng S.-H., Lee C.-H., Wu C.-Y., Yang C.-S., Lo L.W., Lai Y.K. Enhanced chemotherapy of cancer using pH-sensitive mesoporous silica nanoparticles to antagonize P-glycoprotein-mediated drug resistance. Mol. Cancer Ther. 2011;10:761–769. doi: 10.1158/1535-7163.MCT-10-0884. [DOI] [PubMed] [Google Scholar]
- 96.Jiang J., Yang S.J., Wang J.C., Yang L.J., Xu Z.Z., Yang T., Liu X.Y., Zhang Q. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxorubicin. Eur J Pharm Biopharm. 2010;76:170–178. doi: 10.1016/j.ejpb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 97.Yadav S., van Vlerken L.E., Little S.R., Amiji M.M. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother. Pharmacol. 2009;63:711–722. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- 98.Navarro G., Sawant R.R., Biswas S., Essex S., Tros de Ilarduya C., Torchilin V.P. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine (Lond.) 2012;7:65–78. doi: 10.2217/nnm.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janát-Amsbury M.M., Yockman J.W., Lee M., Kern S., Furgeson D.Y., Bikram M., Kim S.W. Combination of local, nonviral IL12 gene therapy and systemic paclitaxel treatment in a metastatic breast cancer model. Mol. Ther. 2004;9:829–836. doi: 10.1016/j.ymthe.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 100.Chen A.M., Zhang M., Wei D., Stueber D., Taratula O., Minko T., He H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5:2673–2677. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saraswathy M., Gong S. Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater. Today. 2014;17:298–306. [Google Scholar]
- 102.Miller T., Breyer S., van Colen G., Mier W., Haberkorn U., Geissler S., Voss S., Weigandt M., Goepferich A. Premature drug release of polymeric micelles and its effects on tumor targeting. Int. J. Pharm. 2013;445:117–124. doi: 10.1016/j.ijpharm.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 103.Mekaru H., Lu J., Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015;95:40–49. doi: 10.1016/j.addr.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giri S., Trewyn B.G., Stellmaker M.P., Lin V.S.Y. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Ed. Engl. 2005;44:5038–5044. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- 105.Lai C.-Y., Trewyn B.G., Jeftinija D.M., Jeftinija K., Xu S., Jeftinija S., Lin V.S. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003;125:4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 106.Torney F., Trewyn B.G., Lin V.S.-Y., Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007;2:295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 107.Li Z., Clemens D.L., Lee B.-Y., Dillon B.J., Horwitz M.A., Zink J.I. Mesoporous silica nanoparticles with pH-sensitive nanovalves for delivery of moxifloxacin provide improved treatment of lethal pneumonic tularemia. ACS Nano. 2015;9:10778–10789. doi: 10.1021/acsnano.5b04306. [DOI] [PubMed] [Google Scholar]
- 108.Ashley C.E., Carnes E.C., Epler K.E., Padilla D.P., Phillips G.K., Castillo R.E., Wilkinson D.C., Wilkinson B.S., Burgard C.A., Kalinich R.M. Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. ACS Nano. 2012;6:2174–2188. doi: 10.1021/nn204102q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ashley C.E., Carnes E.C., Phillips G.K., Padilla D., Durfee P.N., Brown P.A., Hanna T.N., Liu J., Phillips B., Carter M.B. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu J., Wang B., Hartono S.B., Liu T., Kantharidis P., Middelberg A.P., Lu G.Q., He L., Qiao S.Z. Magnetic silica spheres with large nanopores for nucleic acid adsorption and cellular uptake. Biomaterials. 2012;33:970–978. doi: 10.1016/j.biomaterials.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Li X., Xie Q.R., Zhang J., Xia W., Gu H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials. 2011;32:9546–9556. doi: 10.1016/j.biomaterials.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 112.Gao F., Botella P., Corma A., Blesa J., Dong L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J. Phys. Chem. B. 2009;113:1796–1804. doi: 10.1021/jp807956r. [DOI] [PubMed] [Google Scholar]
- 113.Hom C., Lu J., Liong M., Luo H., Li Z., Zink J.I., Tamanoi F. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small. 2010;6:1185–1190. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee M.J., Ye A.S., Gardino A.K., Heijink A.M., Sorger P.K., MacBeath G., Yaffe M.B. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liedtke C., Mazouni C., Hess K.R., André F., Tordai A., Mejia J.A., Symmans W.F., Gonzalez-Angulo A.M., Hennessy B., Green M. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 116.Natarajan K., Xie Y., Baer M.R., Ross D.D. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem. Pharmacol. 2012;83:1084–1103. doi: 10.1016/j.bcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nimesh S. Polyethylenimine as a promising vector for targeted siRNA delivery. Curr. Clin. Pharmacol. 2012;7:121–130. doi: 10.2174/157488412800228857. [DOI] [PubMed] [Google Scholar]
- 119.Taratula O., Kuzmov A., Shah M., Garbuzenko O.B., Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J. Control. Release. 2013;171:349–357. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Won Y.-W., Yoon S.-M., Lee K.-M., Kim Y.-H. Poly(oligo-D-arginine) with internal disulfide linkages as a cytoplasm-sensitive carrier for siRNA delivery. Mol. Ther. 2011;19:372–380. doi: 10.1038/mt.2010.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y., Schwerbrock N.M., Rogers A.B., Kim W.Y., Huang L. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol. Ther. 2013;21:1559–1569. doi: 10.1038/mt.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuckerman J.E., Choi C.H.J., Han H., Davis M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang W.-S., Yang X.-S., Xia M., Jiang H.-Y., Hou J.-Q. Silencing of twist expression by RNA interference suppresses epithelial-mesenchymal transition, invasion, and metastasis of ovarian cancer. Asian Pac. J. Cancer Prev. 2012;13:4435–4439. doi: 10.7314/apjcp.2012.13.9.4435. [DOI] [PubMed] [Google Scholar]
- 124.Liu J.C., Voisin V., Wang S., Wang D.Y., Jones R.A., Datti A., Uehling D., Al-awar R., Egan S.E., Bader G.D. Combined deletion of Pten and p53 in mammary epithelium accelerates triple-negative breast cancer with dependency on eEF2K. EMBO Mol. Med. 2014;6:1542–1560. doi: 10.15252/emmm.201404402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Akar U., Chaves-Reyez A., Barria M., Tari A., Sanguino A., Kondo Y., Kondo S., Arun B., Lopez-Berestein G., Ozpolat B. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4:669–679. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- 126.Leung R.K., Whittaker P.A. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol. Ther. 2005;107:222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phannasil P., Thuwajit C., Warnnissorn M., Wallace J.C., MacDonald M.J., Jitrapakdee S. Pyruvate carboxylase is up-regulated in breast cancer and essential to support growth and invasion of MDA-MB-231 cells. PLoS ONE. 2015;10:e0129848. doi: 10.1371/journal.pone.0129848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li C.-W., Xia W., Huo L., Lim S.-O., Wu Y., Hsu J.L., Chao C.H., Yamaguchi H., Yang N.K., Ding Q. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y., Liu J., Ying X., Lin P.C., Zhou B.P. Twist-mediated Epithelial-mesenchymal Transition Promotes Breast Tumor Cell Invasion via Inhibition of Hippo Pathway. Sci. Rep. 2016;6:24606. doi: 10.1038/srep24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finlay J., Roberts C.M., Dong J., Zink J.I., Tamanoi F., Glackin C.A. Mesoporous silica nanoparticle delivery of chemically modified siRNA against TWIST1 leads to reduced tumor burden. Nanomedicine (Lond.) 2015;11:1657–1666. doi: 10.1016/j.nano.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wan C., Allen T.M., Cullis P.R. Nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv. Transl. Rev. 2014;4:74–83. doi: 10.1007/s13346-013-0161-z. [DOI] [PubMed] [Google Scholar]
- 132.Su S., Tian Y., Li Y., Ding Y., Ji T., Wu M., Wu Y., Nie G. “Triple-punch” strategy for triple negative breast cancer therapy with minimized drug dosage and improved antitumor efficacy. ACS Nano. 2015;9:1367–1378. doi: 10.1021/nn505729m. [DOI] [PubMed] [Google Scholar]
- 133.Rajput S., Puvvada N., Kumar B.N., Sarkar S., Konar S., Bharti R., Dey G., Mazumdar A., Pathak A., Fisher P.B., Mandal M. Overcoming Akt induced therapeutic resistance in breast cancer through siRNA and thymoquinone encapsulated multilamellar gold niosomes. Mol. Pharm. 2015;12:4214–4225. doi: 10.1021/acs.molpharmaceut.5b00692. [DOI] [PubMed] [Google Scholar]
- 134.Kirat Kumar G., Triparna S., Sekhar P., Jaydip B., Amitava C. Studies on Focal Adhesion Kinase in human breast cancer cell MDA-MB-231. Adv. Biol. Chem. 2012;2 [Google Scholar]
- 135.Deng Z.J., Morton S.W., Ben-Akiva E., Dreaden E.C., Shopsowitz K.E., Hammond P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano. 2013;7:9571–9584. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gandhi N.S., Tekade R.K., Chougule M.B. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J. Control. Release. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Horiuchi D., Kusdra L., Huskey N.E., Chandriani S., Lenburg M.E., Gonzalez-Angulo A.M., Creasman K.J., Bazarov A.V., Smyth J.W., Davis S.E. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp. Med. 2012;209:679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kapse-Mistry S., Govender T., Srivastava R., Yergeri M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014;5:159. doi: 10.3389/fphar.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao J., Liu W., Xia Y., Li W., Sun J., Chen H., Li B., Zhang D., Qian W., Meng Y. The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials. 2011;32:3459–3470. doi: 10.1016/j.biomaterials.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 140.Cleator S., Heller W., Coombes R.C. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]