Abstract
This paper represents attempts to observe alterations in the pattern of chromatin protein phosphorylation in Lemna and barley (Hordeum vulgare).
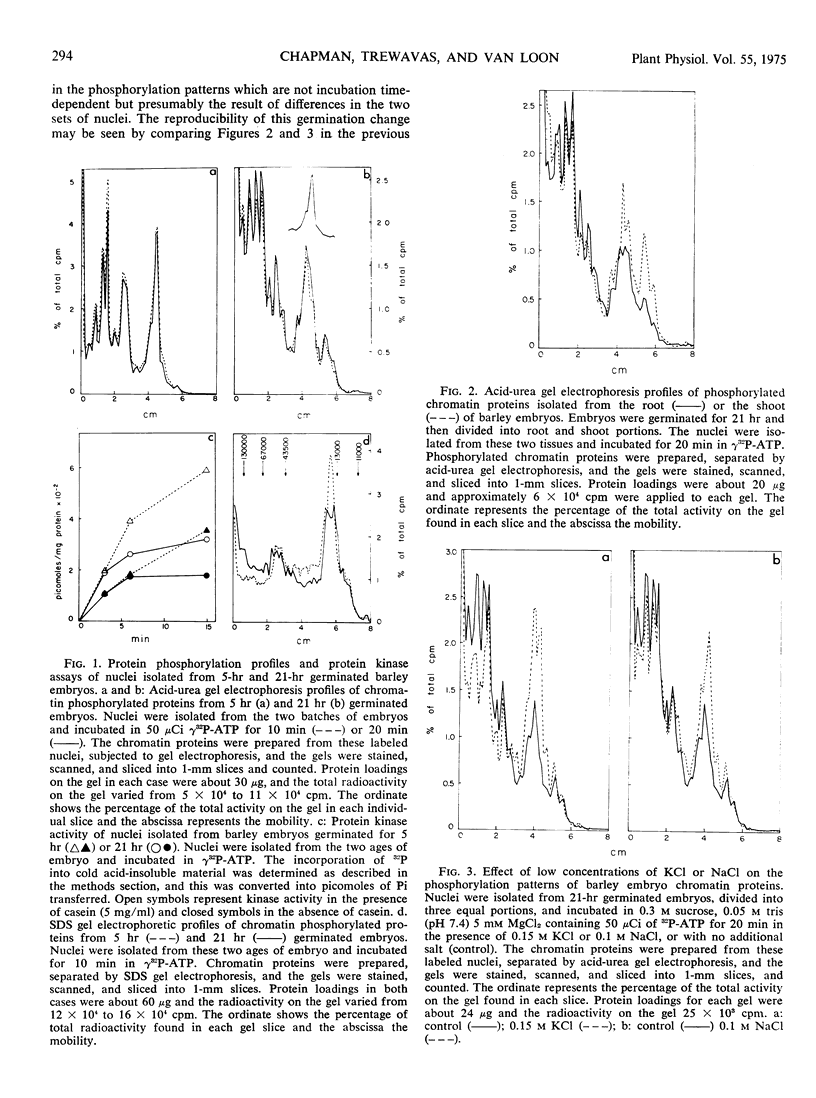
As judged by in vitro labeling the phosphorylation profile is substantially altered during germination. This may not be the result of specific tissue differentiation, however, because phosphorylation does not differ markedly between the embryonic root and shoot. Treatment of nuclei from germinating embryos with low concentrations of sodium or potassium chloride produced phosphorylation patterns similar but not identical to those found in nuclei from ungerminated embryos.
Treatment of Lemna with abscisic acid in vivo causes substantial alterations in the labeling of three protein bands and part of this may be duplicated by labeling isolated nuclei from treated tissue with γ32P-ATP. Some effects of light/dark transition on Lemna chromatin protein phosphorylation are also described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Teng C. S., Teng C. T., Allfrey V. G. Studies of nuclear acidic proteins. Evidence for their phosphorylation, tissue specificity, selective binding to deoxyribonucleic acid, and stimulation effects on transcription. J Biol Chem. 1971 Jun 10;246(11):3597–3609. [PubMed] [Google Scholar]
- van Loon L. C., Trewavas A., Chapman K. S. Phosphorylation of Chromatin-associated Proteins in Lemna and Hordeum. Plant Physiol. 1975 Feb;55(2):288–292. doi: 10.1104/pp.55.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]


