Abstract
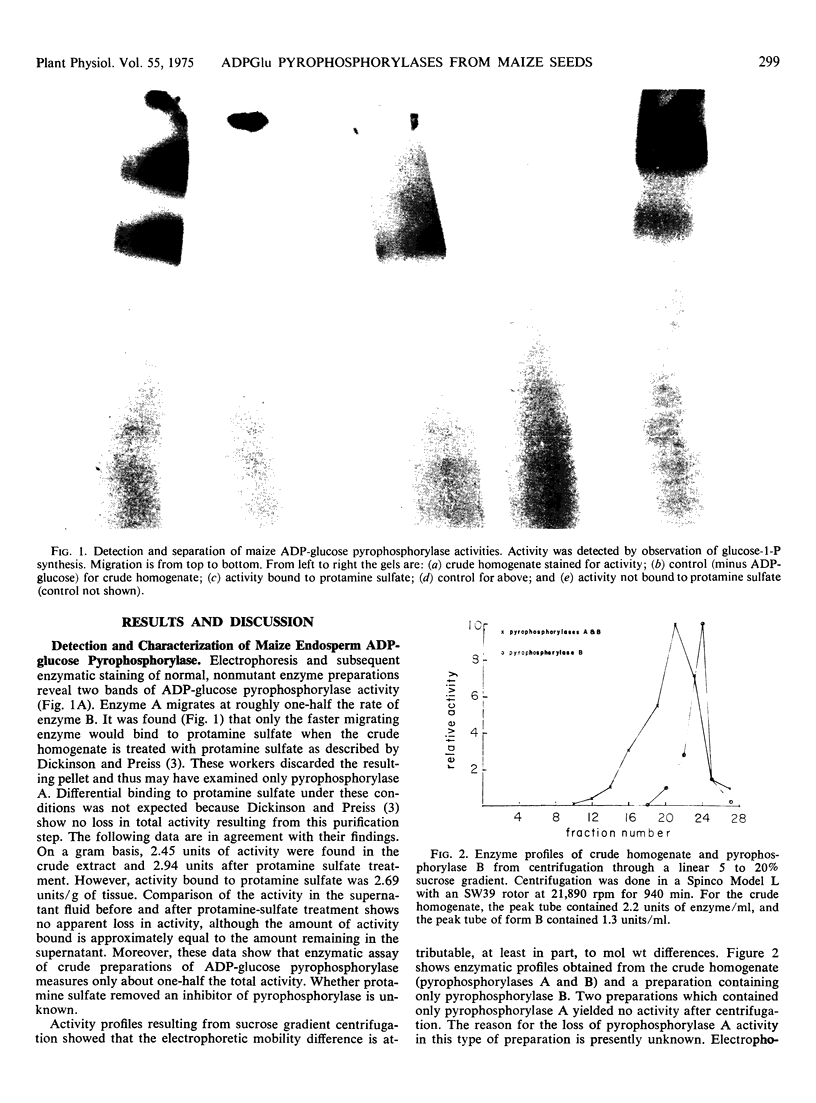
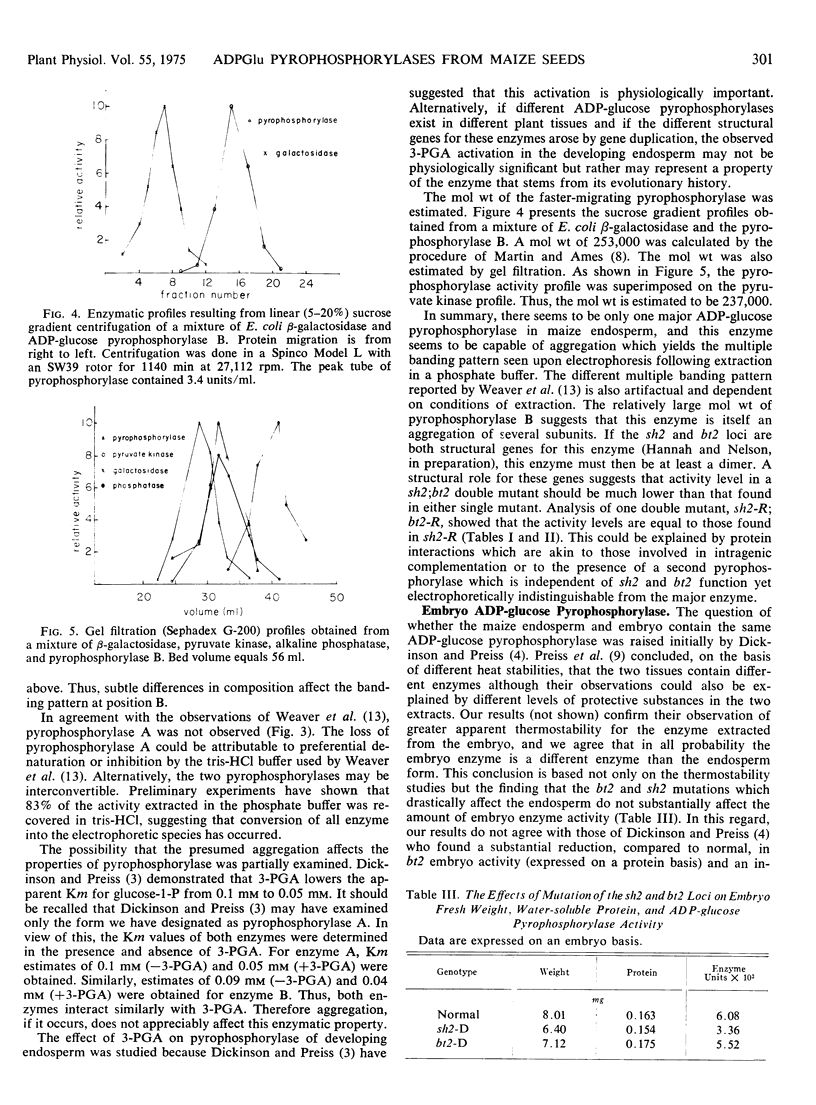
Electrophoretic examination of 22-day-old, normal maize (Zea mays L.) endosperm extracts revealed two zones of adenosine diphosphate glucose pyrophosphorylase activity. The enzymes are identical in terms of Km for glucose 1-phosphate and the effect of 3-phosphoglyceric acid on apparent Km for glucose 1-phosphate. Both enzymatic activities increase with increasing doses of the functional alleles at the shrunken-2 and brittle-2 loci. Molecular weight differences between the two electrophoretic species were inferred from sucrose gradient centrifugation. It is suggested that the two bands of activity represent different aggregation states of the same enzyme because under different extraction conditions, only one enzyme is found. Molecular weight estimates of 237,000 and 253,000 were obtained for the smaller enzyme. It is suggested that this enzyme is an aggregate of several subunits. Comparison of the embryo and endosperm pyrophosphorylases showed the embryo activity to be more heat stable and probably independent of direct shrunken-2 or brittle-2 control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akatsuka T., Nelson O. E. Starch granule-bound adenosine diphosphate glucose-starch glucosyltransferases of maize seeds. J Biol Chem. 1966 May 25;241(10):2280–2285. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. ADP glucose pyrophosphorylase from maize endosperm. Arch Biochem Biophys. 1969 Mar;130(1):119–128. doi: 10.1016/0003-9861(69)90017-4. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. Presence of ADP-Glucose Pyrophosphorylase in Shrunken-2 and Brittle-2 Mutants of Maize Endosperm. Plant Physiol. 1969 Jul;44(7):1058–1062. doi: 10.1104/pp.44.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Dickinson D. B. Partial purification and sugar nucleotide inhibition of UDP-glucose pyrophosphorylase from Lilium longiflorum pollen. Arch Biochem Biophys. 1972 Feb;148(2):523–535. doi: 10.1016/0003-9861(72)90171-3. [DOI] [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Preiss J., Lammel C., Sabraw A. A unique adenosine diphosphoglucose pyrophosphorylase associated with maize embryo tissue. Plant Physiol. 1971 Jan;47(1):104–108. doi: 10.1104/pp.47.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamini F., Tsai C. Y., Nelson O. E. Multiple forms of glucosephosphate isomerase in maize. Plant Physiol. 1972 Aug;50(2):256–261. doi: 10.1104/pp.50.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. Y., Nelson O. E. Starch-deficient maize mutant lacking adenosine dephosphate glucose pyrophosphorylase activity. Science. 1966 Jan 21;151(3708):341–343. doi: 10.1126/science.151.3708.341. [DOI] [PubMed] [Google Scholar]
- Tsai C. Y., Salamini F., Nelson O. E. Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol. 1970 Aug;46(2):299–306. doi: 10.1104/pp.46.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIPSER D. A STUDY OF THE UREA-PRODUCED SUBUNITS OF BETA-GALACTOSIDASE. J Mol Biol. 1963 Aug;7:113–121. doi: 10.1016/s0022-2836(63)80040-6. [DOI] [PubMed] [Google Scholar]