Abstract
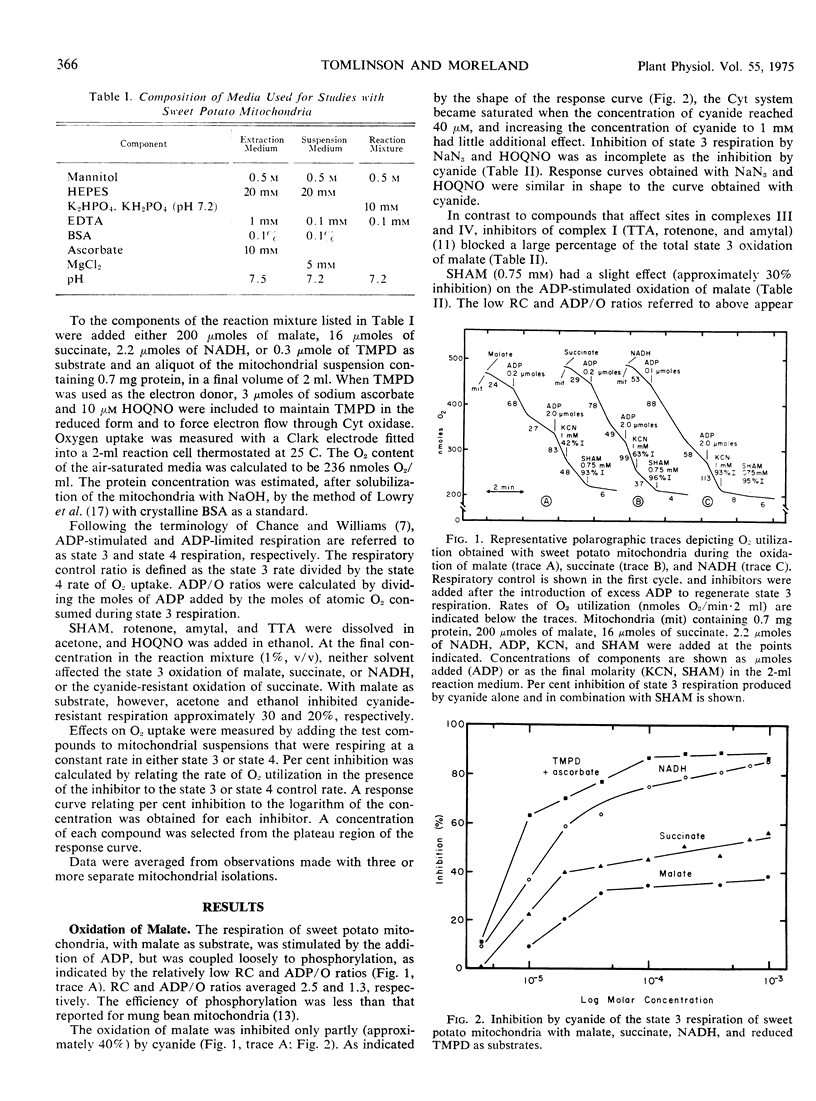
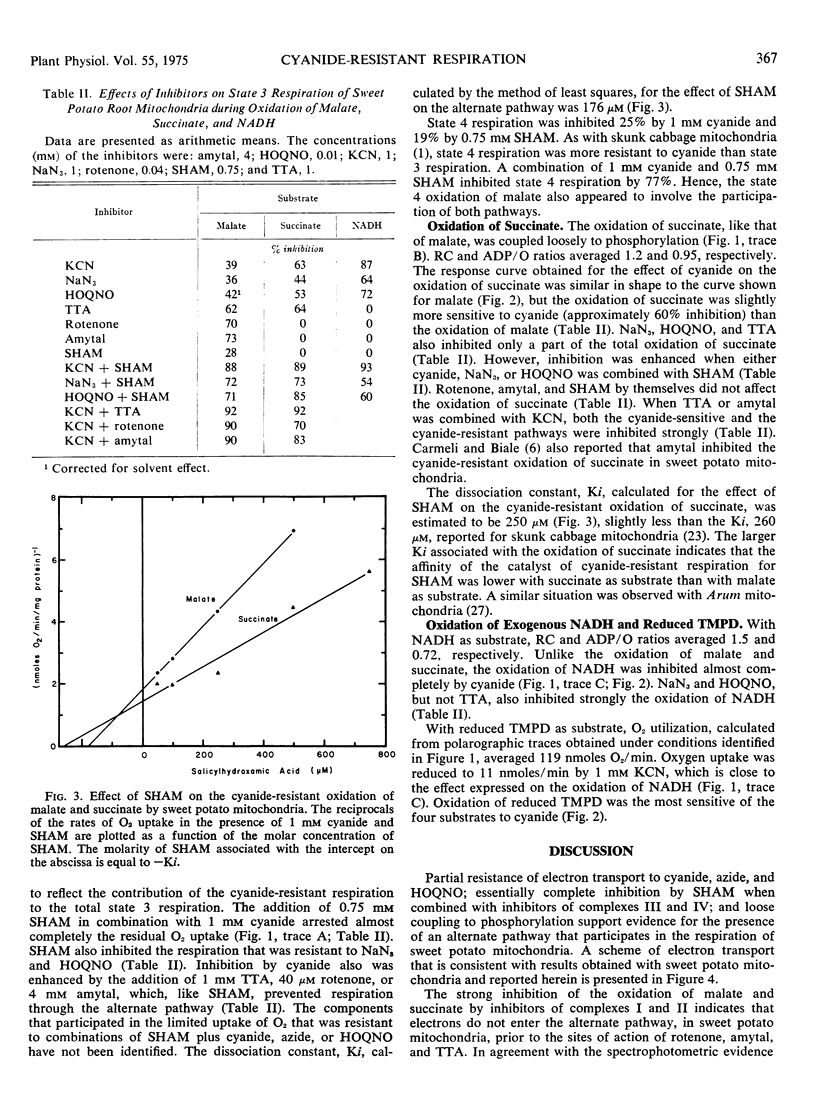
The oxidation of malate and succinate by sweet potato mitochondria (Ipomoea batatas [L.] Lam.) was blocked only partly by inhibitors of complexes III (2-heptyl-4-hydroxyquinoline-N-oxide) and IV (cyanide and azide). The respiration insensitive to inhibitors of complexes III and IV was inhibited by salicylhydroxamic acid. Essentially complete inhibition was obtained with inhibitors of complex I (rotenone, amytal, and thenoyltrifluoroacetone) and complex II (thenoyltrifluoroacetone). The observations indicated that electrons were transferred to the cyanide-resistant pathway from ubiquinone or from nonheme iron (iron-sulfur) proteins of complexes I and II before reaching the b cytochromes. In contrast, the oxidation of exogenous NADH did not involve the alternate pathway, as indicated by complete inhibition by inhibitors of complexes III and IV and the absence of an effect of inhibitors of complexes I and II. Hence, electrons from exogenous NADH appear to pass directly to complex III in sweet potato mitochondria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Baker J. E., Lieberman M. Cytochrome Components & Electron Transfer in Sweet Potato Mitochondria. Plant Physiol. 1962 Jan;37(1):90–97. doi: 10.1104/pp.37.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton C. J., Palmer J. M. Pathways for the oxidation of malate and reduced pyridine nucleotide by wheat mitochondria. Eur J Biochem. 1973 Nov 1;39(1):283–291. doi: 10.1111/j.1432-1033.1973.tb03125.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- HACKETT D. P., RICE B., SCHMID C. The partial dissociation of phosphorylation from oxidation in plant mitochondria by respiratory chain inhibitors. J Biol Chem. 1960 Jul;235:2140–2144. [PubMed] [Google Scholar]
- Hatefi Y. Flavoproteins of the electron transport system and the site of action of amytal, rotenone, and piericidin A. Proc Natl Acad Sci U S A. 1968 Jun;60(2):733–740. doi: 10.1073/pnas.60.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambowitz A. M., Smith E. W., Slayman C. W. Electron transport in Neurospora mitochondria. Studies on wild type and poky. J Biol Chem. 1972 Aug 10;247(15):4850–4858. [PubMed] [Google Scholar]
- Moreland D. E., Boots M. R. Effects of optically active 1-(alpha-methylbenzyl)-3-(3,4-dichlorophenyl)urea on reactions of mitochondria and chloroplasts. Plant Physiol. 1971 Jan;47(1):53–58. doi: 10.1104/pp.47.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPPEL A. L. Inhibition of electron transport by antimycin A, alkyl hydroxy naphthoquinones and metal coordination compounds. Biochem Pharmacol. 1960 Jul;3:289–296. doi: 10.1016/0006-2952(60)90094-0. [DOI] [PubMed] [Google Scholar]
- Verleur J. D. Studies on the Isolation of Mitochondria from Potato Tuber Tissue. Plant Physiol. 1965 Nov;40(6):1003–1007. doi: 10.1104/pp.40.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B. The effect of respiratory inhibitors on NADH, succinate and malate oxidation in corn mitochondria. Plant Physiol. 1969 Sep;44(9):1335–1341. doi: 10.1104/pp.44.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation associated with cyanide-insensitive respiration in plant mitochondria. Biochim Biophys Acta. 1970 Dec 8;223(2):383–387. doi: 10.1016/0005-2728(70)90195-7. [DOI] [PubMed] [Google Scholar]
- Wilson S. B. Studies on the cyanide insensitive oxidase of plant mitochondria. FEBS Lett. 1971 Jun 2;15(1):49–52. doi: 10.1016/0014-5793(71)80077-7. [DOI] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]


