Abstract
Radioactivity from d-glucosamine-14C is incorporated into particulate fractions of hypocotyls of Phaseolus aureus (mung bean) seedlings. Polyacrylamide gel electrophoresis of the sodium dodecyl sulfate-solubilized materials revealed that several polypeptide components varying considerably in molecular weight had become radioactive during the incubation. A considerable amount of 14C was also recovered in lipid. Equilibrium centrifugation of the particulate material, isolated by initial centrifugation at 100,000 times gravity on sucrose density gradients revealed that radioactivity was recoverable in all of the membrane fractions along the gradient. It is suggested that glycoproteins and glycolipids containing amino sugar are normal constituents of such membranes. The ability of the particulate preparations to catalyze the transfer of N-acetyl-d-glucosamine from uridine diphospho-N-acetyl-d-glucosamine to endogenous acceptor material was also tested. Transfer was optimal at around pH 9 and in the presence of 10 mm Mg2+, and it occurred largely into an unidentified lipid fraction. After equilibrium centrifugation of crude membrane material on sucrose gradients, a number of distinct fractions could be detected which would catalyze the transfer reaction. Uridine diphospho-d-glucose transferase activity showed a similar but not identical distribution along the gradient.
Full text
PDF

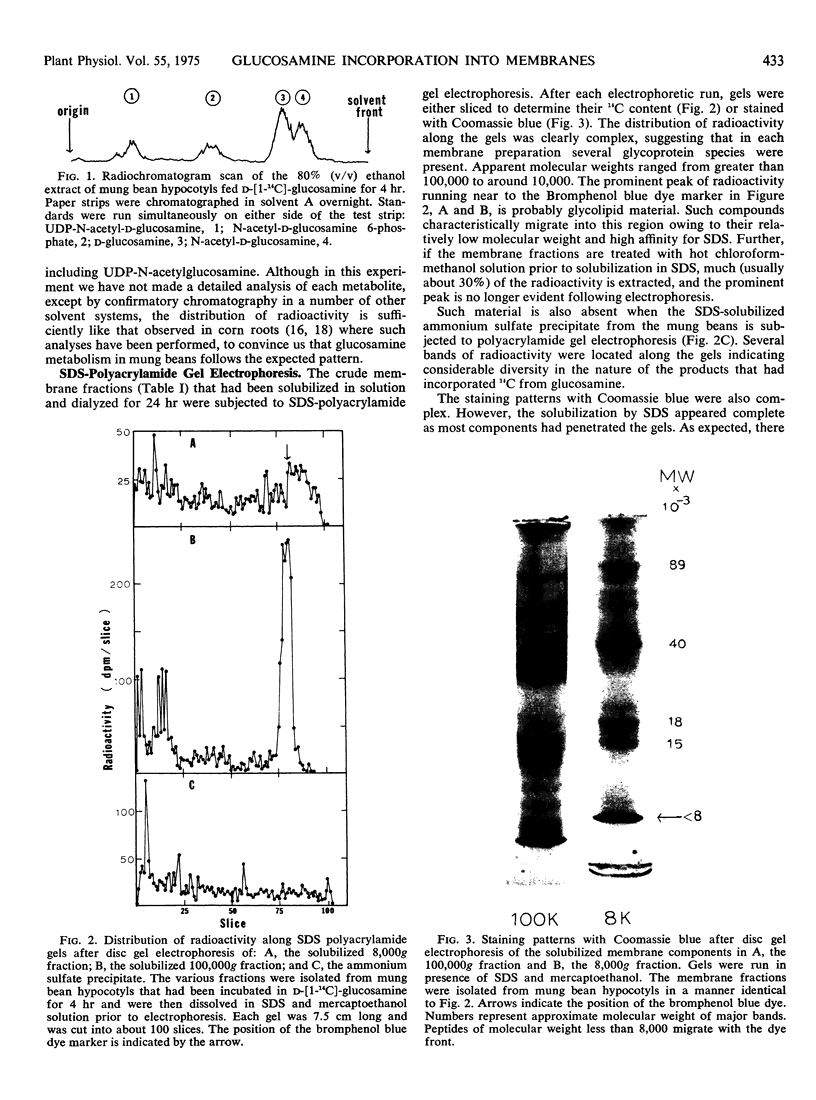
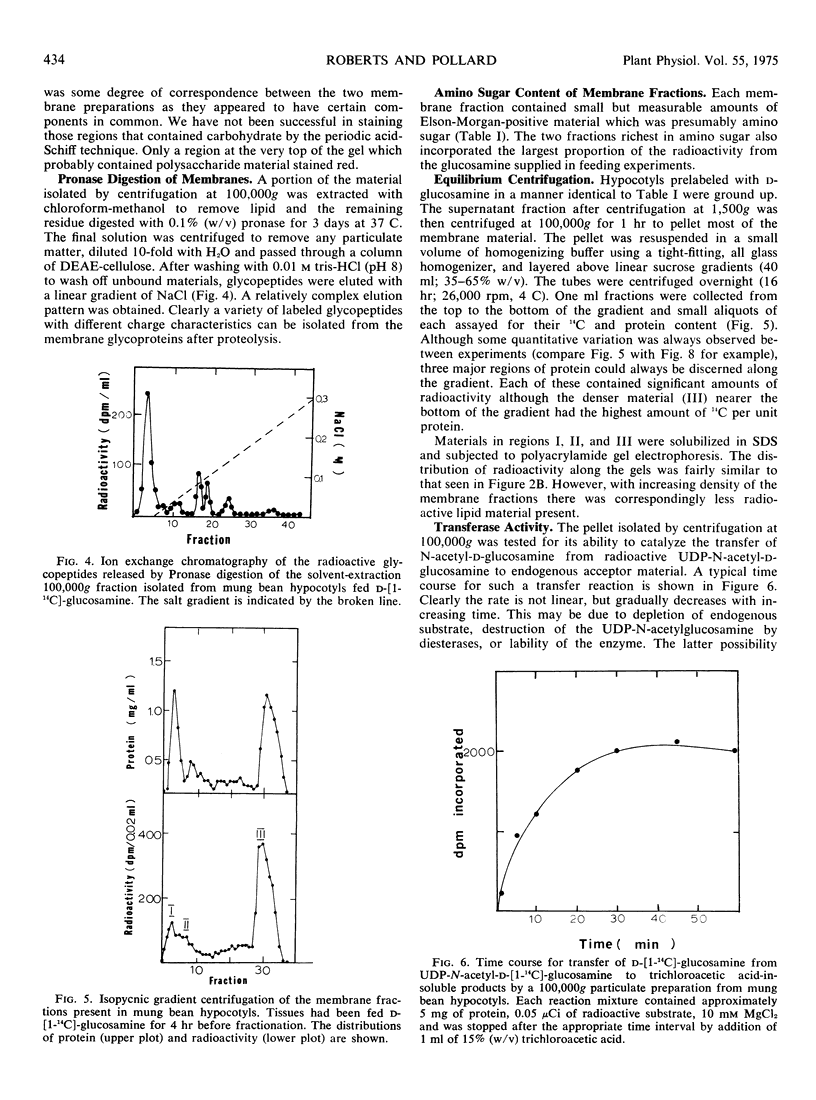
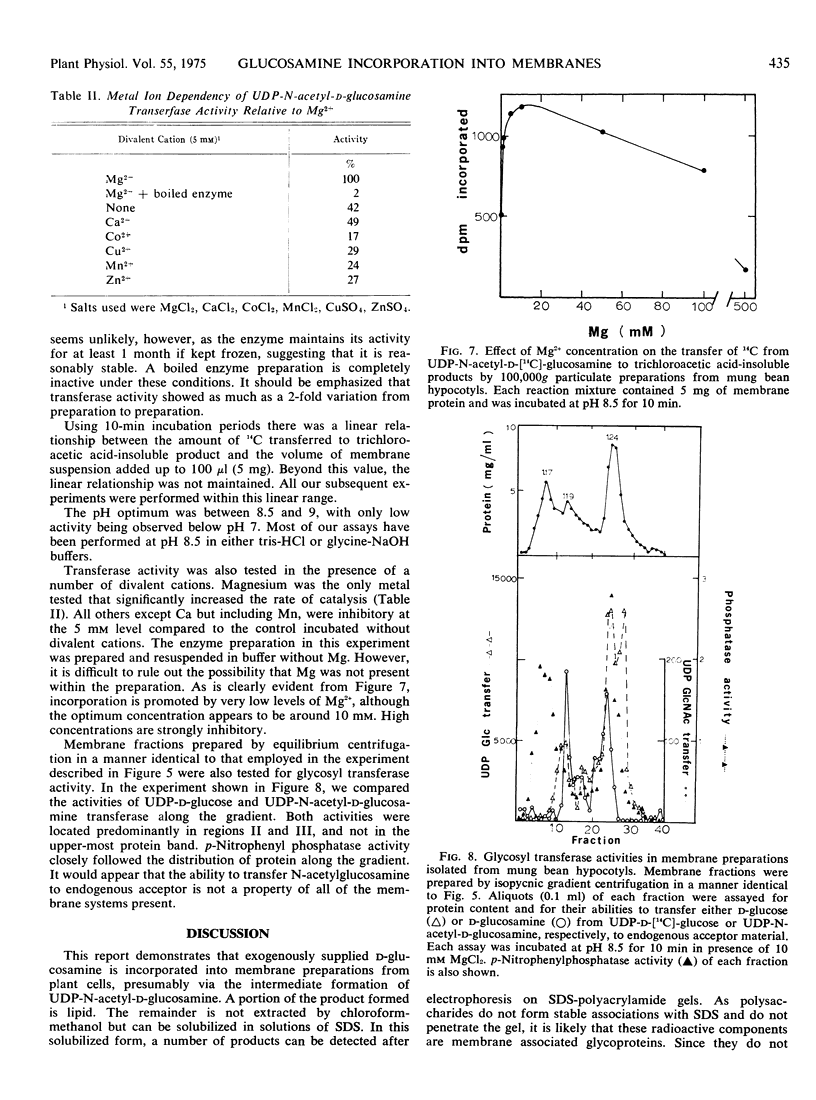

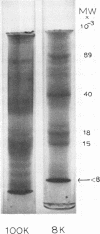
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam S. S., Hemming F. W. Betulaprenol phosphate as an acceptor of mannose from GDP-mannose in Phaseolus aureus preparations. FEBS Lett. 1971 Nov 15;19(1):60–62. doi: 10.1016/0014-5793(71)80604-x. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Hsu A. F., Heath E. C. The role of mannosyl-phosphoryl-dihydropolyisoprenol in the synthesis of mammalian glycoproteins. J Biol Chem. 1973 Aug 25;248(16):5693–5704. [PubMed] [Google Scholar]
- Caccam J. F., Jackson J. J., Eylar E. H. The biosynthesis of mannose-containing glycoproteins: a possible lipid intermediate. Biochem Biophys Res Commun. 1969 May 22;35(4):505–511. doi: 10.1016/0006-291x(69)90375-1. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Hou C. T., Umemura Y., Nakamura M., Funahashi S. Enzymatic synthesis of steryl glucoside by a particulate preparation from immature soybean seeds. J Biochem. 1967 Sep;62(3):389–391. [PubMed] [Google Scholar]
- Kauss H. A plant mannosyl-lipid acting in reversible transfer of mannose. FEBS Lett. 1969 Sep;5(1):81–84. doi: 10.1016/0014-5793(69)80298-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine R. A., Elbein A. D. Steryl glucosides in Phaseolus aureus. Use of gas-liquid chromatography and mass spectrometry for structural identification. Biochemistry. 1971 Jun 22;10(13):2547–2553. doi: 10.1021/bi00789a020. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Ongun A., Mudd J. B. The biosynthesis of steryl glucosides in plants. Plant Physiol. 1970 Mar;45(3):255–262. doi: 10.1104/pp.45.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Roberts R. M., Connor A. B., Cetorelli J. J. The formation of glycoproteins in tissues of higher plants. Specific labelling with D-(1- 14 C)glucosamine. Biochem J. 1971 Dec;125(4):999–1008. doi: 10.1042/bj1250999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M. The incorporation of D-glucosamine-14C into root tissues of higher plants. Plant Physiol. 1970 Mar;45(3):263–267. doi: 10.1104/pp.45.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]