The use of implantable cardioverter-defibrillators (ICDs) has been a major advancement in patients with ischemic cardiomyopathy with reduced ejection fraction <35%.1 Although the data supporting the use of ICDs are robust in patients with ischemic cardiomyopathy, limited randomized controlled clinical trial (RCT) data exist for similar benefit in patients with nonischemic cardiomyopathy (NICM).2 A prior meta-analysis that included both primary and secondary prevention ICD trials in 2004 by Desai et al2 demonstrated a 31% reduction in all-cause mortality with ICD use in patients with NICM. The data became the back bone of the current American College of Cardiology/American Heart Association guidelines for ICD implantation in patients with NICM.3 However, recently, the DANISH trial (Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure), which randomly assigned >1100 patients with NICM on optimal medical therapy (OMT) and cardiac resynchronization therapy (CRT) to ICD versus no ICD for primary prevention of sudden cardiac death, revealed no difference in all-cause mortality between the 2 groups at 5-year follow-up.4 Although the primary results of DANISH were neutral, the ICD group showed reduction in incidence of sudden cardiac death by half, and there was an interaction of survival benefit with ICD use in younger patients with NICM. In light of the recent data, we sought to update the meta-analysis of RCTs assessing the utility of ICD for primary prevention in patients with NICM.
We searched the MEDLINE, PUBMED, and SCOPUS databases using the following keywords: implantable cardioverter defibrillator, ICD, cardiac resynchronization therapy, CRT, heart failure, cardiomyopathy, and randomized controlled trials from their inception to October 20, 2016. After examining 773 relevant studies, we included 6 RCTs that assessed the efficacy of ICD for primary prevention in patients with NICM. Two authors (H.G., N.B.) independently extracted data from each individual study. We calculated risk ratios/hazard ratios and 95% confidence intervals (CIs) using the available event rate data from individual trials when these measures were not reported in the individual trial. The risk measures and 95% CIs were log transformed and combined using random-effects models. Data were analyzed for heterogeneity using the I2 statistic. We also performed a separate analysis of trials with/without CRT use to assess the differential effect of CRT on the efficacy of ICDs. Analyses were performed using Stata V14.1 statistical software.
We identified 6 RCTs enrolling 2970 patients with NICM to study the efficacy of ICDs for primary prevention. Pooled analysis of the 6 RCTs (including those with CRT defibrillator) demonstrated a statistically significant 23% risk reduction in all-cause mortality in favor of ICD therapy (hazard ratio, 0.77; 95% CI, 0.64–0.91) (Figure). In addition, when we performed separate analysis of trials that assessed ICD plus OMT versus OMT alone (after exclusion of trials that involved patients with CRT defibrillator), we found a statistically significant 24% reduction in all-cause mortality with ICD (hazard ratio, 0.76; 95% CI, 0.62–0.94). When we compared the 2 trials (COMPANION [Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure] and DANISH-CRT subgroup) with ICD plus CRT plus OMT versus CRT plus OMT alone, we found a trend toward benefit in terms of all-cause mortality in the ICD group, although it did not meet statistical significance (hazard ratio, 0.70; 95% CI, 0.39–1.26) (Figure).
Figure. Forest plot of all-cause mortality among patients with nonischemic cardiomyopathy randomly assigned to ICD and CRT-D versus optimal medical therapy for primary prevention of sudden cardiac death.
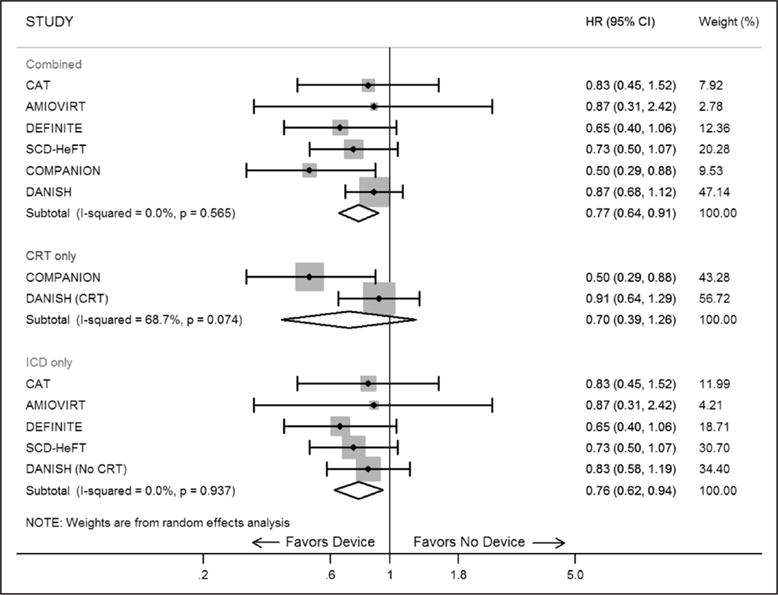
Black marker represents hazard ratio estimate for the study. The gray box around the marker corresponds to the weight of study in the random-effects model. The diamond-shaped box is the summary estimate from the random-effects model. The horizontal black lines denote 95% confidence intervals of hazard ratio of each study. The black vertical line is the line of no effect difference. AMIOVIRT indicates Amiodarone versus Implantable Cardioverter-Defibrillator Randomized Trial; CAT, cardiomyopathy trial; CI, confidence interval; COMPANION, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy defibrillator; DANISH, Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure; DEFINITE, Defibrillator in Nonischemic Cardiomyopathy Treatment Evaluation; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; and SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
Newly diagnosed NICM is a heterogeneous group of patients with heart failure, and prior studies demonstrate that, despite OMT, a subset of these patients remains at risk of sudden cardiac death.5 Our updated meta-analysis combining all available RCTs, including the recently published DANISH trial, demonstrates that ICDs reduce all-cause mortality by 23% in patients with NICM. This incremental reduction of all-cause mortality with ICD is substantial and provides support to the existing American College of Cardiology/American Heart Association guidelines until we acquire additional data.3 In addition, despite the individual subgroup analysis of COMPANION and DANISH trials demonstrating no incremental benefit of ICD in patients with CRT, when we combined the 2 trials, we found that ICDs may still reduce all-cause mortality in patients who are also candidates of CRT therapy, although the results did not meet statistical significance. It may be plausible that, because of the high use of CRT in the DANISH trial (60% in each arm), ICD failed to demonstrate statistically significant effect on all-cause mortality in patients with NICM.
Taken collectively, despite the neutral results of the recently published DANISH trial, our meta-analysis of all the published RCTs to date demonstrates significant clinical benefit on all-cause mortality in favor of ICD use for primary prevention in patients with NICM. Improvement in risk prediction models can help overcome the traditional reliance on ejection fraction for risk stratification of sudden cardiac death in NICM patients. Furthermore, adequately powered randomized studies are needed before recommending any change in existing guidelines, and clinical judgment should prevail while assessing risk of sudden cardiac death in NICM patients with reduced ejection fraction.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by the Walter B. Frommeyer Junior Fellowship in Investigative Medicine that was awarded to Dr Arora.
Footnotes
DISCLOSURES
None.
Circulation is available at http://circ.ahajournals.org.
Contributor Information
Harsh Golwala, Brigham and Women’s Heart and Vascular Institute, Boston, MA.
Navkaranbir Singh Bajaj, Brigham and Women’s Heart and Vascular Institute, Boston, MA.
Garima Arora, Division of Cardiovascular Disease, Department of Medicine, University of Alabama, Birmingham.
Pankaj Arora, Division of Cardiovascular Disease, Department of Medicine, University of Alabama, Birmingham.
References
- 1.Nanthakumar K, Epstein AE, Kay GN, Plumb VJ, Lee DS. Prophylactic implantable cardioverter-defibrillator therapy in patients with left ventricular systolic dysfunction: a pooled analysis of 10 primary prevention trials. J Am Coll Cardiol. 2004;44:2166–2172. doi: 10.1016/j.jacc.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–2879. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b.. [DOI] [PubMed] [Google Scholar]
- 4.Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S, DANISH Investigators Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029.. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]


