Abstract
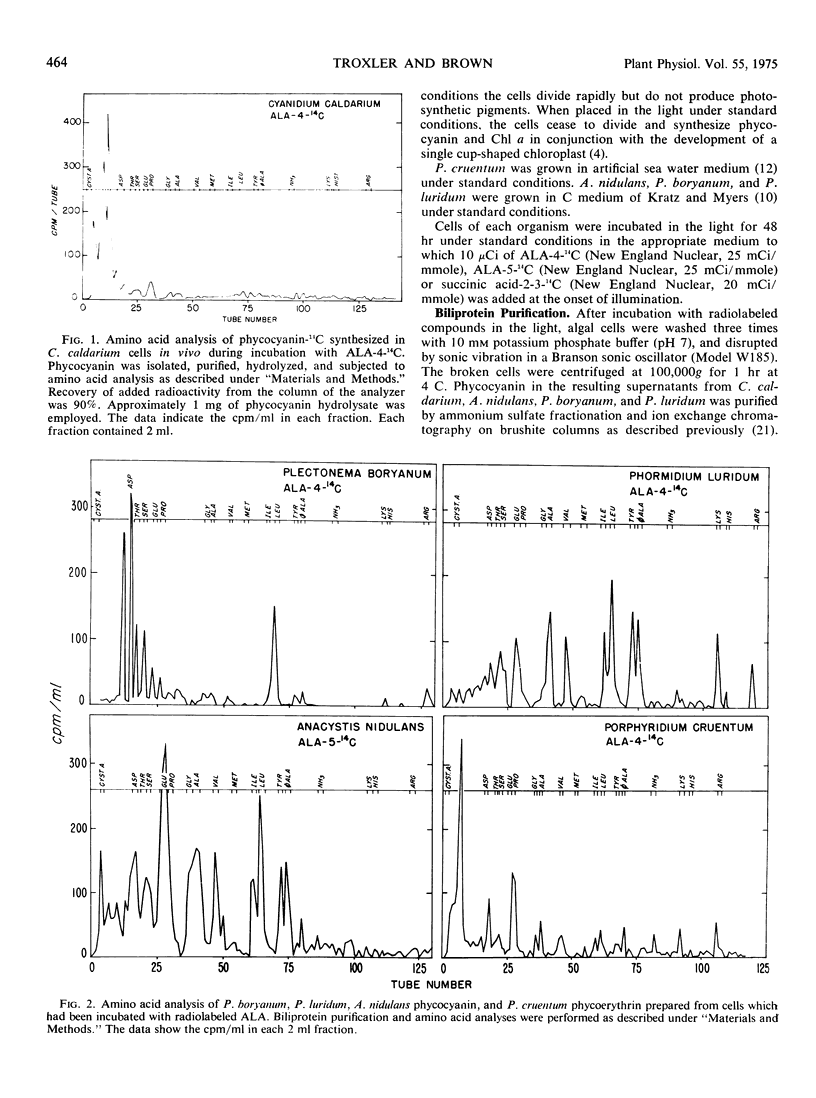
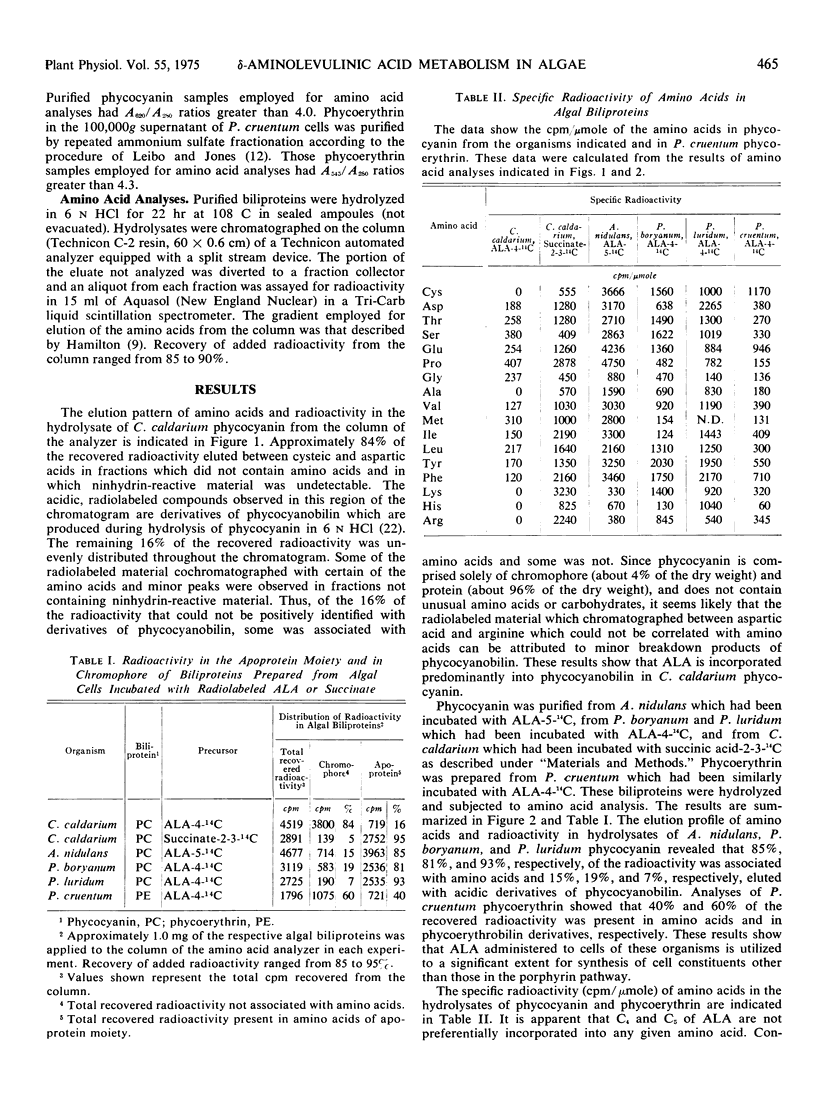
δ-Aminolevulinic acid was incorporated in vivo into C-phycocyanin and B-phycoerythrin in two species of the Rhodophyta (Cyanidium caldarium, Porphyridium cruentum) and three species of the Cyanophyta (Anacystis nidulans, Plectonema boryanum, Phormidium luridum). Amino acid analysis of phycocyanin-14C from C. caldarium cells which had been incubated with δ-aminolevulinate-4-14C showed that 84% of the radioactivity incorporated was present in the phycocyanobilin chromophore and less than 16% of the radioactivity cochromatographed with amino acids. These results indicate that δ-aminolevulinate is utilized predominantly via the porphyrin pathway in C. caldarium. Conversely, analysis of phycocyanin-14C prepared from cells of A. nidulans, P. boryanum, and P. luridum which had been incubated with radiolabeled δ-aminolevulinate demonstrated that 85%, 81%, and 93%, respectively, of the radioactivity incorporated cochromatographed with amino acids. The ratio of incorporated radioactivity in amino acids and phycoerythrobilin was 40:60 in P. cruentum phycoerythrin obtained from cells which had been incubated with δ-aminolevulinate-4-14C. Succinate-2-3-14C appeared to be as good a carbon source of amino acids as did C4 and C5 of δ-aminolevulinate. These data demonstrate a major alternate route (other than the porphyrin pathway) of δ-aminolevulinate metabolism in red and blue-green algae. The factors responsible for the extent to which δ-aminolevulinate is utilized for synthesis of porphyrins and their derivatives and routes of δ-aminolevulinate catabolism in the organisms employed are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Cole W. J., Chapman D. J., Siegelman H. W. The structure and properties of phycocyanobilin and related bilatrienes. Biochemistry. 1968 Aug;7(8):2929–2935. doi: 10.1021/bi00848a033. [DOI] [PubMed] [Google Scholar]
- Crespi H. L., Smith U., Katz J. J. Phycocyanobilin. Structure and exchange studies by nuclear magnetic resonance and its mode of attachment in phycocyanin. A model for phytochrome. Biochemistry. 1968 Jun;7(6):2232–2242. doi: 10.1021/bi00846a028. [DOI] [PubMed] [Google Scholar]
- Gassman M., Pluscec J., Bogorad L. delta-Aminolevulinic Acid Transaminase in Chlorella vulgaris. Plant Physiol. 1968 Sep;43(9):1411–1414. doi: 10.1104/pp.43.9.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaw S. A., Callahan E. W., Jr, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970 May;49(5):914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger W., O Carra P., O hEocha C. Structure of phycoerythrobilin and phycocyanobilin. Nature. 1967 Sep 30;215(5109):1477–1478. doi: 10.1038/2151477a0. [DOI] [PubMed] [Google Scholar]
- SHEMIN D., RUSSELL C. S., ABRAMSKY T. The succinate-glycine cycle. I. The mechanism of pyrrole synthesis. J Biol Chem. 1955 Aug;215(2):613–626. [PubMed] [Google Scholar]
- Shigesada K., Ebisuno T., Katsuki H. 5-Amino-4-hydroxyvaleric acid: a new intermediate in 5-aminolevulinate metabolism of Rhodospirillum rubrum. Biochem Biophys Res Commun. 1970 Apr 8;39(1):135–141. doi: 10.1016/0006-291x(70)90768-0. [DOI] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Brown A. Biosynthesis of phycocyanin in vivo. Biochim Biophys Acta. 1970 Sep 22;215(3):503–511. doi: 10.1016/0304-4165(70)90100-5. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Dokos J. M. Formation of carbon monoxide and bile pigment in red and blue-green algae. Plant Physiol. 1973 Jan;51(1):72–75. doi: 10.1104/pp.51.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Lester R. Biosynthesis of phycocyanobilin. Biochemistry. 1967 Dec;6(12):3840–3846. doi: 10.1021/bi00864a030. [DOI] [PubMed] [Google Scholar]
- Troxler R. F. Synthesis of bile pigments in plants. Formation of carbon monoxide and phycocyanobilin in wild-type and mutant strains of the alga, Cyanidium caldarium. Biochemistry. 1972 Nov 7;11(23):4235–4242. doi: 10.1021/bi00773a007. [DOI] [PubMed] [Google Scholar]


