Abstract
Morphine actions involve the dopamine (DA) D1 and D3 receptor systems (D1R and D3R), and the responses to morphine change with age. We here explored in differently aged wild type (WT) and D3R knockout mice (D3KO) the interactions of the D1R/D3R systems with morphine in vivo at three different times of the animals’ lifespan (2 months, 1 year, and 2 years). We found that: 1) thermal pain withdrawal reflexes follow an aging-associated phenotype, with relatively longer latencies at 2 months and shorter latencies at 1 year, 2) over the same age range, a dysfunction of the D3R subtype decreases reflex latencies more than aging alone, 3) morphine altered reflex responses in a dose-dependent manner in WT animals and changed at its higher dose the phenotype of the D3KO animals from a morphine-resistant state to a morphine-responsive state, 4) block of D1R function had an aging-dependent effect on thermal withdrawal latencies in control animals that, in old animals, was stronger than that of low-dose morphine. Lastly, 5) block of D1R function in young D3KO animals mimicked the behavioral phenotype observed in the aged WT. Our proof-of-concept data from the rodent animal model suggest that, with age, block of D1R function may be considered as an alternative to the use of morphine, to modulate the response to painful stimuli.
Keywords: Morphine tolerance, aging, chronic pain, dopamine receptor interactions, animal model, spinal cord
Introduction
Pain is “the most common reason Americans access the health care system” (NIH, 2010), and an estimated 15% of the US population suffer from a chronic pain condition (Von Korff et al., 2005; Gironda et al., 2006; Gaskin and Richard, 2012). Opiate analgesics such as morphine are the classical first line treatment for strong and persistent pain, but their effectiveness in long-term treatment is limited by the emergence of tolerance (i.e. (Colpaert, 2002; Taylor and Saper, 2004; DuPen et al., 2007; Bekhit, 2010; Joseph et al., 2010)). This tolerance to the drug is thought to arise over time and involve a dysfunction of μ-opioid receptor (MOR)- and dopamine (DA) receptor-mediated second messenger pathways in the brain (Suzuki et al., 2001; Schmidt et al., 2002; Fazli-Tabaei et al., 2006; Le Marec et al., 2011). Further, there is evidence that DA affects the change of pain-related activity induced by morphine (Zhang et al., 2012), and that morphine in turn has a prolonged effect on DA neuron activities (Zhang et al., 2008). However, under this tenet the role of the spinal cord remains entirely overlooked. Both MOR and DA receptors are present in the spinal cord (Mansour et al., 1994; Ji et al., 1995; Ray and Wadhwa, 1999; Abbadie et al., 2001; Abbadie et al., 2002; Ray and Wadhwa, 2004; Zhang et al., 2006; Zhao et al., 2007; Zhu et al., 2007, 2008; Barraud et al., 2010), and excitatory dopamine D1 receptors (D1Rs) can form hetero-dimers with inhibitory D3 receptors (D3Rs) (Surmeier et al., 1996; Fiorentini et al., 2008; Maggio et al., 2009; Missale et al., 2010; Cruz-Trujillo et al., 2013), with both playing a role in opioid tolerance (Lin et al., 1996; Cook et al., 2000; Fazli-Tabaei et al., 2006).
Chronic pain becomes more prevalent with age (Saastamoinen et al., 2005), when up to 50–60% of the population 70 years or older suffers from the symptoms (McCarthy et al., 2009), possibly mediated in part by an decrease in pain thresholds and a decrease in pain tolerance (Bicket and Mao, 2015), or as a result of pathological conditions that have developed over time (Jones et al., 2016). In parallel with this aging-related increase in pain prevalence, morphine treatment is often only poorly tolerated in the elderly (Abdulla et al., 2013), which may be in part due to an age-related decline in kidney function that may make it more difficult for older patients to eliminate opioids and their metabolites (Goldstein and Morrison, 2005). Thus different treatment approaches and alternative options may be required to improve treatment of chronic pain in the elderly.
In the rodent animal model, we have recently shown that normal (normative) aging is associated with a strong increase of excitatory D1R expression levels in both striatum and spinal cord, whereas inhibitory D3R levels remain stable (Keeler et al., 2016), suggesting an upregulation of the overall excitability of the underlying neural circuits. We have further shown that a dysfunction of the D3R in rodents is associated with a lack of responsiveness to low levels of morphine both in vivo and in the isolated spinal cord in vitro (Brewer et al., 2014).
Using behavioral and pharmacological approaches in wild type (WT) and dopamine D3 receptor knockout mice (D3KO) across their life span, we here demonstrate that a dysfunction of the D3R is associated with a morphine-tolerant phenotype that can be rescued by block of D1 receptor (D1R) function. We further show that young, 2 month-old D3KO mimic the behavioral pain phenotype of aged, 2 year-old WT, and we present evidence that block of D1R function may be an alternative to opioid treatment of pain with age. Together with our recent findings on an altered D1R/D3R ratio with normal aging, these data suggest that, with increasing age, the dopamine D1R/D3R system plays an important role in the modulation of morphine responsiveness.
Methods
Animals
All experimental procedures were approved by the East Carolina University Institutional Animal Care and Use Committee and were fully compliant with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 80–23). All efforts were made to minimize the number of animals used. In vivo behavioral testing was performed on male dopamine D3 receptor knockout mice (D3KO; strain B6.129S4-Drd3tm1dac/J (stock # 002958, Jackson Laboratory, Bar Harbor, ME)) and their appropriate male wild-type (WT) controls (C57BL/6). WT animals were purchased at 2 months of age and as retired breeders (~12 months of age). Young animals were kept in groups of 2–4 animals per cage and tested immediately. The older animals were kept separated to prevent fighting and were tested at 1 year and 2 years of age. We did not keep track of the responses of the individual animals over time. D3KO animals were bred and raised in-house. Prior to the experiments, animals were housed in standard cages with food and water available ad libitum with a 12 hr light/dark cycle at room temperature of ~ 20° C.
Behavioral Assessments
Thermal withdrawal latencies (Hargreaves’ method) were obtained from WT and D3KO in each cohort by using the IITC plantar analgesia meter (IITC Series 8, IITC Inc., Woodland Hills, CA). Experiments were performed between 9 am and 1 pm, to minimize the potential role of a circadian influence. The week before their first testing, animals were acclimated 4 to 5 times to the experimental room (maintained at 20–22° C and an average light intensity of 50–100 lux) and the Hargreaves’ system, by placing them individually into the Plexiglas cubicles for an average of 2 hours. Following this acclimation period, the effects of vehicle injections (0.9% NaCl, i.p., ~ 90–120 μl per animal) were tested in week 1 of the experimental setting. Animals were tested 5 times per session, with resting periods for each individual animal between tests of 5 to 10 min. The light was positioned under the plantar surface of the right hindlimb, and the thermal withdrawal latency reflex, defined as the time between the onset of the high intensity light beam and the removal of the targeted foot from the light path, was measured and recorded by the experimenter. The instrument was set to provide a ceiling temperature of 50–52° C on the glass pane after ~ 10 s, and the stimulation cut-off for each test was set to 30 s test duration, to prevent the possibility of a heat-induced injury. Once initiated, recording sessions for all 5 trials lasted no longer than 60 to 90 min for all animals tested that day. We subsequently compared all drug effects against the data obtained after the respective vehicle injections in each animal cohort. After vehicle assessments, drug treatments began the following week. Animals were injected with one of the drugs (dopaminergics and/or morphine) and allowed to recover in the Hargreaves cubicles at low-light conditions for 1 hr before testing. Each drug test was separated from the next drug treatment by an at least 3-day recovery period, to minimize any potential drug interactions possibly skewing the latency measurements. Animals were sacrificed at the end of the testing paradigms.
Drugs
We used the following compounds to test their effects on thermal pain withdrawal latencies: 0.9 % NaCl (vehicle control (sham), inj. USP, Hospira, Lake Forest, IL); morphine (Morphine sulfate salt pentahydrate, M8777, Sigma-Aldrich, St. Louis, MO) at 2 mg/kg, or 5 mg/kg; and the D1R antagonist, SCH 39166 (SCH 39166 hydrobromide, Tocris, Minneapolis, MN) at 0.1 mg/kg. Both morphine doses and SCH 39166 were tested both individually and in combinations of SCH 39166 + 2 mg/kg morphine, and SCH 39166 + 5 mg/kg morphine, respectively. All drugs were administered via intraperitoneal (i.p.) injections and all treatments, including vehicle controls, were administered to all the mice in each age-dependent cohort and genetic strain. Each age group of animals (WT and D3KO) received multiple treatments, which in turn were spaced over subsequent weeks, to avoid the possibility of a carry-over drug effect. We also tested reflex responses under sham conditions prior and after the drug treatments and found no significant differences between these recording sessions (data not shown).
Statistical Analysis
Following the experiments, acquired data were transferred and stored in Excel format on a dedicated 4 TB data storage server (Netgear ReadyNas NV+), then analyzed and plotted offline with SigmaPlot (version 11, Systat, San Jose, CA). For statistical comparisons between multiple age groups, we employed parametric or non-parametric comparisons as appropriate (One-Way ANOVA or ANOVA on Ranks) with relevant post-hoc comparisons (Holms-Sidak, Dunn’s); t-tests were used for comparison between paired sets of data (treatment against respective control vehicle treatment). Significance levels were set at p<0.05.
Results
Aging-related changes in thermal withdrawal latencies
We first tested if aging alone led to any changes in thermal withdrawal latencies in WT or D3KO animals that would mimic the trend of an increased sensitivity to pain with age observed in the clinic. We found that, in WT animals (Figure 1A), withdrawal latencies to a thermal stimulus decreased from 11.05 ± 0.55 s at 2 months of age (n=8) to 8.45 ± 0.24 s at 1 year (n=7), but then increased back to 10.51 ± 0.15 s at 2 years (n=8). A One-Way ANOVA coupled to a Holm-Sidak posthoc test revealed that the difference between 2 months and 1 year, and 1 year and 2 years was significant (p=0.002, power at p=0.05: 0.993). The decrease in thermal withdrawal latencies from 2 months to 1 year suggests an increase in sensitivity in 1 year-old animals that, towards the end of the lifespan, returns to values similar as in the 2 month-old animals.
Figure 1.
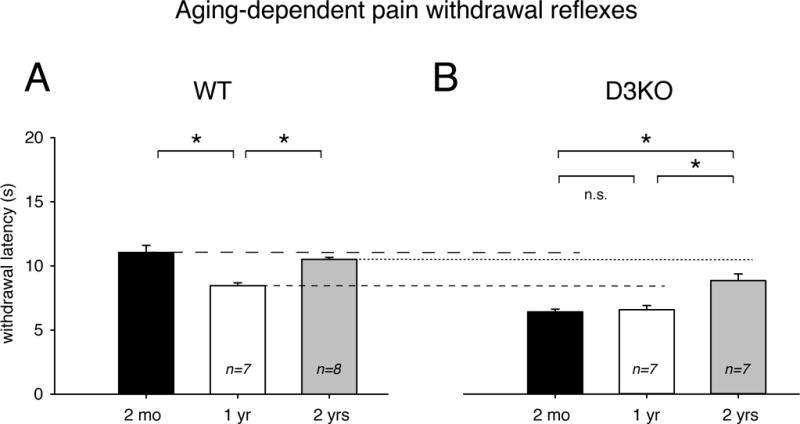
Aging-associated changes in thermal pain withdrawal latencies after vehicle injection. A. Wild-type animals. Normal aging is associated with a decrease in withdrawal latencies from 2 months (black bar) to 1 year of age (white bar), which then increases again at 2 years of age (grey bar). B. D3KO animals. Young (2 months, black bars) and middle-aged (1 year, white bar) animals do not differ in their thermal withdrawal latencies, but as in WT, there is an increase in withdrawal latencies at 2 years of age (grey bar). Dashed / dotted lines in B refer to average values of the corresponding age groups in A. Abbreviations: 2 mo: 2 months; 1 yr: 1 year; 2 yrs: 2 years, WT: wild-type; D3KO: D3 receptor knockout. *: denotes significant difference between groups.
In D3KO mice (Figure 1B), we observed that withdrawal latencies at 2 months of age (6.37 ± 0.22 s, n=5) were similar to those at 1 year of age (6.53 ± 0.33 s, n=7), but, as in WT, increased from 1 to 2 years (8.81 ± 0.52 s, n=7). A One-Way ANOVA coupled to a Holm-Sidak posthoc test revealed that the difference between 2 months and 2 years, and 1 year and 2 years was significant (p<0.001, power: 0.982). T-test comparisons between age-matched WT and D3KO cohorts revealed that, at each age, D3KO reacted consistently faster to the thermal stimuli than their WT counterparts, as indicated by the dotted / dashed lines between Figure 1A and 1B (2 months: p=0.002, power: 1; 1 year: p<0.001, power: 0.993; 2 years: p=0.009, power: 0.85). This confirms earlier findings obtained in young D3KO (Keeler et al., 2012; Brewer et al., 2014) and suggests that D3KOs consistently have a lower threshold for thermal pain over age-matched WT.
Effects of low and high dose of morphine on thermal withdrawal latencies
We next tested if morphine, a common treatment for chronic pain, had differential effects with aging (Figure 2). We first used a low dose of 2 mg/kg (single i.p. injection) and tested withdrawal latencies 1 hour after the injections. This low dose of morphine provides acute analgesia in naïve animals (Yu et al., 1997; Saghaei et al., 2012). All animals were naïve to morphine, i.e. had never been exposed to the drug before. We found that in WT, a single low-dose morphine injection significantly increased withdrawal latencies at each age over its respective vehicle control (Figure 2A). Specifically, at 2 months, latencies rose to 117 ± 4.01 % of control (n=8, p=0.017, t-test, power: 0.651), in 1 year-old animals latencies were at 137 ± 7.03 % of control (n=7, p<0.001, t-test, power: 0.996), and in the 2 year-old animals latencies were at 112.8 ± 2.3 % of control (n=8, p=0.003, t-test, power: 0.993). We did not detect any significant differences in the relative increase of withdrawal latencies induced by the injection of 2 mg/kg morphine between the different age groups (p=0.099, One-Way ANOVA).
Figure 2.
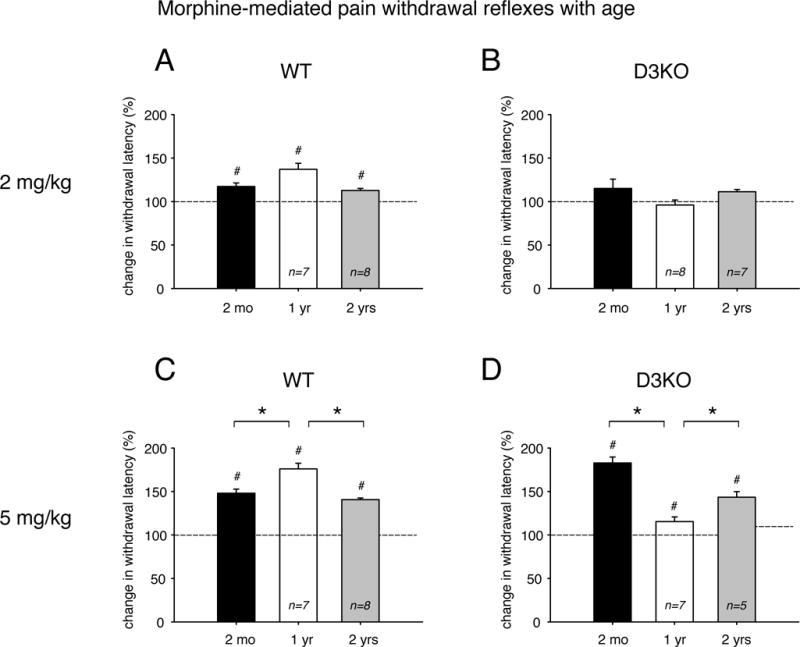
Effects of low and high doses of morphine on thermal pain withdrawal latencies in differently aged WT and D3KO. Displayed data represent changes in withdrawal latency compared to respective control vehicle injections, with dashed lines representing relative vehicle control levels. A. Effects of low morphine (2 mg/kg) in WT. Treatment with low morphine significantly increased thermal withdrawal latencies at all ages, and there was no significant difference between age groups. B. Effects of low morphine (2 mg/kg) in D3KO. Treatment with low morphine had no significant effect at all age, and there was no significant difference between the groups. C. Effects of high morphine (5 mg/kg) in WT. Treatment with high morphine significantly increased thermal withdrawal latencies at all ages, and this effect was significantly increased at 1 year of age over the effects at 2 months and 2 years of age. D. Effects of high morphine (5 mg/kg) in D3KO. Similar to WT, treatment with high morphine significantly increased thermal withdrawal latencies at all ages, but this effect was significantly increased at 2 months of age over the effects at 1 year and 2 years of age. Abbreviations and colors as in Figure 1. *: denotes significant difference between groups; #: denotes significant difference from vehicle control (dashed line).
In contrast, in D3KO, injection of 2 mg/kg morphine did not significantly alter thermal withdrawal latencies, regardless of the age of the animals (Figure 2B). Specifically, at 2 months, latencies were at 115.13 ± 10.73 % of control (n=5, p=0.26, t-test), at 1 year they were at 98.87 ± 6.85 % (n=8, p=0.21, t-test), and at 2 years they were at 111.31 ± 2.7 % of control (n=7, p=0.11, t-test).
To test if the lack of responsiveness to low-dose morphine in D3KO across the life span represents a morphine-tolerant phenotype, we repeated the experiment with 5mg/kg morphine, a dose that also provides acute analgesia in naïve animals (Bruins Slot et al., 2002). At the higher dose of 5 mg/kg, the effects of morphine became more differentiated in WT and D3KO. In WT (Figure 2C), withdrawal latencies in 2 month-old animals rose to 148.41 ± 4.71 % of control (n=8), at 1 year they were at 176.03 ± 6.58 % (n=7), and at 2 years they were at 140 ± 1.9 % (n=8). At each age, these increases were significantly different over vehicle control (p<0.001, t-tests, power: 1, for all three comparisons, respectively), and a One-Way ANOVA coupled to a Dunn’s Method posthoc test revealed significant differences between 2 months and 1 year, and 1 year and 2 years (p=0.003, power: 0.998), but not between 2 months and 2 years.
Similar to WT, and unlike to the low-dose effect of the drug, 5 mg/kg morphine was effective in extending the withdrawal latencies in D3KO for each age group (Figure 2D). In 2 month-old animals, thermal withdrawal latencies rose to 182.87 ± 6.8 % of control (n=5), at 1 year they were at 115.42 ± 5.4 % (n=7), and at 2 years of age they were at 143.42 ± 6.5 % (n=5). At each age, withdrawal latencies were significantly increased over their appropriate vehicle controls (p<0.001, power: 1, 1, 0.994, respectively). Furthermore, a One-Way ANOVA revealed significant differences with age (p<0.001), with the Holm-Sidak comparisons reaching significance between all comparisons (2 months vs. 1 year vs. 2 years, p<0.001, power: 1). Of note that, in D3KO, the strongest effect of the higher dose of morphine was observed in the young, 2 month-old animal, unlike in WT, where morphine had its biggest impact at 1 year of age, independent of the dose.
Together, these data suggest that morphine exerts a dose-dependent effect in WT, and that it can recruit at the higher dose a modulatory effect in a D3KO animal, even if the animals are non-responsive to morphine at the lower dose.
Differential effects of D1 receptor block on thermal withdrawal latencies
We recently reported that D3KO show an increased D1R protein expression in the spinal cord (Brewer et al., 2014) and that D1R protein levels increase with age in WT (Keeler et al., 2016). We, therefore, tested if blocking D1R function using a single i.p. injection of the D1R antagonist, SCH 39166 (0.1 mg/kg) would modify thermal pain withdrawal latencies differently with aging (Figure 3). We found that, in WT (Figure 3A), withdrawal latencies were not altered in 2 month-old animals (100.93 ± 4.63 % of control, p=0.89, n=8), but they increased significantly over their respective vehicle controls in 1 year and 2 year old animals to 117.49 ± 3.4 % (p=0.015, t-test, n=7, power: 0.953) and 129.1 ± 7.5 % (p=0.014, t-test, n=8, power: 0.964). A One-Way ANOVA analysis coupled to Holm-Sidak multiple comparisons further revealed that the results from 2 month- and 2 year-old animals were significantly different to each other (p=0.005, power: 0.836).
Figure 3.
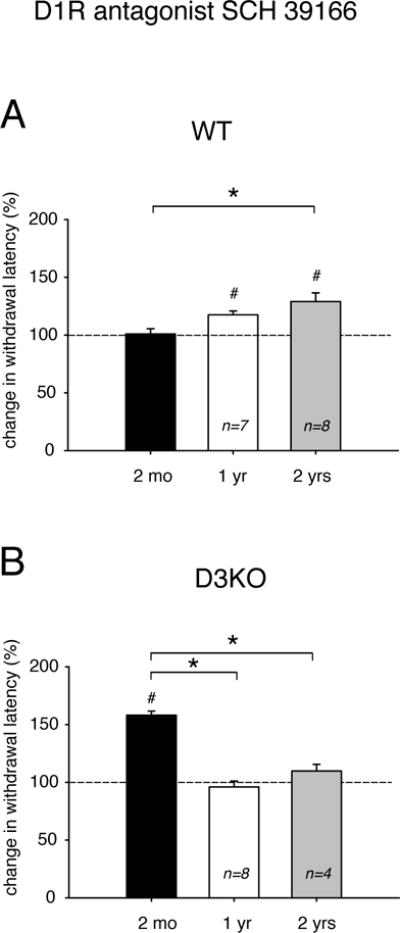
Effects of the D1R antagonist, SCH 39166, on thermal pain withdrawal latencies in differently aged WT and D3KO. Displayed data represent changes in withdrawal latency compared to respective control vehicle injections, with dashed lines representing relative vehicle control levels. A. WT animals. The D3R antagonist SCH 39166 had no modulatory effect in 2 month-old animals, but increased withdrawal latencies significantly at 1 year and 2 years. The difference between 2 months and 2 years is significant. B. D3KO animals. Unlike WT, SCH 39166 strongly and significantly increased thermal pain withdrawal latencies in 2 month-old animals, but had no effect at 1 year or 2 years. The difference between 2 months and 1 year, and 2 months and 2 years is significant. Abbreviations as in Figure 1. *: denotes significant difference between groups; #: denotes significant difference from vehicle control (dashed line).
Conversely, in D3KO (Figure 3B), we found that application of SCH 39166 had a very strong effect at 2 months of age, when it significantly increased withdrawal latencies to 158.13 ± 3.6 % of control (p<0.001, n=5, power: 1). However, the D1R antagonist had no effect over the age-matched vehicle controls at 1 or 2 years of age (1 year: 96.05 ± 5.09 %, p=0.59, n=8; 2 years: 109.79 ± 5.76 %, p=0.28, n=5). The One-Way ANOVA analysis coupled to Holm-Sidak multiple comparisons further revealed that the data of 2 month-old animals were significantly different from those in 1 year- and 2 year-old animals (p<0.001, power: 1), while there was no difference between 1 and 2 years. These data show that blocking of D1R function has differential, aging-associated effects on thermal pain withdrawal latencies in WT and D3KO.
Differential effects of combined morphine treatment and D1 receptor block on thermal withdrawal latencies
Since morphine and D3R actions are mediated via inhibitory Gi second messenger pathways, while excitatory Gs pathways control D1R actions, we next addressed the question of whether blocking D1R function in the presence of low or high morphine doses would strengthen the opioid-induced lengthening of the withdrawal response (Figure 4). We found that, in WT animals, the combined treatment of low morphine (2 mg/kg) and SCH 39166 (0.1 mg/kg) led to an aging-associated increase in thermal withdrawal latencies from 125 ± 3.5 % of control at 2 months (p<0.001, t-test, power: 0.973, n=8), to 138.02 ± 4.44 % at 1 year (p<0.001, t-test, power: 1, n=7), to 165.34 ± 9.3 % at 2 years (p<0.001, t-test, power: 1, n=7, Figure 4A); and a One-Way ANOVA revealed significant differences between 2 month- and 2 year-old animals (p=0.004, power: 0.978). Note that the low-morphine / D1R block combination increased the withdrawal latencies in the 2 year-old animals more than each of the individual treatments alone (cf. Figure 2A, Figure 4A). In contrast, in D3KO (Figure 4B), the same combination treatment led to an opposing effect with age; we observed a strong and significant increase of withdrawal latencies in the 2 month-old animals, but no effect in the older cohorts. Specifically, latencies at 2 months were 135.25 ± 6.7 % of control (p=0.005, t-test, power: 0.903, n=5), at 1 year they were at 109.89 ± 3.38 % (p=0.12, n=8), and at 2 years they were at 101.47 ± 3.9 % (p=0.87, n=4). The differences between 2 months and 1 year, and 2 months and 2 years were significant (p<0.001, One-Way ANOVA, power: 0.979).
Figure 4.
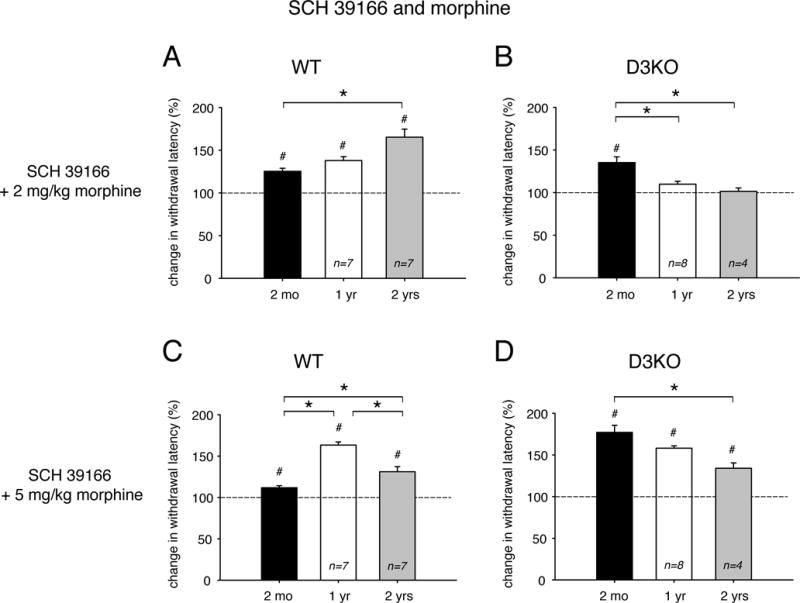
Effects of combinatory treatments of the dopamine D1R antagonist, SCH 39166, and low (2 mg/kg) or high (5 mg/kg) morphine on thermal pain withdrawal latencies in differently aged WT and D3KO. Displayed data represent changes in withdrawal latency compared to respective control vehicle injections, with dashed lines representing relative vehicle control levels. A. WT animals, SCH 39166 + low morphine. The combination of SCH 39166 and low morphine was effective across all ages in significantly increasing withdrawal latencies over the respective controls, and the increase at 2 years of age was significantly enhanced over the data at 2 months. B. D3KO animals, SCH 39166 + low morphine. In D3KO, the combination of SCH 39166 and low morphine was effective only at 2 months, but not at 1 year or 2 years. The increase at 2 months of age was significantly enhanced over the data at both 1 year and 2 years. C. WT animals, SCH 39166 + high morphine. Similar to the combination of SCH 39166 and low morphine, SCH 39166 + high morphine was effective across all ages in significantly increasing withdrawal latencies over the respective controls. Further, the increase at 1 year was significantly enhanced over that at 2 years, which in turn was larger than that at 2 months. D. D3KO animals, SCH 39166 + high morphine. In D3KO, the combination of SCH 39166 and high morphine was effective across all ages in significantly increasing withdrawal latencies over the respective controls, and there was a significant difference between 2 months, 1 year, and 2 years. Abbreviations as in Figure 1. *: denotes significant difference between groups; #: denotes significant difference from vehicle control (dashed line).
Increasing morphine to 5 mg/kg in the presence of the D1R antagonist modified thermal withdrawal responses in an aging-related manner in both WT and D3KO. In WT (Figure 4C), the high morphine/SCH 39166 treatments had no significant effect on withdrawal latencies at 2 months (111.83 ± 2.5 % of control, p=0.1, t-test, n=8), but increased significantly to 163.32 ± 3.8 % at 1 year (p<0.001, power: 1, n=7), and 131.15 ± 6.2 % at 2 years (p<0.001, power: 0.999, n=7). The increase in latencies at 1 year and the subsequent decrease at 2 years was similar to the effects after high morphine alone (cf. Fig. 2C); all 3 age groups were significantly different from one another (p<0.001, One-ANOVA with Holm-Sidak multiple comparisons, power: 1). Unlike in WT, combining high morphine with the D1R antagonist led in D3KO to a significant increase in withdrawal latencies at 2 months of 177 ± 8.4 % over control (p<0.001, power: 1, n=5), at 1 year of 158.1 ± 2.9 % (p<0.001, power: 1, n=8), and at 2 years of 134.2 ± 6.3 % (p=0.005, power: 0.899, n=4, Figure 4D). The effects at 2 months and 2 years were significantly different from each other (p=0.005, ANOVA on Ranks with Dunn’s comparison, power: 1). Together, these data suggest a possible interaction between block of D1R function and morphine that has its strongest effects for low doses of morphine in aged WT and young D3KO, and for the higher dose in 1 year-old WT and again young 2 month-old D3KO.
Blocking / reducing D1 receptor function alters morphine responsiveness in aged WT and young D3KO
Lastly, we compared the effects of a block of D1R function on thermal withdrawal latencies alone with those obtained with low or high doses of morphine (Figure 5). The original datasets for theses analyses stem from the experiments presented in Figures 2 and 3. In WT (Figure 5A), SCH 39166 induced a linear aging-related increase in thermal withdrawal latencies (2 months: 100.93 ± 4.6 %; 1 year: 117.48 ± 3.4 %; 2 years: 129.1 ± 7.5 %, black bars). In contrast, both low (2 mg/kg) and high (5 mg/kg) morphine showed an increase in withdrawal latencies from 2 months to 1 year that was followed by a decrease at 2 years (2 mg/kg: 2 months: 117.4 ± 4 %; 1 year: 137.1 ± 7 %; 2 years: 112.8 ± 2.3 %; 5 mg/kg: 2 months: 148.1 ± 4.7 %; 1 year: 176 ±6.6 %; 2 years: 140.7 ±1.9 %). Specifically, in 2 month-old and 1 year-old WT, the effect of 5 mg/kg morphine was significantly different from both the 2 mg/kg morphine and the SCH 39166 treatments, and there was also a significant difference between SCH and low morphine (for both comparisons: p<0.001, ANOVA on Ranks, power: 1). At 2 years, morphine treatments remained significantly different only from each other (p<0.01, ANOVA on Ranks).
Figure 5.
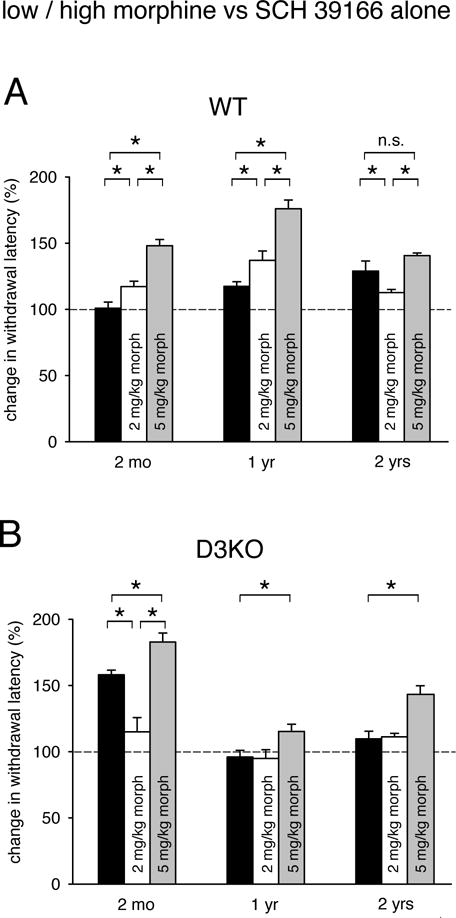
Comparison of treatments of SCH 39166 versus low or high doses of morphine. Displayed data represent changes in withdrawal latency compared to respective control vehicle injections, with dashed lines representing relative vehicle control levels. A. WT animals. Treatment with low (white bars) or high dose of morphine (grey bars) had a significantly larger effect over SCH 39166 (black bars) at 2 months and at 1 year, but at 2 years of age, SCH 39166 was more effective than low morphine, and as effective as high morphine. B. D3KO animals. Treatment with high morphine (gray bars) had at every age the strongest effect on withdrawal latencies, and at 1 year and 2 years, the effects of SCH 39166 (black bars) or low morphine (white bars) were not different from each other, and neither significantly altered withdrawal latencies. In contrast, at 2 months, SCH 39166 was highly effective, and significantly more so than low morphine. Note that the data from the 2 month-old D3KO mirrored those of the 2 year-old WT group. Abbreviations as in Figure 1. *: denotes significant difference between groups; n.s.: not significant.
However, we also observed a significant increase in the effectiveness of SCH 39166 from 2 months to 2 years (p=0.005, One-Way ANOVA, power: 0.836), Moreover, at 2 years, SCH 39166 was significantly more effective than low dose (2 mg/kg) morphine (p=0.047, t-test, power: 0.431) and there was no significant difference between treatments with SCH 39166 and high (5 mg/kg) morphine (p=0.054, t-test). These analyses suggest that, in aged WT, blocking D1R function has a stronger outcome on thermal pain withdrawal latencies than the use of low morphine, and that it is as effective as high morphine.
In D3KO (Figure 5B), the effects of SCH 39166 alone were mixed when compared to low and high morphine. At 2 months, treatment with SCH 39166 led to an increase to 158 ± 3.6 % of vehicle control, which was significantly higher than that induced by 2 mg/kg morphine (115.1 ± 10.7 %), but also significantly less than that caused by 5 mg/kg morphine (p<0.001, One-Way ANOVA, power: 0.999). At 1 year of age, neither SCH 39166 nor low morphine alone had any significant effect on thermal withdrawal latencies, while high morphine led to a slight but significant increase in withdrawal latencies (p=0.036, One-Way ANOVA, power: 0.503). The effects for SCH 39166 and 2 mg/kg morphine remained similar at 2 years of age (SCH: 109.8 ± 5.8 %, 2 mg/kg: 111.3 ± 2.7 %), while high morphine increasing its efficiency to 143 ± 6.5 % (p<0.001, One-Way ANOVA, power: 0.994). Together, these data indicate that block of D1R function can have, in young 2 month-old D3KO, a similar strong effect on thermal pain withdrawal latencies as observed in aged 2 year-old WT.
Discussion
The role of normal aging on withdrawal latencies
Normal healthy physiological aging is associated with a gradual decline in sensory processing and motor functions (Rossini et al., 2007). We observed that withdrawal latencies of untreated WT animals decreased significantly from 2 months to 1 year of age, suggesting a heightened sensitivity to the thermal pain stimulus at the 1-year stage, whereas latencies remained similar in D3KO. However, at 2 years of age, both WT and D3KO latency responses increased again, and in WT, to levels close to those observed in the young animals (cf. Figure 1). We speculate that the decrease in WT withdrawal latencies from 2 months to 1 year is a result of the gradual change in sensory nerve conduction velocities, which peak in mice at about 1 year of age (Verdu et al., 2000). In contrast, nerve conduction velocities (both: sensory and motor) start to drop at about 20 months of age (Walsh et al., 2015), which may underlie the increase in withdrawal latencies observed at 2 years of age over that of 1 year-old animals.
Dopamine modulation of withdrawal latencies with aging
Aging is also associated with a decrease in DA levels (Haycock et al., 2003), and a reduction in the expression levels of inhibitory Gi-coupled D2 receptors (Mesco et al., 1991; Valerio et al., 1994), but not D3 receptors (Valerio et al., 1994). In contrast, we have recently shown that, with age, expression levels of excitatory and Gs-coupled D1 receptors significantly increases in both striatum and spinal cord at 1 and 2 years (Keeler et al., 2016), suggesting an overall gradual disinhibition of the DA system in these areas with age.
Both D1 and D3 receptors are widely expressed throughout the spinal gray matter of the lumbar spinal cord, including in putative motoneurons (Zhu et al., 2007), and DA can up- or downregulate cellular and network functions in a dose-dependent manner (Missale et al., 1998; Thirumalai and Cline, 2008; Clemens et al., 2012). Moreover, modeling studies of mammalian dopamine neurons suggest that tonic versus burst firing can result in differences in the relative occupancies of the different receptor subtypes (Dreyer et al., 2010; Kishore and McLean, 2015). D3R and D1R can display functional interactions that are based on different hetero-dimer (Fiorentini et al., 2008; Marcellino et al., 2008; Cruz-Trujillo et al., 2013) or hetero-tetramer configurations (Guitart et al., 2014). In the hetero-tetrameric model, D1R and D3R co-activation can lead to both antagonistic and synergistic interactions at the level of adenylyl-cyclase and MAPK activation, respectively (Guitart et al., 2014). Experimental evidence supports the idea that D1R-D3R antagonistic interactions play an important role at the spinal cord level [67], while synergistic interactions might be more involved at the striatal level [63]. For example, activation of D1R tends to increase the excitability or the performance of neural networks that underlie or control fictive locomotion in different animal models (Han and Whelan, 2009; Clemens et al., 2012), while activation of the D3R pathway reduces overall motor excitability (Sharples et al., 2015). As D1R and D3R can co-localize or form heterodimers and -tetramers (Marcellino et al., 2008; Guitart et al., 2014; Ferre, 2015), thus oppositely regulating cAMP/PKA-mediated second messenger pathways, it is conceivable that the age-associated increase in D1 but not D3 receptor expression levels (Keeler et al., 2016) might be a contributing factor to the decrease in withdrawal latencies at 1 year, which then is buffered in 2-year old animals by a sharp decrease in nerve conduction velocities (Walsh et al., 2015).
Opioid modulation of withdrawal latencies with aging
We previously reported that a dysfunction of D3R alone prevented the modulation of thermal pain behavior by morphine, provided that morphine was applied at low doses (2 mg/kg) only (Brewer et al., 2014). In the same study we also found that D1R protein expression levels were increased in the spinal cord of D3KO animals, and we proposed a model whereby the upregulation of the D1R, in the absence of the D3R, could drive the beta-arrestin-dependent internalization of the μ-opioid receptor (MOR) (Brewer et al., 2014). A critical point we add to those findings here is that D3R dysfunction does not completely block morphine actions. At the higher dose of 5 mg/kg, morphine was able to increase thermal withdrawal latencies in D3KO, and this increase was most pronounced in the 2-months old D3KO animals (cf. Figure 2), but present across all ages tested. As D1R protein expression levels are relatively low in young animals (Keeler et al., 2016), it is conceivable that the high dose of morphine can recruit the low amount of un-phosphorylated MORs (Brewer et al., 2014) to exert its inhibitory actions. It is also conceivable that with an increase in age, morphine pharmacokinetics or metabolism may change as well, however, we did not test for these possibilities in the present study. Our data do indicate that that the young naïve D3KO mouse may provide a morphine-tolerant phenotype and serve as a model to better understand the role of the DA system in opioid tolerance, while comparing the mechanisms underlying low- and high-dose effects in these animals.
D1 receptor modulation of withdrawal latencies with aging
D1R expression is not only increased in the spinal cord of young D3KO animals, its expression levels also increase in WT with normal aging (Keeler et al., 2016). Since D1R pathways are Gi-coupled and promote excitatory actions, we therefore tested if blocking D1R function can modify thermal withdrawal latencies. Contrary to our expectations, we found that blocking D1R function was most effective in the 2 year-old WT, but had no effect in the 2 month-old animals (cf. Figure 3A). In contrast, the effects of the D1R antagonist were only significant in the young D3KO, but not in the older cohorts (cf. Figure 3B). We speculate that the reason for this could depend on the conformation configurations between D1R and D3Rs; if the receptors are facultative but not obligatory heterodimers or –tetramers (Guitart et al., 2014), an increase in D1R protein expression with age would lead to an increase of D1R monomers. This then could serve as the preferred target of the D1R antagonist, thereby leading to the increase in thermal withdrawal latencies and thus the decrease in thermal pain sensitivity. Notably, these data highlight again that the young D3KO mouse mimics a behavioral phenotype observed in the aged WT, underlying its potential role in deciphering aging-related changes in a young animal model.
D1 receptor and opioid interactions with aging
As both D1R-mediated and MOR-mediated pathways modulate adenylate cyclase and consequently cAMP-dependent signaling cascades, we sought to address if a combination of D1R block and MOR activation might have synergistic effects in controlling thermal pain withdrawal latencies (cf. Figure 4). In WT, such a synergistic effect was only evident with the low dose of morphine and SCH 39266 in the 2 year-old animals, while in D3KO, we observed such a synergistic action between SCH 39166 and morphine only at 5 mg/kg in the 1 year-old animals. More intriguingly however, blocking D1R function led to a loss of the dose-dependent effect of morphine in 2 month- and 2 year-old WT, but not D3KO (cf. Figure 4). This suggests that a combination treatment of low-dose morphine with D1R block can have a stronger effect on thermal withdrawal latencies then a high dose. This outcome was particularly obvious when we compared the individual effects of D1R block with those of low and high dose of morphine in the 2 year-old WT animals (cf. Figure 5). With age, the modulatory actions of the D1R block became more efficient than those of low morphine, and they were at 2 years of age as successful in increasing thermal withdrawal latencies as high morphine.
Block of D1R receptor function with SCH 39166 in the clinic – promises and pitfalls
Several studies have shown that the D1R antagonist used in our study, SCH 39166, also known as ecopipam, is well tolerated in the clinic (Gilbert et al., 2014), and studies on the effectiveness of this compound in humans point to its effectiveness in treating Tourette’s syndrome in juveniles (Chipkin, 2014) or self-injurious behavior in Lesch-Nyhan disease (Khasnavis et al., 2016b; Khasnavis et al., 2016a). These data support the idea that a block or a reduction of D1R function might reduce increased excitability. Furthermore, earlier studies point to an attenuating effect of ecopipam of the euphoric and stimulating effects of cocaine, while at the same time reducing the desire to take cocaine (Romach et al., 1999). In contrast, chronic use of the D1R antagonist may lead to increased cocaine self-administration and hence may not be a useful approach for the treatment of cocaine abuse (Haney et al., 2001). Our data in the aging rodent were obtained under acute testing conditions and, while highlighting possible interactions between D1R, D3R, and MOR pathways in this model, will have to undergo in future studies additional testing under both chronic conditions and in animal models of chronic pain.
The caveat of using the D3KO mouse model
It is tempting to speculate that the 2 month-old D3KO mouse may provide a model to assess the mechanisms of altered dopamine / morphine receptor interactions in 2 year-old WT animals, but this concept must be taken with caution. The D3KO used in this study is an unconditional knockout (Accili et al., 1996), hence any of the changes to the behavior we observed in the D3KO may potentially also stem from alterations to D1/D3 heterodimer or –tetramer configurations (Moreno et al., 2011; Guitart et al., 2014), including in organ systems other than the neural sensorimotor pathways we assessed in this study (Asico et al., 1998; Johnson et al., 2013). However, we have observed an increase in D1R protein expression levels in the spinal cord in both D3KO (Brewer et al., 2014) and aged wild type animals (Keeler et al., 2016), suggesting that the fundamental pieces are in place at the spinal reflex circuitry level to mediate the behavioral changes we reported. However, the lack of a D1R-mediated effect in older (1 yr and 2yrs) D3KO suggest that additional mechanisms might be involved. Therefore, future experiments will have to test, both in vivo and in the isolated spinal cord in vitro, WT animals with intact D1R and D3R components and configurations in combination with different selective D1R and D3R agonists and antagonists at the different aging stages against D3KO.
Conclusions
These data suggest that thermal pain withdrawal reflexes can be modulated in an aging-dependent manner by both morphine and D1R block, and that with increasing age, block of D1R function can be a successful surrogate for morphine treatment. Furthermore, the side-by-side comparison of these drugs in D3KO manifests that young (2 month-old) animals show the same qualitative phenotypical response as the 2 year-old WT, indicating that the young D3KO animal model to deconstruct the mechanisms of opiate responsiveness that may also emerge with normal aging.
Highlights.
Pain withdrawal reflexes are age-dependent and dopamine D3 receptor–mediated
Block of dopamine D1 receptor in young D3KO animals mimics aged wild-type controls
In old animals, dopamine D1 receptor block can mimic analgesic effects of morphine
Acknowledgments
Funding for this study was provided in part by the Office of Research & Graduate Studies of the Brody School of Medicine and a National Institute of Health grant (R01NS082244).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Pasternak GW, Aicher SA. Presynaptic localization of the carboxy-terminus epitopes of the μ opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience. 2001;106:833–842. doi: 10.1016/s0306-4522(01)00317-7. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI. Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res. 2002;930:150–162. doi: 10.1016/s0006-8993(02)02242-4. [DOI] [PubMed] [Google Scholar]
- Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, Knaggs R, Martin D, Sampson L, Schofield P. Guidance on the management of pain in older people. Age and ageing. 2013;42(Suppl 1):i1–57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renindependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud Q, Obeid I, Aubert I, Barriere G, Contamin H, McGuire S, Ravenscroft P, Porras G, Tison F, Bezard E, Ghorayeb I. Neuroanatomical study of the A11 diencephalospinal pathway in the non-human primate. PloS one. 2010;5:e13306. doi: 10.1371/journal.pone.0013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhit MH. Opioid-induced hyperalgesia and tolerance. American journal of therapeutics. 2010;17:498–510. doi: 10.1097/MJT.0b013e3181ed83a0. [DOI] [PubMed] [Google Scholar]
- Bicket MC, Mao J. Chronic Pain in Older Adults. Anesthesiology clinics. 2015;33:577–590. doi: 10.1016/j.anclin.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Baran CA, Whitfield BR, Jensen AM, Clemens S. Dopamine D3 receptor dysfunction prevents anti-nociceptive effects of morphine in the spinal cord. Frontiers in Neural Circuits. 2014;8:62–01. 62–10. doi: 10.3389/fncir.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins Slot LA, Tarayre JP, Koek W, Ribet JP, Colpaert FC. Experimental conditions for the continuous subcutaneous infusion of four central analgesics in rats. Pharmacol Biochem Behav. 2002;72:943–951. doi: 10.1016/s0091-3057(02)00760-8. [DOI] [PubMed] [Google Scholar]
- Chipkin RE. Ecopipam Treatment of Tourette’s Syndrome in Subjects 7–17 Years. 2014 ClinicalTrials.gov.
- Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol. 2012;107:2250–2259. doi: 10.1152/jn.00366.2011. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Mechanisms of opioid-induced pain and antinociceptive tolerance: signal transduction. Pain. 2002;95:287–288. doi: 10.1016/S0304-3959(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Syvanthong C, Picker MJ. Modulatory effects of dopamine D3/2 agonists on kappa opioid-induced antinociception and diuresis in the rat. Psychopharmacology (Berl) 2000;152:14–23. doi: 10.1007/s002130000519. [DOI] [PubMed] [Google Scholar]
- Cruz-Trujillo R, Avalos-Fuentes A, Rangel-Barajas C, Paz-Bermudez F, Sierra A, Escartin-Perez E, Aceves J, Erlij D, Floran B. D3 dopamine receptors interact with dopamine D1 but not D4 receptors in the GABAergic terminals of the SNr of the rat. Neuropharmacology. 2013;67:370–378. doi: 10.1016/j.neuropharm.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPen A, Shen D, Ersek M. Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nurs. 2007;8:113–121. doi: 10.1016/j.pmn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Fazli-Tabaei S, Yahyavi SH, Nouri M, Zartab H, Javid G, Loghavi S, Zarrindast MR. Dopamine receptor mechanism(s) and antinociception and tolerance induced by swim stress in formalin test. Behav Pharmacol. 2006;17:341–347. doi: 10.1097/01.fbp.0000224383.63744.69. [DOI] [PubMed] [Google Scholar]
- Ferre S. The GPCR heterotetramer: challenging classical pharmacology. Trends Pharmacol Sci. 2015;36:145–152. doi: 10.1016/j.tips.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clin Neuropharmacol. 2014;37:26–30. doi: 10.1097/WNF.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7:339–343. doi: 10.1111/j.1526-4637.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- Goldstein NE, Morrison RS. Treatment of pain in older patients. Critical reviews in oncology/hematology. 2005;54:157–164. doi: 10.1016/j.critrevonc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez-Soto M, Kumar-Barodia S, Naidu YT, Mallol J, Cortes A, Lluis C, Canela EI, Casado V, McCormick PJ, Ferre S. Functional Selectivity of Allosteric Interactions within G Protein-Coupled Receptor Oligomers: The Dopamine D1-D3 Receptor Heterotetramer. Mol Pharmacol. 2014;86:417–429. doi: 10.1124/mol.114.093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Whelan PJ. Modulation of AMPA currents by D(1)-like but not D(2)-like receptors in spinal motoneurons. Neuroscience. 2009;158:1699–1707. doi: 10.1016/j.neuroscience.2008.11.040. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law P-Y, Low HH, Elde R, Hökfelt T. Expression of μ-, δ-, and κ-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Tulis DA, Keeler BE, Virag JA, Lust RM, Clemens S. The dopamine D3 receptor knockout mouse mimics aging-related changes in autonomic function and cardiac fibrosis. PloS one. 2013;8:e74116. doi: 10.1371/journal.pone.0074116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Ehrhardt KP, Ripoll JG, Sharma B, Padnos IW, Kaye RJ, Kaye AD. Pain in the Elderly. Current pain and headache reports. 2016;20:23. doi: 10.1007/s11916-016-0551-2. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Reichling DB, Levine JD. Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci. 2010;30:4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler BE, Baran CA, Brewer KL, Clemens S. Increased excitability of spinal pain reflexes and altered frequency-dependent modulation in the dopamine D3-receptor knockout mouse. Exp Neurol. 2012;238:273–283. doi: 10.1016/j.expneurol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Keeler BE, Patel MM, Lallemand P, de Castro Brás LE, Clemens S. Opposing aging - related shift of excitatory dopamine D1and inhibitory D3 receptor protein expression in striatum and spinal cord. J Neurophysiol. 2016;115:363–369. doi: 10.1152/jn.00390.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnavis T, Torres RJ, Sommerfeld B, Puig JG, Chipkin R, Jinnah HA. A double-blind, placebo-controlled, crossover trial of the selective dopamine D1 receptor antagonist ecopipam in patients with Lesch-Nyhan disease. Molecular genetics and metabolism. 2016a;118:160–166. doi: 10.1016/j.ymgme.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Khasnavis T, Reiner G, Sommerfeld B, Nyhan WL, Chipkin R, Jinnah HA. A clinical trial of safety and tolerability for the selective dopamine D1 receptor antagonist ecopipam in patients with Lesch-Nyhan disease. Molecular genetics and metabolism. 2016b;117:401–406. doi: 10.1016/j.ymgme.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Kishore S, McLean DL. Neuromodulation: letting sources of spinal dopamine speak for themselves. Curr Biol. 2015;25:R146–148. doi: 10.1016/j.cub.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Le Marec T, Marie-Claire C, Noble F, Marie N. Chronic and intermittent morphine treatment differently regulates opioid and dopamine systems: a role in locomotor sensitization. Psychopharmacology (Berl) 2011;216:297–303. doi: 10.1007/s00213-011-2223-6. [DOI] [PubMed] [Google Scholar]
- Lin CW, Bianchi BR, Miller TR, Stashko MA, Wang SS, Curzon P, Bednarz L, Asin KE, Britton DR. Persistent activation of the dopamine D1 receptor contributes to prolonged receptor desensitization: studies with A-77636. J Pharmacol Exp Ther. 1996;276:1022–1029. [PubMed] [Google Scholar]
- Maggio R, Aloisi G, Silvano E, Rossi M, Millan MJ. Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat Disord. 2009;15(Suppl 4):S2–7. doi: 10.1016/S1353-8020(09)70826-0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. Journal of the American Geriatrics Society. 2009;57:115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesco ER, Joseph JA, Blake MJ, Roth GS. Loss of D2 receptors during aging is partially due to decreased levels of mRNA. Brain Res. 1991;545:355–357. doi: 10.1016/0006-8993(91)91314-q. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Collo G, Spano P. The neurobiology of dopamine receptors: evolution from the dual concept to heterodimer complexes. J Recept Signal Transduct Res. 2010;30:347–354. doi: 10.3109/10799893.2010.506192. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moreno E, Hoffmann H, Gonzalez-Sepulveda M, Navarro G, Casado V, Cortes A, Mallol J, Vignes M, McCormick PJ, Canela EI, Lluis C, Moratalla R, Ferre S, Ortiz J, Franco R. Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem. 2011;286:5846–5854. doi: 10.1074/jbc.M110.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Pain Management. In: NINR, editor. NIH Fact Sheets Home. US Department of Health and Human Services; 2010. [Google Scholar]
- Ray SB, Wadhwa S. Mu opioid receptors in developing human spinal cord. J Anat. 1999;195(Pt 1):11–18. doi: 10.1046/j.1469-7580.1999.19510011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SB, Wadhwa S. Expression of mu-opioid receptors in developing rat spinal cord: an autoradiographic study. Indian journal of experimental biology. 2004;42:533–537. [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56:1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Prog Neurobiol. 2007;83:375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Saastamoinen P, Leino-Arjas P, Laaksonen M, Lahelma E. Socio-economic differences in the prevalence of acute, chronic and disabling chronic pain among ageing employees. Pain. 2005;114:364–371. doi: 10.1016/j.pain.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Saghaei E, Moini Zanjani T, Sabetkasaei M, Naseri K. Enhancement of Antinociception by Co-administrations of Nefopam, Morphine, and Nimesulide in a Rat Model of Neuropathic Pain. The Korean journal of pain. 2012;25:7–15. doi: 10.3344/kjp.2012.25.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, Gear RW. Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci. 2002;22:6773–6780. doi: 10.1523/JNEUROSCI.22-15-06773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples SA, Humphreys JM, Jensen AM, Dhoopar S, Delaloye N, Clemens S, Whelan PJ. Dopaminergic modulation of locomotor network activity in the neonatal mouse spinal cord. J Neurophysiol. 2015;113:2500–2510. doi: 10.1152/jn.00849.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Ozaki S, Narita M. Mechanism of opioid dependence and interaction between opioid receptors. Eur J Pain. 2001;5(Suppl A):63–65. doi: 10.1053/eujp.2001.0282. [DOI] [PubMed] [Google Scholar]
- Taylor FR, Saper JR. Opioid tolerance or rather opioid-induced pain sensitivity. Headache. 2004;44:839–840. doi: 10.1111/j.1526-4610.2004.04158_3.x. [DOI] [PubMed] [Google Scholar]
- Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J Neurophysiol. 2008;100:1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Belloni M, Gorno ML, Tinti C, Memo M, Spano P. Dopamine D2, D3, and D4 receptor mRNA levels in rat brain and pituitary during aging. Neurobiology of aging. 1994;15:713–719. doi: 10.1016/0197-4580(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Walsh ME, Sloane LB, Fischer KE, Austad SN, Richardson A, Van Remmen H. Use of Nerve Conduction Velocity to Assess Peripheral Nerve Health in Aging Mice. The journals of gerontology. 2015;70:1312–1319. doi: 10.1093/gerona/glu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Hao JX, Xu XJ, Wiesenfeld-Hallin Z. The development of morphine tolerance and dependence in rats with chronic pain. Brain Res. 1997;756:141–146. doi: 10.1016/s0006-8993(97)00132-7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang H, Jin GZ, Zhang K, Zhen X. Single dose of morphine produced a prolonged effect on dopamine neuron activities. Molecular pain. 2008;4:57. doi: 10.1186/1744-8069-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pan YX, Kolesnikov Y, Pasternak GW. Immunohistochemical labeling of the mu opioid receptor carboxy terminal splice variant mMOR-1B4 in the mouse central nervous system. Brain Res. 2006;1099:33–43. doi: 10.1016/j.brainres.2006.04.133. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang F, Yang C, Jin H, Yang Y, Xu M. Dopamine affects the change of pain-related electrical activity induced by morphine dependence. Neurochem Res. 2012;37:977–982. doi: 10.1007/s11064-011-0690-0. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhu W, Pan T, Xie W, Zhang A, Ondo WG, Le W. Spinal cord dopamine receptor expression and function in mice with 6-OHDA lesion of the A11 nucleus and dietary iron deprivation. J Neurosci Res. 2007;85:1065–1076. doi: 10.1002/jnr.21207. [DOI] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk M, Hochman S. Expression and distribution of all dopamine receptor subtypes (D1 – D5) in the mouse lumbar spinal cord: A real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885–897. doi: 10.1016/j.neuroscience.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk M, Hochman S. Unaltered D1, D2, D4, and D5 dopamine receptor mRNA expression and distribution in the spinal cord of the D3 receptor knockout mouse. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:957–962. doi: 10.1007/s00359-008-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


