Abstract
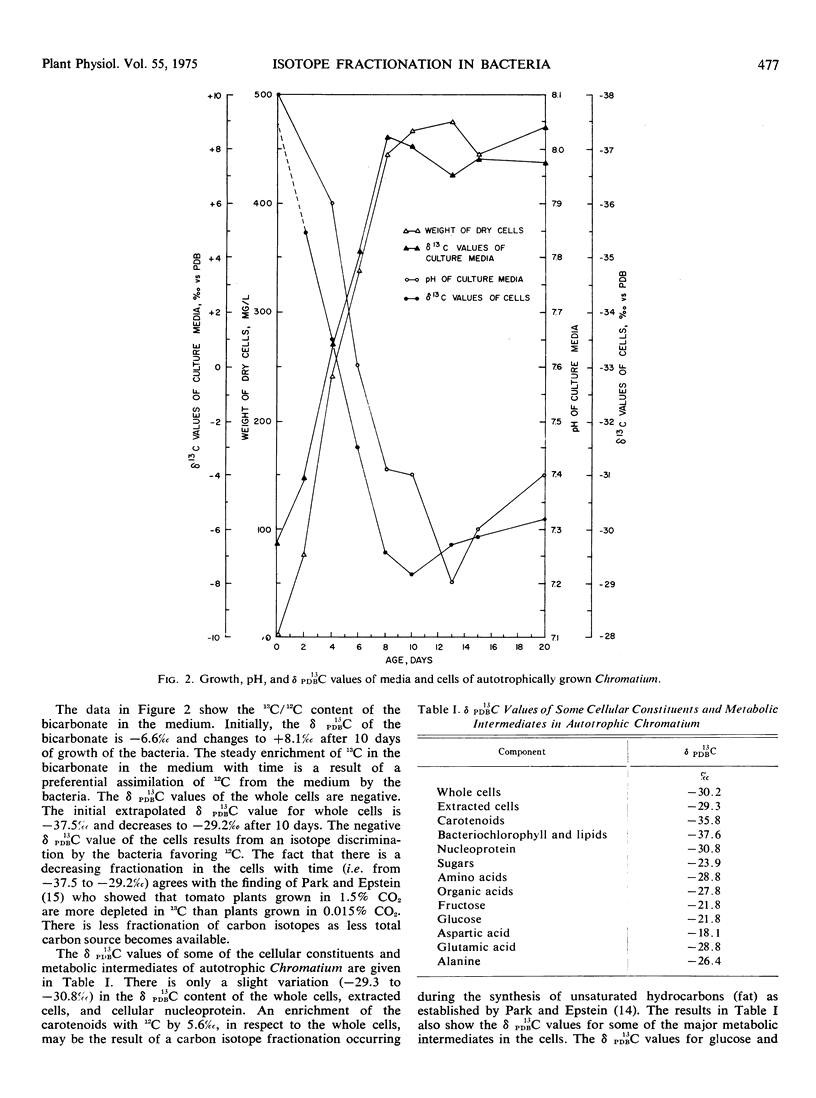
The δ PDB13C values have been determined for the cellular constituents and metabolic intermediates of autotrophically grown Chromatium vinosum. The isotopic composition of the HCO3- in the medium and the carbon isotopic composition of the bacterial cells change with the growth of the culture. The δ PDB13C value of the HCO3- in the media changes from an initial value of −6.6‰ to +8.1‰ after 10 days of bacterial growth and the δ PDB13C value of the bacterial cells change from −37.5‰ to −29.2‰ in the same period. The amount of carbon isotope fractionation during the synthesis of hexoses by the photoassimilation of CO2 has a range of −15.5‰ at time zero to −22.0‰ after 10 days. This range of fractionation compares to the range of carbon isotope fractionation for the synthesis of sugars from CO2 by ribulose 1,5-diphosphate carboxylase and the Calvin cycle.
The amount of carbon isotope fractionation during the synthesis of aspartic acid from CO2 is −24.9‰ at time zero and −15.0‰ after 10 days of bacterial growth. This amount of fractionation is in the range of carbon isotope fractionation for the synthesis of C4 amino acids by a double carboxylation through ribulose 1,5-diphosphate and phosphoenolpyruvate carboxylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. M. C/C ratio changes in crassulacean Acid metabolism plants. Plant Physiol. 1973 Nov;52(5):427–430. doi: 10.1104/pp.52.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- Cooper T. G., Wood H. G. The carboxylation of phosphoenolpyruvate and pyruvate. II. The active species of "CO2" utilized by phosphoenlpyruvate carboxylase and pyruvate carboxylase. J Biol Chem. 1971 Sep 10;246(17):5488–5490. [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., SISLER E. C., KORNBERG H. L. Carbon metabolism in Chromatium. J Biol Chem. 1961 Jul;236:2140–2149. [PubMed] [Google Scholar]
- LOSADA M., TREBST A. V., OGATA S., ARNON D. I. Equivalence of light and adenosine triphosphate in bacterial photosynthesis. Nature. 1960 Jun 4;186:753–760. doi: 10.1038/186753a0. [DOI] [PubMed] [Google Scholar]
- Lerman J. C. Variation in the carbon isotope composition of a plant with crassulacean Acid metabolism. Plant Physiol. 1974 Apr;53(4):581–584. doi: 10.1104/pp.53.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKINNEY C. R., McCREA J. M., EPSTEIN S., ALLEN H. A., UREY H. C. Improvements in mass spectrometers for the measurement of small differences in isotope abundance ratios. Rev Sci Instrum. 1950 Aug;21(8):724–730. doi: 10.1063/1.1745698. [DOI] [PubMed] [Google Scholar]
- Rinne R. W., Buckman R. W., Benedict C. R. Acetate and bicarbonate metabolism in photosynthetic bacteria. Plant Physiol. 1965 Nov;40(6):1066–1073. doi: 10.1104/pp.40.6.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. N., Epstein S. Two categories of c/c ratios for higher plants. Plant Physiol. 1971 Mar;47(3):380–384. doi: 10.1104/pp.47.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan T., Sackett W. M., Benedict C. R. Carbon isotope discrimination in a plant possessing the C4 dicarboxylic acid pathway. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1205–1210. doi: 10.1016/0006-291x(70)90214-7. [DOI] [PubMed] [Google Scholar]
- Whelan T., Sackett W. M. Enzymatic fractionation of carbon isotopes by phosphoenolpyruvate carboxylase from c(4) plants. Plant Physiol. 1973 Jun;51(6):1051–1054. doi: 10.1104/pp.51.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]


