Abstract
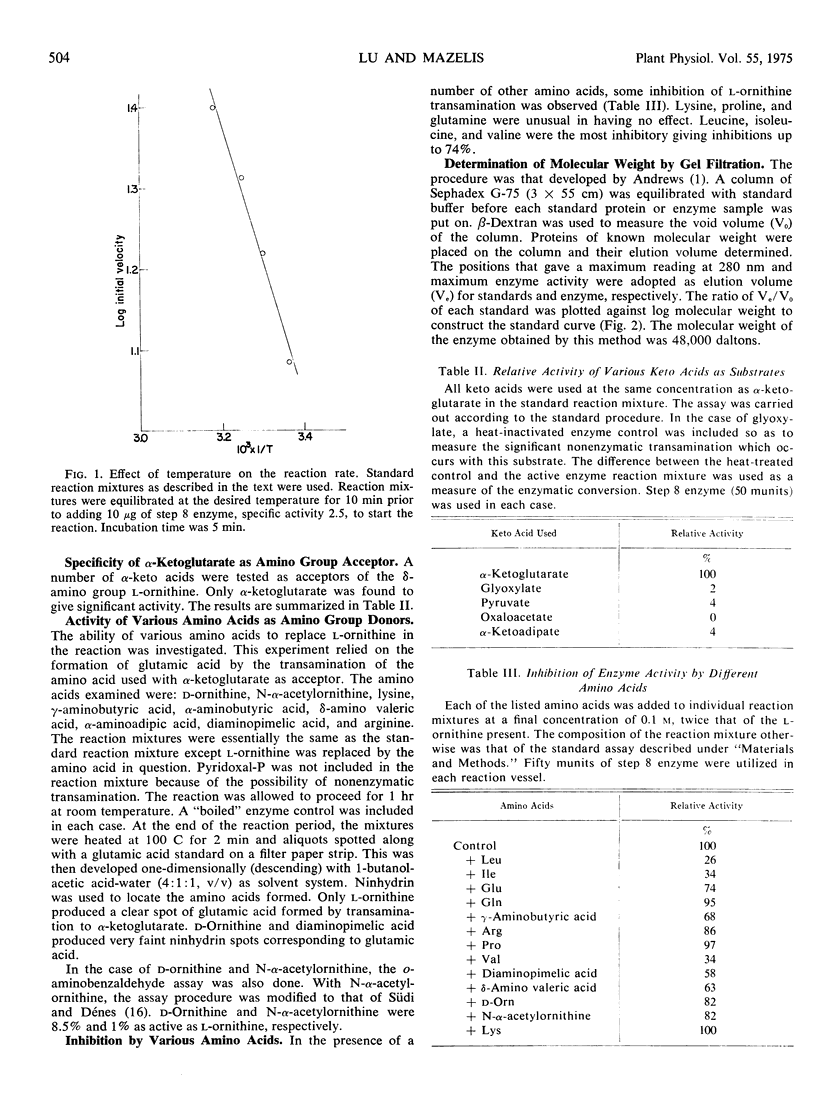
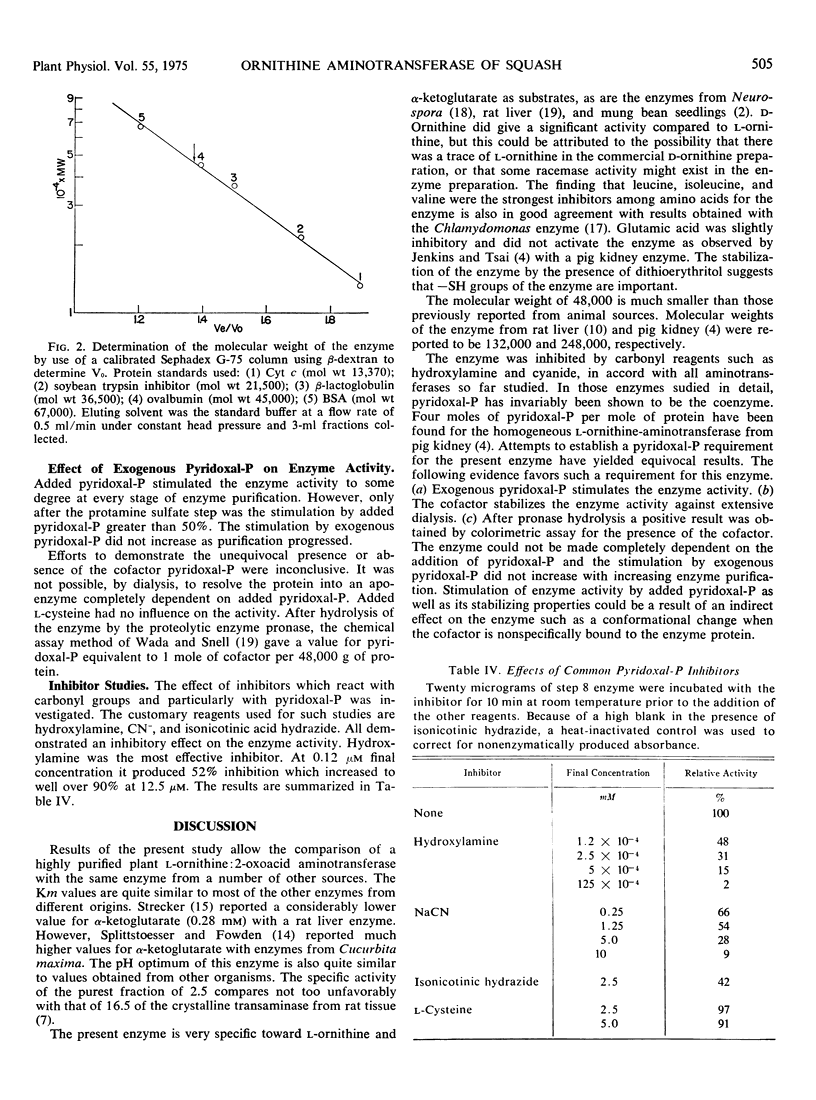
Ornithine: 2-oxoacid aminotransferase (EC 2.6.1.13) has been purified over 400-fold with a total recovery of 14% from acetone powders of cotyledons of germinating squash (Cucurbita pepo, L.) seedlings. The pH optimum of the transamination between l-ornithine and α-ketoglutarate is 8 and the Michaelis constants are 4.7 mm and 6.3 mm, respectively. The enzyme has a molecular weight of 48,000 as determined by gel filtration. The reaction is essentially specific for α-ketoglutarate as the amino group acceptor. The enzyme is inhibited very strongly by hydroxylamine, and less severely by NaCN and isonicotinylhydrazide. No inhibition is observed in the presence of 10 mml-cysteine. The energy of activation is 7.6 kcal/mole. The stability of the enzyme preparation is enhanced by the presence of dithioerythritol and glycerol. The enzyme activity of the most purified fraction is stimulated 30% by the addition of pyridoxal phosphate; however, the evidence for the unequivocal involvement of pyridoxal phosphate was inconclusive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone D. H. Metabolism of Citrulline and Ornithine in Mung Bean Mitochondria. Plant Physiol. 1959 Mar;34(2):171–175. doi: 10.1104/pp.34.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuzawa T., Katsunuma T., Katunuma N. Crystallization of ornithine transaminase and its properties. Biochem Biophys Res Commun. 1968 Jul 26;32(2):161–166. doi: 10.1016/0006-291x(68)90363-x. [DOI] [PubMed] [Google Scholar]
- Peraino C., Bunville L. G., Tahmisian T. N. Chemical, physical, and morphological properties of ornithine Aminotransferase from rat liver. J Biol Chem. 1969 May 10;244(9):2241–2249. [PubMed] [Google Scholar]
- SMITH J. E. Mitochondrial transamination in sunflower hypocotyls. Biochim Biophys Acta. 1962 Feb 12;57:183–185. doi: 10.1016/0006-3002(62)91106-x. [DOI] [PubMed] [Google Scholar]
- STRECKER H. J. PURIFICATION AND PROPERTIES OF RAT LIVER ORNITHINE DELTA-TRANSAMINASE. J Biol Chem. 1965 Mar;240:1225–1230. [PubMed] [Google Scholar]
- WADA H., SNELL E. E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J Biol Chem. 1961 Jul;236:2089–2095. [PubMed] [Google Scholar]


