Abstract
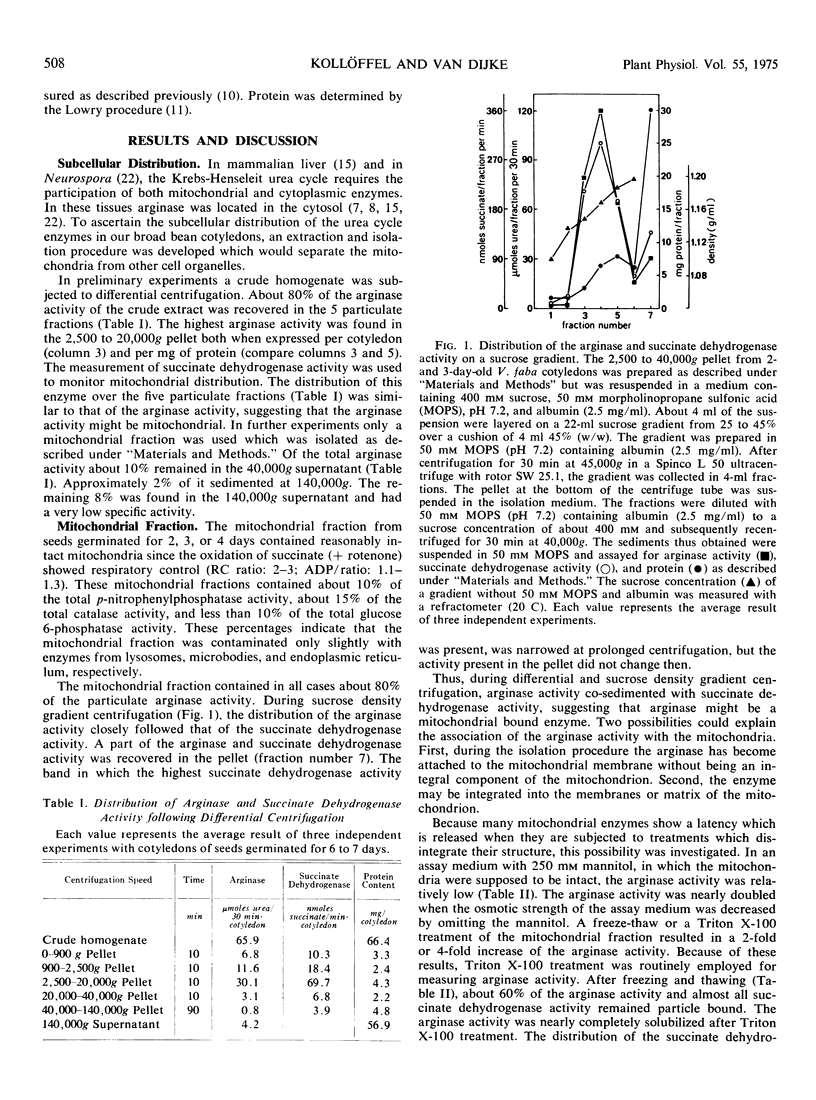
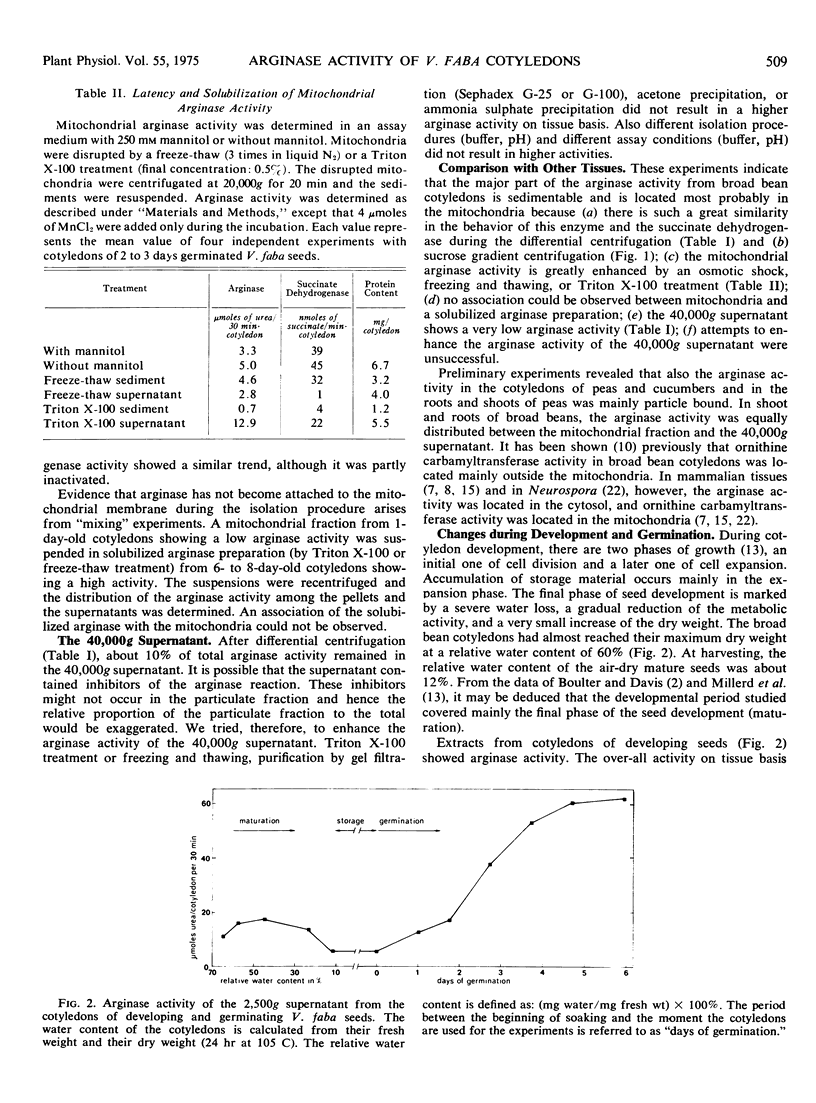
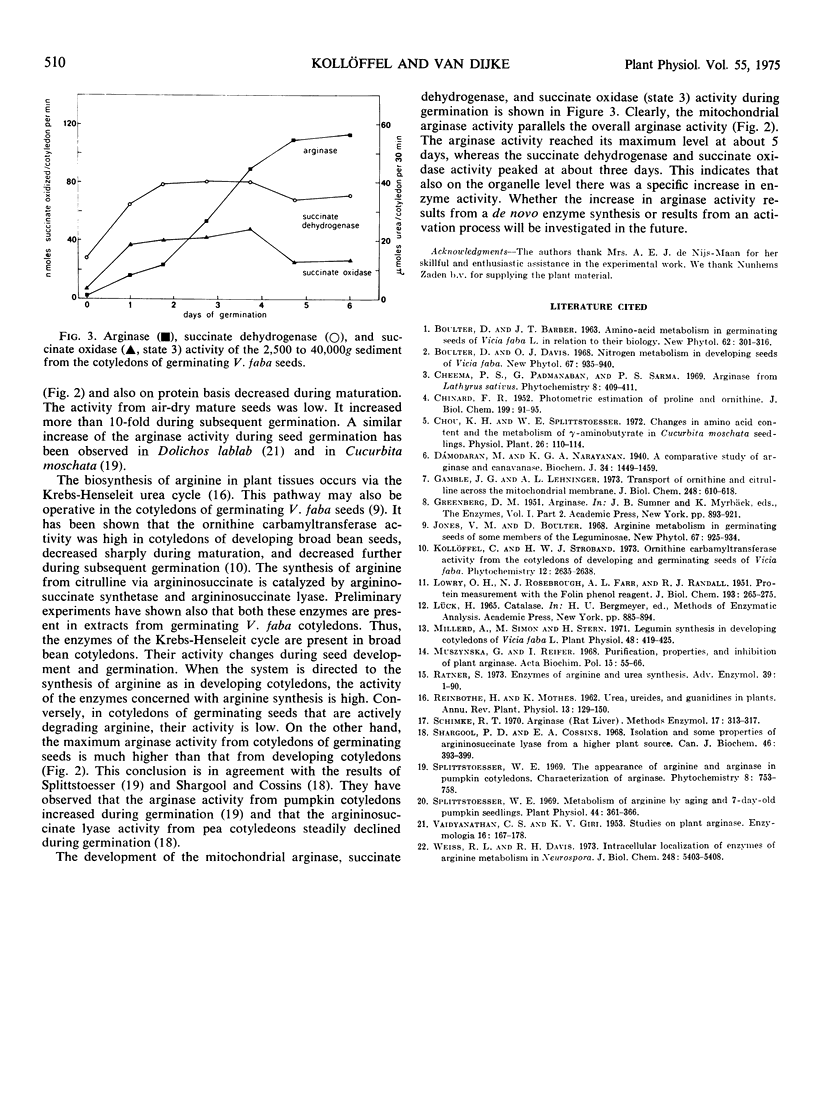
Differential and sucrose density gradient centrifugation established that about 80% of the total arginase activity (EC 3.5.3.1) in cotyledons of germinating broad bean seeds (Vicia faba L.) was present in the mitochondrial fraction. The mitochondrial arginase activity was enhanced considerably by exposure to osmotic shock, by freezing and thawing, or by Triton X-100 treatment. About 10% of the total arginase activity was recovered from the 40,000g supernatant fraction. During seed maturation, arginase activity in the cotyledons decreased to about one-third of its maximal activity, while increasing over 10-fold during subsequent germination. The time courses of mitochondrial arginase, succinate oxidase, and succinate dehydrogenase activities differed considerably during germination.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- Damodaran M., Narayanan K. G. A comparative study of arginase and canavanase. Biochem J. 1940 Nov;34(10-11):1449–1459. doi: 10.1042/bj0341449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millerd A., Simon M., Stern H. Legumin Synthesis in Developing Cotyledons of Vicia faba L. Plant Physiol. 1971 Oct;48(4):419–425. doi: 10.1104/pp.48.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszyńska G., Reifer I. Purification, properties and inhibition of plant arginase. Acta Biochim Pol. 1968;15(1):55–66. [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Shargool P. D., Cossins E. A. Isolation and some properties of argininosuccinate lyase from a higher plant source. Can J Biochem. 1968 May;46(5):393–399. doi: 10.1139/o68-060. [DOI] [PubMed] [Google Scholar]
- Splittstoesser W. E. Metabolism of arginine by aging and 7 day old pumpkin seedlings. Plant Physiol. 1969 Mar;44(3):361–366. doi: 10.1104/pp.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAIDYANATHAN C. S., GIRI K. V. Studies on plant arginase. I. Arginase from field beans (Dolichos lablab); general properties and the effect of metallic ions. Enzymologia. 1953 Sep 30;16(3):167–178. [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]


