Abstract
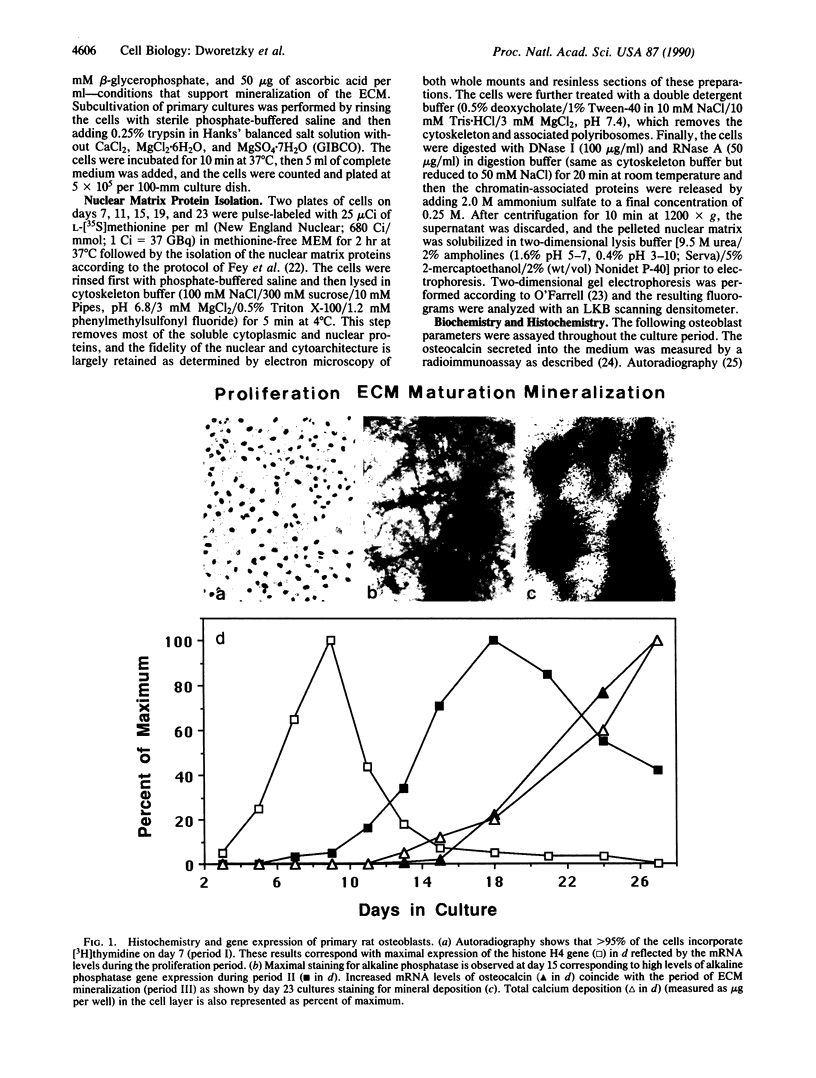
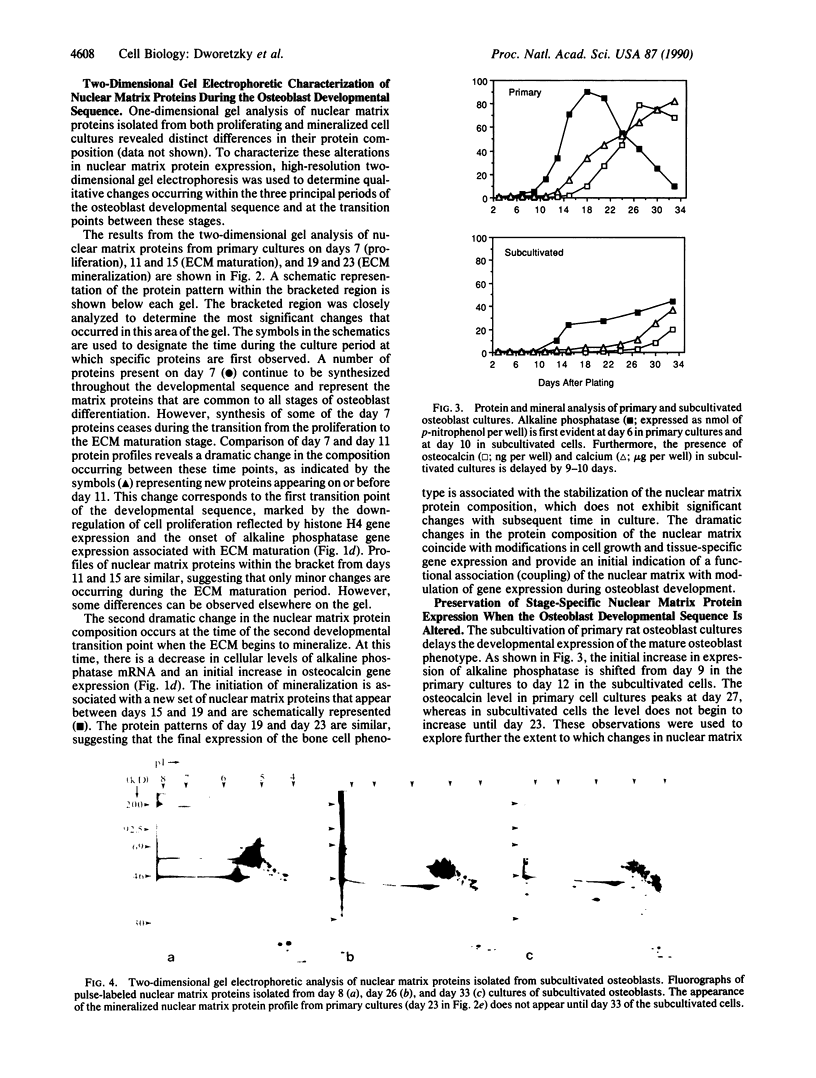
Primary cultures of fetal rat calvarial osteoblasts undergo a developmental sequence with respect to the temporal expression of genes encoding osteoblast phenotypic markers. Based on previous suggestions that gene-nuclear matrix associations are involved in regulating cell- and tissue-specific gene expression, we investigated the protein composition of the nuclear matrix during this developmental sequence by using high-resolution two-dimensional gel electrophoresis. The nuclear matrix was isolated at times during a 4-week culture period that represent the three principal osteoblast phenotypic stages: proliferation, extracellular matrix (ECM) maturation, and mineralization. The most dramatic changes in the nuclear matrix protein patterns occurred during transitions from the proliferation to the ECM maturation stage and from ECM maturation to the mineralization period, with only minor variations in the profiles within each period. These stage-specific changes, corresponding to the major transition points in gene expression, indicate that the nuclear matrix proteins reflect the progressive differentiation of the bone cell phenotype. Subcultivation of primary cells delays mineralization, and a corresponding delay was observed for the nuclear matrix protein patterns. Thus, the sequential changes in protein composition of the nuclear matrix that occur during osteoblast differentiation represent distinct stage-specific markers for maturation of the osteoblast to an osteocytic cell in a bone-like mineralized ECM. These changes are consistent with a functional involvement of the nuclear matrix in mediating modifications of developmental gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow M. A., Gerstenfeld L. C., Owen T. A., Tassinari M. S., Stein G. S., Lian J. B. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990 May;143(2):213–221. doi: 10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E., Heersche J. N., Antosz M. E. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986 Mar;38(3):143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Glorieux F. H., van der Rest M., Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol. 1983 Mar;96(3):639–643. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Shepard N., Charette G., Grynpas M., Glorieux F. H. Mineralization in osteoblast cultures: a light and electron microscopic study. Bone. 1988;9(3):147–154. doi: 10.1016/8756-3282(88)90004-x. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Penman S. Nuclear matrix proteins reflect cell type of origin in cultured human cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):121–125. doi: 10.1073/pnas.85.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Chipman S. D., Kelly C. M., Hodgens K. J., Lee D. D., Landis W. J. Collagen expression, ultrastructural assembly, and mineralization in cultures of chicken embryo osteoblasts. J Cell Biol. 1988 Mar;106(3):979–989. doi: 10.1083/jcb.106.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundberg C. M., Hauschka P. V., Lian J. B., Gallop P. M. Osteocalcin: isolation, characterization, and detection. Methods Enzymol. 1984;107:516–544. doi: 10.1016/0076-6879(84)07036-1. [DOI] [PubMed] [Google Scholar]
- He D. C., Nickerson J. A., Penman S. Core filaments of the nuclear matrix. J Cell Biol. 1990 Mar;110(3):569–580. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara-Siri M. H., Shapiro L. E., Surks M. I. Association of the 3,5,3'-triiodo-L-thyronine nuclear receptor with the nuclear matrix of cultured growth hormone-producing rat pituitary tumor cells (GC cells). J Biol Chem. 1986 Feb 25;261(6):2844–2852. [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., Collart D., Zambetti G., Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefussi J. R., Boy-Lefevre M. L., Boulekbache H., Forest N. Mineralization in vitro of matrix formed by osteoblasts isolated by collagenase digestion. Differentiation. 1985;29(2):160–168. doi: 10.1111/j.1432-0436.1985.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Pardoll D. M., Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucleic Acids Res. 1980 Dec 11;8(23):5623–5633. doi: 10.1093/nar/8.23.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Owen T. A., Aronow M., Shalhoub V., Barone L. M., Wilming L., Tassinari M. S., Kennedy M. B., Pockwinse S., Lian J. B., Stein G. S. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990 Jun;143(3):420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Shalhoub V., Gerstenfeld L. C., Collart D., Lian J. B., Stein G. S. Downregulation of cell growth and cell cycle regulated genes during chick osteoblast differentiation with the reciprocal expression of histone gene variants. Biochemistry. 1989 Jun 27;28(13):5318–5322. doi: 10.1021/bi00439a002. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Lian J. B., Gerstenfeld L. G., Shalhoub V., Aronow M., Owen T., Markose E. The onset and progression of osteoblast differentiation is functionally related to cellular proliferation. Connect Tissue Res. 1989;20(1-4):3–13. doi: 10.3109/03008208909023869. [DOI] [PubMed] [Google Scholar]
- Stuurman N., Van Driel R., De Jong L., Meijne A. M., Van Renswoude J. The protein composition of the nuclear matrix of murine P19 embryonal carcinoma cells is differentiation-stage dependent. Exp Cell Res. 1989 Feb;180(2):460–466. doi: 10.1016/0014-4827(89)90072-4. [DOI] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Parent A., Silverstein S., Efstratiadis A. Pre-mRNA splicing and the nuclear matrix. Mol Cell Biol. 1987 Jan;7(1):111–120. doi: 10.1128/mcb.7.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]