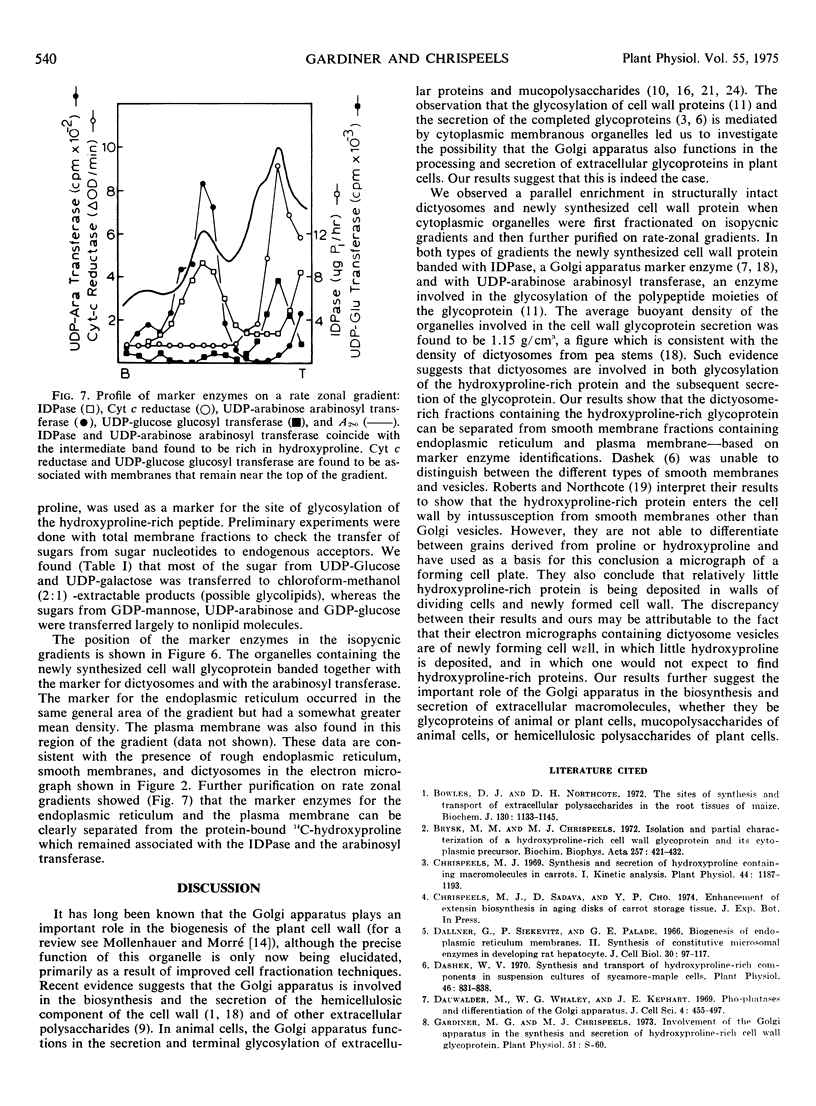
Abstract
Pulse labeling of carrot root phloem parenchyma (Daucus carota L. cv. Nantes) tissue with 14C-proline followed by fractionation of the cytoplasmic organelles on sucrose gradients was used to determine the identity of the membranous organelles involved in the secretion of the hydroxyproline-rich glycoproteins of the cell wall. Identification of the organelles was done through electron-microscopical observations and through the localization of marker enzymes on the sucrose gradients. Enrichment of the organelles involved in secretion was determined by measuring the percentage of the incorporated radioactivity present as 14C-hydroxyproline. The Golgi apparatus (dictyosome) was found to be a major site of glycoprotein transport. This identification was based on the observed enrichment of dictyosomes paralleling the purification of newly synthesized cell-wall glycoproteins. A marker enzyme for the Golgi apparatus, inosinediphosphatase, banded with the newly synthesized cell wall glycoproteins on sequential isopycnic and rate zonal sucrose gradients. Marker enzymes for the endoplasmic reticulum and the plasma membrane were clearly separated from the dictyosome-rich fraction. UDP-arabinose arabinosyl transferase, an enzyme involved in the glycosylation of the peptide moiety of this glycoprotein, also banded with the dictyosomes on both kinds of gradients. The results suggest an important role of the Golgi apparatus in the biosynthesis and the secretion of the cell wall glycoproteins of higher plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashek W. V. Synthesis and Transport of Hydroxyproline-rich Components in Suspension Cultures of Sycamore-Maple Cells. Plant Physiol. 1970 Dec;46(6):831–838. doi: 10.1104/pp.46.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder M., Whaley W. G., Kephart J. E. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969 Mar;4(2):455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Polysaccharide formation in plant Golgi bodies. Biochim Biophys Acta. 1971 Apr 20;237(1):56–64. doi: 10.1016/0304-4165(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Role of the Golgi complex in the intracellular transport of secretory proteins. Proc Natl Acad Sci U S A. 1966 Feb;55(2):424–431. doi: 10.1073/pnas.55.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr A. L. Isolation of an enzyme system which will catalyze the glycosylation of extensin. Plant Physiol. 1972 Aug;50(2):275–282. doi: 10.1104/pp.50.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H., CHAMBERS J. E. GLUTARALDEHYDE STABILIZATION AS AN AID TO GOLGI APPARATUS ISOLATION. Exp Cell Res. 1965 Jun;38:672–675. doi: 10.1016/0014-4827(65)90392-7. [DOI] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. Radioautographic comparison of the uptake of galactose-H and glucose-H3 in the golgi region of various cells secreting glycoproteins or mucopolysaccharides. J Cell Biol. 1966 Jul;30(1):137–150. doi: 10.1083/jcb.30.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. C. Proteins and Plant Cell Walls. Proline to Hydroxyproline in Tobacco Suspension Cultures. Plant Physiol. 1964 Jul;39(4):543–550. doi: 10.1104/pp.39.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline biosynthesis in plant cells. Peptidyl proline hydroxylase from carrot disks. Biochim Biophys Acta. 1971 Feb 10;227(2):278–287. doi: 10.1016/0005-2744(71)90060-x. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Wagner R. R., Cynkin M. A. Glycoprotein biosynthesis. Incorporation of glycosyl groups into endogenous acceptors in a Golgi apparatus-rich fraction of liver. J Biol Chem. 1971 Jan 10;246(1):143–151. [PubMed] [Google Scholar]