Abstract
Background:
Eucalyptus spp. and Pistascia lentiscus are among the Palestinian trees that are traditionally used in folkloric medicine in treating many diseases; leaves of which are thought to have anti-inflammatory, antibacterial and antioxidant effects. The goal of this study is to evaluate the in vitro inhibitory effect of Eucalyptus spp. and Pistascia lentiscus extracts on Lipopolysacaride (LPS)-induced Interlukin-6 (Il-6) and Tumor Necrosis Factor-α (TNF-α) by polymorphonuclear Cells (PMNCs).
Materials and Methods:
Polymorphonuclear cells were isolated from the whole blood using Histopaque (Ficol-1077) method and then cultured in an enriched Roswell Park Memorial Institute (RBMI) medium. Supernatants’ Interlukin-6 (IL-6) and Tumor Necrosis Factor (TNF-α) levels were determined 24 hour after LPS stimulation. HPLC was employed to determine the concentration of phenolic compounds in the extracts. The concentrations of TNF-α and IL-6 were compared using paired-samples t test.
Results:
Eucalyptus spp. and Pistascia lentiscus leaves extracts have shown significant reduction in the levels of both Il-6 and TNF-α Gallic acid; a strong anti-inflammatory agent was found to be the major phenolic compound in both leaf extracts. However, other anti-inflammatory phenolic compounds were detected in Pitascia lentiscus extract including syringic acid and p-coumaric acid, while chlorogenic acid was detected in Eucalyptus spp. leaf extract.
Conclusion:
Reduction in the levels of Il-6 and TNF-α upon the effect of both Eucalyptus spp. and Pistascia lentiscus extract is an indication of their anti-inflammatory effects. Our results may also indicate that the observed anti-inflammatory effect of the above extracts may be due to the presence of gallic acid and other phenolic compounds.
List of Abbreviations and Nomenclature: LPS: Lipopolysacaride, Il-6: Interlukin-6, TNF-α: Tumor Necrosis Factor-α, PMNCs: Polymorphonuclear Cells, HPLC: High Performance Liquid Chromatography, ELISA: Enzyme Linked Immune Sorbent Assay, EDTA: Ethylene Diamine Tetra Acetic acid, PBS: phosphate buffered saline, RPMI: Roswell Park Memorial Institute medium FBS: Fetal Bovine Serum.
Keywords: Anti-inflammatory, Eucalyptus spp, Pistascia lentiscus, HPLC, TNF-alpha, IL-6
Introduction
Human communities in the alternative medicine have traditionally used herbal plants since the ancient time. They contain active ingredients that may help in recovering from many diseases including many infectious and chronic ones. The leaf extract of Eucalyptus spp. and Pistascia lentiscus are well known examples of trees that were used as anti-inflammatory, antibacterial and antioxidant agents (Silverstein et al. 2000; Salari et al. 2006; Mueller et al. 2010; Bampouli et al. 2014).
Despite the fact that high diversity of herbal plants or trees is used worldwide, a small number among them was analyzed pharmacologically and phytochemically for medical applications. Many of the active ingredients were reported out of many medicinal plants or trees that may act as anti-inflammatory, anti-microbial and free radicals scavenging agents. Such ingredients may include phenolics, anthocyanins, carotenoids, and thiols (Qabaha et al. 2013; Gbenou et al. 2013).
Inflammation is part of a self-protection used by human body against pathogens and helps in healing injured tissues. Pro-inflammatory cytokines as Interlukin-6 (IL-6) and Tumor Necrosis Factor (TNF-α) may cause injury to normal tissues upon triggering of inflammation anywhere in the human body. Excessive production of the pro-inflammatory cytokines may evolve into many chronic inflammatory diseases such as rheumatoid arthritis, asthma, and atherosclerosis. Anti-inflammatory drugs decrease the production of such pro-inflammatory cytokines, and therefore improve the symptoms of inflammation (Gaestel et al. 2009; Nathan 2002; Hong et al.).
Il-6 and TNF-α are produced by Monocytes, T-cells, B-cells, endothelial cells, and others as pro-inflammatory mediators. Production of such pro-inflammatory cytokines could be stimulated by lipopolysacharide (LPS), an endotoxin and part of an outer cell membrane component of Gram-negative bacteria. Therefore, LPS initiates inflammation and may lead to septic shock (Mihara et al. 2012, Oe et al. 2015; Cho et al. 2000; et al. 2013). Anti-inflammatory effect was examined by measuring the level of IL-6 and TNF-α by the mono nucleated white blood cells upon effect of LPS with different concentrations. Levels of the cytokines were measured using Enzyme Linked Immune Sorbent Assay (ELISA) method. As part of our ongoing research about the medical effect of the plant extracts, anti-inflammatory of both Eucalyptus spp. and Pistascia lentiscus were investigated along with their phenolic compounds analysis using HPLC with UV detection.
Materials and Methods
Preparation of Plant Extracts
Eucalyptus and Pistascia lentiscus are well known trees in Palestine. Mature leaves of Eucalyptus spp. and Pistascia lentiscus grown in Jenin area determined by their Mediterranean climate were harvested in November-2013, shade dried at room temperature and grinded to pass 1mm sieve, from which a fifty-gram sample was ball-milled to produce a powdered plant material. 50 g of air-dried powdered plant material was soaked in 500 ml of 96% ethanol for five days. The extracts were filtered through ashless filter paper (Whatman blue ribbon No.41). The filtrate was dried completely using rotary evaporator.
Isolation of Polymorphonuclear Cells from Whole Blood
Five ml of freshly transfused whole blood was collected in an EDTA tube and diluted with equal volume of phosphate buffered saline (PBS) in 1:1 ratio, then gently mixed under completely sterile condition. Three ml Histopaque (Ficol-1077) were pipetted into a sterile 15 ml conical tube. The mixture of blood and PBS were added gently and the tube was spun at 400 G for 20 min. Four distinct layers were separated: The lower one was the red blood cells, then the Ficol layer then the polymorphonuclear cells (PMNCs), and the upper one was the PBS and the plasma. The PMN cells were aspirated and washed with 10 ml of PBS in 12 ml conical tubes three times at 100 G for 10 minutes each time, then the supernatant was discarded and the cells were used for our study.
Cell Culture
The cells were grown in Roswell Park Memorial Institute medium (RPMI) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS), 100^g ml-1 streptomycin and 100 Uml-1 penicillin. The cultures were maintained in 12 wells trays in 37° C humidified atmosphere of 5% CO2 and 95% air for 24 hours. Each well contains one million of cells. Cells were exposed to various concentrations of plant extract in medium in both the absence and presence of LPS (1 μg/ well).
Trypan Blue Exclusion Test
The trypan blue dye exclusion test was used to determine the number of viable cells present in a cell suspension (Avelar-Freitas et al. 2014). The sample was diluted in a 0.4% Trypan Blue dye of an acid azo exclusion medium by preparing a 1:1 dilution of the cell suspension. Using the hemocytometer and after 2 minutes, non-viable cells were blue, viable cells were unstained. Trypan Blue was sterile and filtered before using it in order to get rid of particles in the solution that would have disturbed the counting process.
Immunoassay for Cytokines
Commercial enzyme-linked immunosorbent assay (ELISA) was used to quantify IL-6 and TNF- α according manufacturer’s instructions.
HPLC Conditions
C18 column (250mm × 4.6 mm I.D., 5 μm) was used for chromatographic separation, UV detection was employed at 280 nm, isocratic elution was used at a flow rate of 1.0 ml/min, and injection volume was set to 20 μΙ The mobile phase was prepared by mixing 180 ml of methanol with 820 ml of water for HPLC, and adding 2 ml of acetic acid. Standard solution of the eight phenolic compounds with a concentration of 100 ppm was prepared by dissolving 10 mg of each phenolic compound in 100 mL of mobile phase.
Statistical Analysis
The means of the concentrations of IL-6 and TNF-α were compared using Paired-samples t test using SPSS version 19. The differences with p < 0.05 were considered significant.
Results
Cytotoxicity of the Extracts
Eucalyptus spp. and Pistascia lentiscus leaf extracts at concentration of 480 μg/ ml and LPS at concentration of 1 μg/ ml have no obvious effect on the viability of the PMNCs when compared with the control group. Table 1 represents these results.
Table 1.
Effects of Eucalyptus, Pistascia lentiscus extracts on PMNCs viability
| Contents | % Viability |
|---|---|
| PMNCs only | 95.5±1.2 |
| PMNCs with LPS | 93.93±1.3 |
| PMNCs with LPS and 480 μg/ml Eucalyptus Extract | 90.1±1.0 |
| PMNCs with LPS and 480 μg/ml P. lentiscus Extract | 91.3±1.3 |
Anti-Inflammatory Activities of the Plant Extracts
Production of TNF-α and IL-6 increased significantly after 24 h by LPS-stimulated PMNCs. However when it was treated with Eucalptus spp. and P. lentiscus extracts with concentrations ranged from 60 μg/ml to 480 μg/ml, TNF-α and IL-6 in cell culture medium were all significantly reduced and the greater the concentration, the stronger the inhibition (Table 2).
Table 2.
Effect of Eucalyptus spp. and P. lentiscus extracts on production of IL-6 and TNf-α by PMNCs.
| Amount of IL-6 and TNf-α (pg/ml) | ||||
|---|---|---|---|---|
| Eucalyptus extract | P.lentiscus extract | |||
| IL-6 | TNf-α | IL-6 | TNf-α | |
| 1 | 9.7 | 60.3 | 11.3 | 61.7 |
| 2 | 565.0 | 1021.3 | 528.3 | 1000.7 |
| 3 | 451.0 | 444.3 | 229.7 | 385.3 |
| 4 | 304.3 | 334.0 | 148.0 | 311.0 |
| 5 | 238.7 | 208.7 | 74.0 | 184.7 |
| 6 | 44.7 | 149.3 | 36.0 | 115.3 |
HPLC Analysis of the Phenolic Compounds
Figure 1 shows the chromatogram of the eight phenolic compounds, while Figures 2 and 3 show chromatograms for the phenolic compounds detected in Pistascia lentiscus and Eucalyptus spp. leaf extracts respectively. Table 3 shows the phenolic compounds detected in the two tree species extracts. As we can see from Table 3, and figures 2, 3, Gallic acid is the main phenolic compound detected in both extracts with a concentration of 14.7, and 11.9 mg per 100 g of dry weight of the leaves, for Pistascia lentiscus and Eucalyptus spp., respectively.
Figure 1.
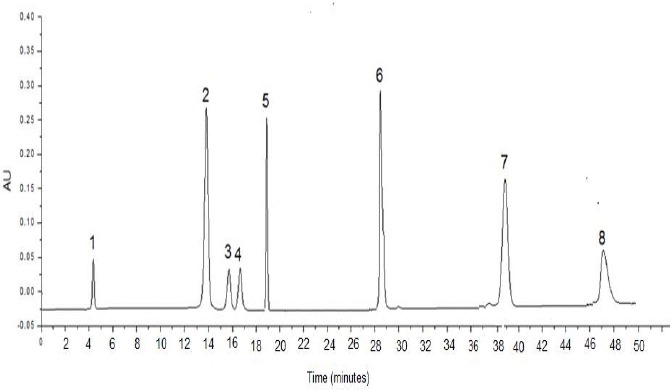
Chromatogram of the eight phenolic compounds investigated in this study. Mobile phase: methanol/water with 2% of acetic acid (18:82, v/v), flow rate 1.0 mL/min, injection volume 20 μL. Column: C18, 5 μm, 25 cm length, 4.6 mm inner diameter. UV detection: 280 nm. Analytes separated: (1) Gallic acid, (2) chlorogenic acid (3) Vanillic acid, (4) Caffeic acid, (5) Syringic acid, (6) p-coumaric acid, (7) ferulic acid, and (8) sinapic acid.
Figure 2.
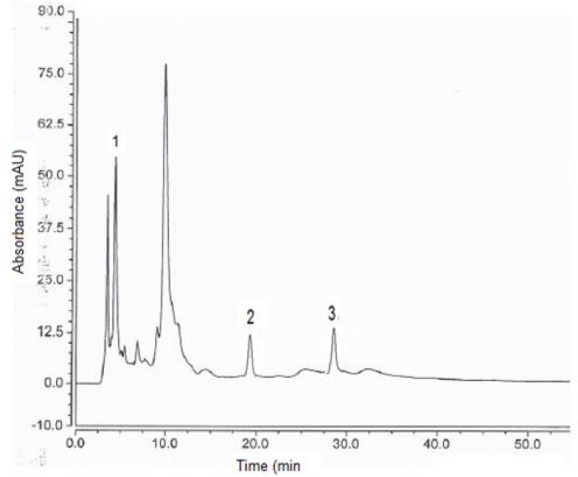
Chromatogram of Pistascia lentiscus ethanolic leaf extract. Analytes separated: (1) Gallic acid, (2) Syringic acid, (3) p-coumaric acid. For other experimental conditions, see legend of Figure 1.
Figure 3.
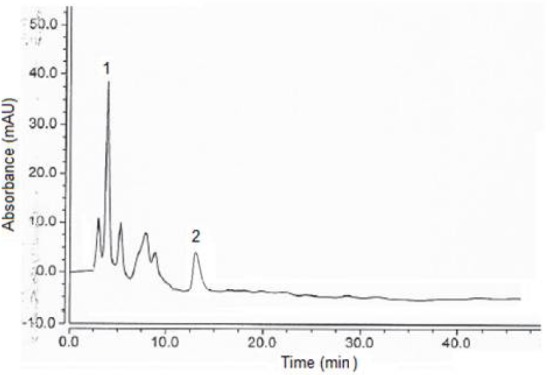
Chromatogram of Eucalyptus spp. ethanolic leaf extract. Analytes separated: (1) Gallic acid, (2) chlorogenic acid (3). For other experimental conditions, see legend of Figure 1.
Table 3.
Phenolic compounds detected in the extracts of the two plants (results are expressed as mg/100g DW*).
| Extract type | Gallic acid | Chlorogenic acid | Vanillic acid | caffeic acid | syringic acid | p-coumaric acid | Ferulic acid | sinapic acid |
|---|---|---|---|---|---|---|---|---|
| P.lentiscs | 14.7 | ND** | ND | ND | 0.16 | 0.17 | ND | ND |
| Eucalypts | 11.9 | 2.62 | ND | ND | ND | ND | ND | ND |
Dry Weight
Not Detected
Discussion
The extracts of Pisstascia lentiscus and Eucalyptus spp. were analyzed for their inhibitory effects on of IL-6 and TNF-α production from PMNCs, induced by LPS. The inhibition in the production of IL-6 and TNF-α increased in a dose-dependent manner. Furthermore, the differences in the inhibition of the cytokine production upon the effect of each dose were significant. It was shown that both extracts at concentration of 480 ml were not toxic to the PMNCs (Table 1). Such results show the strong ability of Pistascia lentiscus and Eucalyptus spp. leaf extracts to inhibit IL-6 and TNF-α production by the LPS-induced PMNCs while maintaining cell viability comparable to the control group.
The results obtained suggest that leaf extracts of both tree species have an anti-inflammatory effects by inhibiting the production of IL-6 and TNF-α from the LPS-induced PMNCs. Such results are consistent with the findings of Vigo et al. (Vigo et al. 2004), Grassmann et al. (Grassmann et al. 2000), Janakat et al. (Janakat et al. 2002), and Landau et al. (Landau et al. 2014). However, Pistascia lentiscus leaf extract has shown stronger effect in the inhibition of the release of both IL-6 and TNF-α than the extract of Eucalyptus spp.
Both extracts were analyzed by HPLC to identify their phenolic ingredients. Gallic acid was found to be the main phenolic compound in both extracts. It has a strong anti-inflammatory capability (Lin et al. 2015; Kang et al. 2015). However, Gallic acid concentration in Pistascia lentiscus extract is higher than in Eucalyptus extract (Table 2). Such finding supports our finding that Pistascia lentiscus is stronger anti-inflamtory agent than Eucalyptus spp.
Chlorogenic acid, an anti-inflammatory compound (Hwang et al. 2014) was revealed to be a phenolic component of Eucalyptus spp. extract while syringic acid and p-coumaric acid, which have a strong anti-inflammatory effects (Lin et al. 2014; Taofiq et al. 2015), were found to be a phenolic components of Pistascia lentiscus extracts.
Conclusion
Pistascia lentiscus and Eucalyptus spp. extracts are rich in phenolic components. These extracts also showed a strong reduction in the production of IL-6 and TNF-α from the LPS-induced PMNCs, indicating strong anti-inflammatory effects. Such results make the leaf extracts of Pistascia lentiscus and Eucalyptus spp. trees promising source of anti-inflammatory candidates to be used in the pharmacological industry.
References
- 1.Avelar-Freitas B.A, Almeida V.G, Pinto MCX, Mouräo FAG, Massensini A.R, Martins-Filho O.A, Rocha-Vieira E, Brito-Melo G.E.A. Trypan blue exclusion assay by flow cytometry. Braz. J. Med. Biol. Res. 2014;47(4):307–3015. doi: 10.1590/1414-431X20143437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bampouli A, Kyriakopoulou K, Papaefstathiou G, Louli V, Aligiannis N, Magoulas K, Magoulas K, Krokida M. Evaluation of total antioxidant potential of Pistacia lentiscus var. chia leaves extracts using UHPLC-HRMS. J. Food Eng. 2015;167:25–31. [Google Scholar]
- 3.Cho J.Y, Kim P.S, Park J, Yoo E.S, Baik K.U, Kim Y.K, Park M.H. Inhibitor of tumor necrosis factor-αproduction in lipopolysaccharide-stimulated RAW264.7 cells from Amorpha fruticosa. J. Ethnopharmacol. 2000;70(2):127–133. doi: 10.1016/s0378-8741(99)00154-3. [DOI] [PubMed] [Google Scholar]
- 4.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009;8(6):480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 5.Gbenou J.D, Ahounou J.F, Akakpo H.B, Laleye A, Yayi E, Gbaguidi F, Baba-Moussa L, Darboux R, Dansou P, Moudachirou M, Kotchoni S.O. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep. 2013;40(2):1127–1134. doi: 10.1007/s11033-012-2155-1. [DOI] [PubMed] [Google Scholar]
- 6.Grassmann J, Hippeli S, Dornisch K, Rohnert U, Beuscher N, Elstner E.F. Antioxidant properties of essential oils. Possible explanations for their anti-inflammatory effects. Arzneimittelforschung. 2000;50(2):135–139. [PubMed] [Google Scholar]
- 7.Hong J.W, Yang G.E, Kim Y, Eom S, Lew J.H, Kang H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complement. Altern. Med. 2012;12(1):237. doi: 10.1186/1472-6882-12-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang S, Kim Y.W, Park Y, Lee H.J, Kim K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014;63(1):81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- 9.Janakat S, Al-Merie H. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. J. Ethnopharmacol. 2002;83(1-2):135–138. doi: 10.1016/s0378-8741(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 10.Kang J, Thakali K, Jensen G, Wu X. Phenolic Acids of the Two Major Blueberry Species in the US Market and Their Antioxidant and Anti-inflammatory Activities. Plant Food Hum Nutr. 2015;70(1):56–62. doi: 10.1007/s11130-014-0461-6. [DOI] [PubMed] [Google Scholar]
- 11.Landau S, Muklada H, Markovics A, Azaizeh H. Traditional Uses of Pistacia lentiscus in Veterinary and Human Medicine. In: Yaniv Z, Dudai N, editors. Medicinal and Aromatic Plants of the Middle-East. Med. Arom. Plant World. Vol. 2. Springer; Netherland: 2014. pp. 163–80. [Google Scholar]
- 12.Lin W.H, Kuo H.H, Ho L.H, Tseng M.L, Siao A.C, Hung C.T, Jeng K.C, Hou C.W. Gardenia jasminoides extracts and gallic acid inhibit lipopolysaccharide-induced inflammation by suppression of JNK2/1 signaling pathways in BV-2 cells 2015. Iran. J. Basic Med. Sci. 2015;18(6):555–562. [PMC free article] [PubMed] [Google Scholar]
- 13.Lin J.T, Liu C.W, Chen Y.C, Hu C.C, Juang L.D, Shiesh C.C, Yang D.J. Chemical composition, antioxidant and anti-inflammatory properties for ethanolic extracts from Pleurotus eryngii fruiting bodies harvested at different time. LWT - Food Sci. Technol. 2014;55(1):374–382. [Google Scholar]
- 14.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 15.Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122(4):987–996. [Google Scholar]
- 16.Nathan C. Points of control in inflammation. (2002) Nature. 420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 17.Oe Y, Mochizuki K, Miyauchi R, Misaki Y, Kasezawa N, Tohyama K, Goda T. Plasma TNF-αIs Associated with Inflammation and Nutrition Status in Community-Dwelling Japanese Elderly. J. of Nut. Sci. and Vitaminol. 2015;61(3):263–269. doi: 10.3177/jnsv.61.263. [DOI] [PubMed] [Google Scholar]
- 18.Qabaha K.I. Antimicrobial and Free Radical Scavenging Activities of Five Palestinian Medicinal Plants. Afr. J. Tradit. Complement. Altern. Med. 2013;10(4):101–108. doi: 10.4314/ajtcam.v10i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salari M.H, Amine G, Shirazi M.H, Hafezi R, Mohammadypour M. Antibacterial effects of Eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin. Microbiol. Infect. 2006;12(2):194–196. doi: 10.1111/j.1469-0691.2005.01284.x. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez M. E, Pérez R.J, Fresán O.C, Pérez G.S. Anti-Inflammatory Effects of Hyptis albida Chloroform Extract on Lipopolysaccharide-Stimulated Peritoneal Macrophages. ISRN Pharmacol. 2013;2013:1–8. doi: 10.1155/2013/713060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverstein F.E, Faich G, Goldstein J.L, et al. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The class study: a randomized controlled trial. JAMA. 2000;284(10):1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 22.Taofiq O, Calhelha R.C, Heleno S, Barros L, Martins A, Santos-Buelga C, Queiroz M.J.R.P, Ferreira I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Internat. 2015;76(3):821–827. doi: 10.1016/j.foodres.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 23.Vigo E, Cepeda A, Perez-Fernandez R, Gualillo O. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A. 1 murine macrophages. J. Pharm. and Pharmacol. 2004;56(2):257–263. doi: 10.1211/0022357022665. [DOI] [PubMed] [Google Scholar]


