Abstract
Background:
Vascular dementia (VD) is the most frequent psychiatric complication of stroke, and is often difficult to treat. Incidence rate of vascular cognition impairment is still 70% after stroke in one year (Sui R et al.2011). Stroke patients with VD suffer from a higher mortality rate and have worse functional outcomes and quality of life. However, despite the extensive literatures on this topic, there is no agreement on the causal mechanisms and effective therapy for VD. The objective of this study is to examine if electroacupuncture at the Wangu acupoint (GB 12), whose position is similar to the cerebellar fastigial nucleus, could reduce inflammatory cytokines in the hippocampus of rats with vascular dementia (VD).
Materials and Methods:
The 54 healthy, male, Sprague-Dawley (SD) rats, 9 months old, and of clean grade (300-450) g, were randomly divided into three groups: sham surgery group, VD group and electro-acupuncture group. The ethology scores of VD rats were evaluated and the mRNA expressions of inflammatory cytokines (TNF-α, IL-6 and IL-1β) in the hippocampus were assessed and the hippocampal tissues were observed by hematoxylin-eosin staining.
Results:
Compared with the VD group, in the electroacupuncture group, the rats’ learning ability improved significantly and the mRNA expression of TNF-α, IL-6 and IL-1β decreased. Simultaneously, the damage extent of nerve cells in the hippocampal tissues decreased, with their morphology recovered to nearly normal.
Conclusions:
Electro-acupuncture at the Wangu acupoint can decrease the levels of inflammatory cytokines in the hippocampus, reduce the damage extent of nerve cells in the hippocampus, and thus provide a new neuroprotective method in VD
Keywords: Vascular Dementia, Hippocampus, Cytokines, Electroacupuncture, “Wangu” acupoint
Introduction
Vascular dementia (VD) is the most frequent psychiatric complication of stroke, and is often difficult to treat. Incidence rate of vascular cognition impairment is still 70% after stroke in one year (Sui et al.2011). Stroke patients with VD suffer from a higher mortality rate and have worse functional outcomes and quality of life. However, despite the extensive literatures on this topic, there is no agreement on the causal mechanisms and effective therapy for VD.
Neurobiochemistry studies (Li et al. 2005; Zhang et al. 2005), cellular and molecular mechanisms studies (Jones et al. 1989; Boulanger et al. 1990) have all reported the frequent involvement of hippocampal damage in the physiopathology of VD in stroke patients, thus the hypothesis of neuroanatomy has been raised by researchers. Besides, recent years’ studies have shown that abnormal cytokines network play an important role on nerve cells damage in cognition impairment after stroke(Angelopoulos et al.2008; Rosenberg 2009; Trollor et al.2010). Batti L et al (Batti L et al.2010) found that TNF-α could impair the recovery of synaptic transmission from hypoxia in rat hippocampal slices. Zuliani et al (Zuliani et al.2007) further found that levels of cytokines increased significantly in VD patients and there was strong correlation between the cytokines and the severity of VD. In addition to theoretical studies mentioned above, many treatment methods have been introduced for VD therapy, but few medicines could result in effective result.
The Wangu acupoint (GB 12), which has important effect on brain function, is the converging acupoint of the bladder meridian and the gall bladder meridian. The reason the present study selected the Wangu acupoint to treat VD is based on the fact that the cerebellar fastigial nucleus electric stimulation has a good therapeutic effect on focal cerebral ischemia (Huang et al.2008) which acts as the trigger factor of VD, and the position and manner of cerebellar fastigial nucleus are similar to the Wangu acupoint in Chinese medicine. In fact, the mode by which the fastigial nucleus electric stimulation works is that the Wangu acupoint is substituted with an electrode for fastigial nucleus electric stimulation. Up till now, there have been many studies on the neuroprotective effect of electroacupuncture, but the studies on VD by electroacupuncture are scarce. And studies on the effect of electroacupuncture at the Wangu acupoint on inflammatory cytokines in the hippocampal tissues, and the damage of nerve cells in the hippocampus of rats with VD have not yet been reported. Following the pathogenesis of disorder in hippocampus and changes in cytokines for VD, this study is to observe the effect of electroacupuncture at the Wangu acupoint on mRNA expression of inflammatory cytokines (TNF-α, IL-6 and IL-β) in the hippocampus of rats with VD.
Materials and Methods
Animals and VD Rats Model
Sprague-Dawley rats (Experimental Animal Center of Jinzhou Medical University, Jinzhou, China), maintained at an ambient temperature of 22-24 °C under a 12 h: 12 h light: dark cycle, were used in this experiment. Animals were divided into three groups: sham surgery group (control group) (only the middle cerebral artery was exposed), VD group and electroacupuncture group (VD +EA). Rats in the latter two groups were used for the VD models. Rats in the electroacupuncture group were given electroacupuncture at the Wangu acupoint. The electric stimulation kept on 20 minutes per day. Subsequently, 2 and 4 month post-stimulation, hippocampus tissues in each group were collected for further study, 54 rats were included in the final analysis.
Rat models of VD were induced by the Left middle cerebral artery occlusion (MCAO)-reperfusion method as described previously. Ischemia was induced by occluding the middle cerebral artery with intraluminal filament (18.5±0.5mm) for 1 h, the circulation was then restored for 48 h (VD group). Rats in the sham surgery group were treated with short intraluminal filament (10mm), which cannot contact the MCA. A neurological evaluation was performed 24 h after the reperfiision and scored on a 6-point scale (Longa et al. 1989): 0=no neurological deficit, 1 = failure to extend left forepaw fully, 2 = circling to the left, 3 = falling to the left, 4 = no spontaneous walking with depressed level of consciousness and 5 = death. All experiments complied with the Guidelines on Ethical Standards for the investigation in animals; the study was approved by Jinzhou Medical University Committee for the care and use of laboratory animals.
Electroacupuncture Parameter and Acupoint Location
The Wangu acupoint was located according to a previously published method (anatomical location: concave on posterior inferior of post aurem and mastoid region, depth: 0.3–0.5 cm) (Shen et al.2007). Electronic acupuncture needle (SDZ-II, Huatuo, Suzhou Medical Products Factory, China) was used. In this study, its parameter was regulated as follows: sparse wave 30 Hz, dense wave 100 Hz, and intensity 6–15 voltage. Slight vibration indicated successful location of the acupoint. The stimulation was about 20 minutes each time, once a day for 4 month.
Assessment of Learning by Morris Water Maze
The Morris water maze procedure was based on literature (Morris et al. 1984).
Behavioral Test
The behavioral test was conducted using Morris water maze. A circular tank (150 cm diameter, 50 cm deep) was filled with 17-cm deep water of 22~26°C. Four entering points were marked on the wall of the pool, and the pool was divided into four quadrants. The platform (12.5 cm diameter, 15 cm high) was placed in the third quadrant, and was submerged 2 cm below the water surface. The behavioral test began immediately right after treatment.
Navigation Test
On 2 and 4 month, rats in all groups experienced Morris water maze test for 4 days with four trials per day. The animals were trained 4 times a day, every training interval 20 minutes. Each trial began by releasing the rat into the water, with its face toward the pool wall at the one of the four placement points. The time to find the platform within a 2-min limit was recorded. If the rat failed to find the platform within 2 min, the experimenter moved the animal to the platform, where it remained for 10 seconds. The escape latency was recorded as 2 min.
Spatial Probe Test
The platform was removed from the pool, and rats were allowed to search for the platform for 120 seconds. Swim paths were recorded to determine search strategies, which including search along the edge (edge type), random swim path (random type), a trend toward a straight swim path (trend type), and a linear swim path (line type).
The PCR Experiments
2 and 4 month later, after assessment of learning by Morris water maze, hippocampus (according to rat brain atlas (Paxinos et al.2007)) of rats in each group were separated rapidly from brain tissues. The total RNA was then extracted from the hippocampus samples using Trizol (TaKaRa) reagent according to the manufacturer’s instructions. The polymerase chain reaction (PCR) procedure started with an initial step at 50° for 2 min, then 95° for 2 min, followed by 40 cycles of denaturation at 94° for 15 s, annealing at 58–62° for 15 s and extension at 72° for 15 s. Melt curve analyses were performed at the end of each PCR; if multiple peaks were observed, the data were not used. Each threshold cycle (Ct) value was calculated by taking an average of values obtained from triplicates samples. The mean of the control Ct values were computed and the AC t values were determined by subtracting the average βactin Ct value from control and experimental Ct values for each sample. Gene expression was evaluated using a comparative Ct method (User Bulletin #2 ABI PRISM 7900HT sequence detection system, PE Applied Biosystems) using the formula2 (ΔC t for exp from the control mean)— (ΔC t for control from the control mean)
Hippocampal Tissues of Rats
Simultaneously, the hippocampal tissues of rats in each group on 4 month were embedded in paraffin, sliced, dewaxed and hydrated. Subsequently, the sections were mounted by buffered glycerine, and observed by optical microscope (x400).
Statistical Analysis
The differences among experimental groups were detected by one-way analysis of variance (ANOVA). Between groups, variance was determined using the LSD and Student–Newman–Keuls post hoc test.
Results
Effect on Escape Latency Time (ELT) using Morris Water Maze
In this study, rats in all groups experienced Morris water maze test. The sham surgery group showed a downward trend in their escape latency time (ELT), and there were significant fall in day 5 ELT when compared with day 1 ELT of these rats, reflecting normal learning ability. In contrast, compared with the sham group, VD rats showed a significant increase in day 4 ELT, indicating impairment of acquisition. However, compared with the VD group, rats in the electroacupuncture group showed a significant decrease in day 4, reflecting electroacupuncture can improve the VD rats’ learning ability. (Fig. 1)
Figure 1.
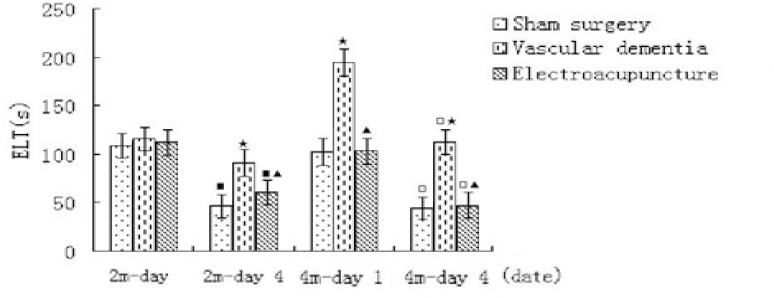
Escape latency time (ELT) on Morris water maze in each group on 2 and 4 month. ■P<0.01, compared with the corresponding group in day 1 ELT on 2 month; □P<0.01, compared with the corresponding group in day 1 ELT on 4 month; ▲ P<0.01, compared with the VD group in day 4 ELT on 2 month, and in day 1ELT and 4 ELT on 4 month respectively.
Electroacupuncture Effects on Nerve Cells in Hippocampus
Results from HE staining of the hippocampus after 4 month showed that normal and abnormal nerve cells were evident in all groups by optical microscope (x400). The normal nerve cells were relatively round with a clear boundary. The damaged nerve cells were swollen and displayed altered symmetry, or were concentrated and trachychromatic. All the damaged nerve cells were triangular which signifies ischemic necrosis. The characteristics of the damaged nerve cells were evident in the VD group. Treatment intervention in the electroacupuncture group mitigated the damage of nerve cells, lessened tissue edema, reverting morphologies of the nerve cells to nearly normal, and increasing the numbers of nerve cells (Fig. 2).
Figure 2.
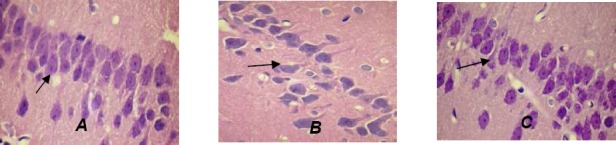
Changes in nerve cells in hippocampal CA1 region in each group (hematoxylin-eosin staining, x400). (A) Sham surgery group: nerve cells showed normal shapes, and were lined up orderly and closely. (B) VD group: nerve cells were lined up sparsely, with enlarged intercellular space and large number of pyramidal cells absent. (C) EA + VD groups: nerve cells were lined up in approximately normal order with parts of cells slightly absent. The damaged nerve cells (arrows) were triangular.
Levels of TNF-α, IL-6and IL-Ιβ mRNA by Real Time PCR Technique
After month 2 and month 4, the expressions of TNF-α mRNA, IL-6 mRNA and IL-1β mRNA were determined by real time PCR technique. The mRNA expressions of TNF-α, IL-6 and IL-1β in the VD group were significantly greater than that of the stroke group (P < 0.01). In contrast, the expression of these inflammatory factors mRNA in the electroacupuncture group (EA group) was lower than that of the VD group (P < 0.01 or P < 0.05) (Fig.3 A-C).
Figure 3.
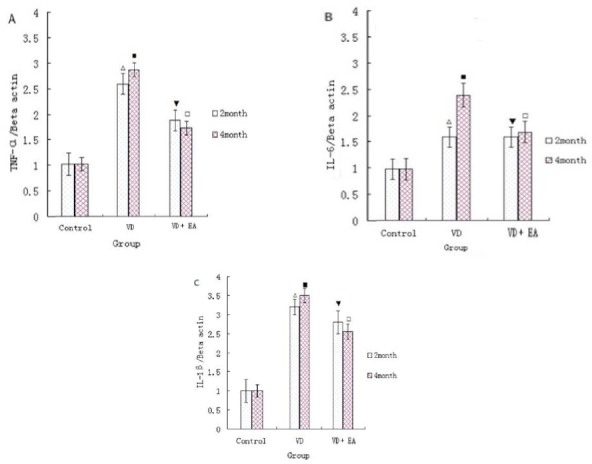
(A)-(C): The mRNA expression of TNF-α mRNA, IL-6 mRNA and IL-1β mRNA in the hippocampal tissues on 2 and 4 month respectively. ΔP<0.01, compared with the the control group (sham surgery group) on 2 month, and ■P<0.01, compared with the control group (sham surgery group) on 4 month; ▼P<0.05, compared with the VD group on 2 month, and □P<0.01, compared with the VD group on 4 month.
Discussion
The results from the present study showed that electroacupuncture at the Wangu acupoint significantly decreased VD rats’ day 4 ELT, indicating the rats’ amelioration in learning ability, and thus improves behaviors in VD rats. Zhou et al.(Zhou et al. 2011)similarly showed that appropriate electroacupuncture treatment protects the brain from cerebral ischemia by increasing blood flow to the ischemic brain region via neural regulation. However, till now, studies on VD by electroacupuncture are strikingly scarce.
The present study mainly focused on observing the effect of electroacupuncture at the Wangu acupoint on mRNA expression of inflammatory cytokines (TNF-α, IL-6 and IL-1β) in the hippocampal tissues, and evaluated cellular pathology in the hippocampus of VD rats. Compared with the VD group, the electroacupuncture groups decreased the mRNA expression of inflammatory cytokines, and relieved nerve cells damage in the hippocampus. The results showed that electroacupuncture at the Wangu acupoint produced a good therapeutic effect for VD. The mechanism that inflammatory cytokines are reduced by electroacupuncture at the Wangu acupoint remains unclear. Ni et al.(Ni et al 2010) provided evidence that cerebellar fastigial nucleus lesions accelerate the differentiation of thymocytes into mature helper T lymphocytes in the thymus and enhance function of the helper T cells in the peripheral immune tissue. Their result suggests a substantial modulation of immune system by the cerebellar fastigial nucleus. Previous study has also shown that electric stimulation at the cerebellar fastigial nucleus not only inhibits the excessive proliferation of astrocytes, but also relieves the structural damage of astrocytes in neonatal rats following hypoxic-ischemic brain damage (Li et al. 2011). Therefore, the stimulation of cerebellar fastigial nucleus (Wangu acupoint) might inhibit immune system and thus prevent inflammatory cytokines from being excessively produced. Thus, electroacupuncture at the Wangu acupoint may stabilize the cytokines network, lessen the impairments of hippocampus and therefore improve symptoms of VD.
To date, the pathogenesis of VD remains unclear and diverse theories of VD have been proposed. At present, it is accepted that the occurrence of VD is closely associated with neuroanatomy (especially for hippocampal neuronal injury) (Li et al. 2005;Zhang et al. 2005;Jones et al. 1989; Boulanger et al. 1990), and cytokines network disorder (Zuliani et al.2007). However, there are no correlations among these different hypotheses of VD. In the present study, the results revealed that there is not only the increased expression of inflammatory cytokines but also the damaged nerve cells in hippocampal tissues in the VD group.
The result is meaningful because it provides evidence of a potential relationship between the theory of cytokines and mechanism of neuroanatomy for VD. Thus, the findings provide evidence that inflammatory factors cause damage to nerve cells in the hippocampus, and then the dysfunctional hippocampus results in VD. Recently, Seguin et al.(Zuliani et al.2007) assessed whether IL-1β and TNF-1β influenced cellular proliferation within the hippocampus in rats. Their result showed that systemic administration of TNF-α reduced 5-bromo-2-deoxyuridine labeling within the hippocampus, whereas IL-1β had no such effect. Actually, the IL-1β increased cellular proliferation within the hippocampus. The different effects of cytokines on nerve cells in the hippocampus may be partly a reflection of the different diseases (such as AD versus VD) and/or different physiological status (such as in vitro versus in vivo) of diseases, suggesting that cytokines may differentially regulate hippocampal plasticity in different neuropsychiatric conditions. The discovery of the relative relationships among the two important mechanisms of VD may lead to new theories on the pathogenesis and new therapeutic approaches for VD. Furthermore, to detect levels of inflammatory cytokines in hippocampal tissues of VD is essential and necessary for investigating the mechanism underlying VD and evaluating different intervention methods, including electroacupuncture.
The limitation of the current study is that due to the relatively short time of observation of changes in inflammatory cytokines, the long-term effects of them on hippocampus are absent. In addition, other parts of brain tissues of rats with VD should also be observed in future research.
The findings of this study indicate that electroacupuncture at the Wangu acupoint can improve symptoms of VD in rats by decreasing expression of inflammatory cytokines and therefore alleviating damage extent of nerve cells of brain tissues in VD rats. These findings may provide a new avenue for exploring the pathogenesis and selecting therapeutic targets for VD.
Competing Interests
The authors declare that they have no competing interest.
Authors’ Contribution
SRB conceptualized the design and conduct of the experiment, FYN conceptualized the design of the study, assisted with data analysis, interpretation and writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr Dianzhu Pan and Dr Xidong Li for assisting in the data entry and analysis. We thank Lei Zhang for indicating the test. The study was supported by National Natural Science Foundation of China (NO: 81241050 and NO: 81371461)
References
- 1.Angelopoulos P, Agouridaki H, Vaiopoulos H, Siskou E, Doutsou K, Costa V. Baloyiannis SI: Cytokines in Alzheimer’s disease and vascular dementia. Int J Neurosci. 2008;118:1659–1672. doi: 10.1080/00207450701392068. [DOI] [PubMed] [Google Scholar]
- 2.Batti L, O’Connor JJ. Tumor necrosis factor-alpha impairs the recovery of synaptic transmission from hypoxia in rat hippocampal slices. J Neuroimmunol. 2010;18:21–27. doi: 10.1016/j.jneuroim.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger C, Luseher TF. Release of endothelin from the porcine aorta: Inhibition by endothelium-derived nitric oxide. Clin Invest. 1990;85:587–591. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YJ, Luo Y. Effect of electrical stimulating to fastigial nucleus onproliferation of neural stem cell in brain of adult rat after focal cerebral ischemia/reperfusion. Chinese journal of rehabilitation medicine. 2008;23:211–215. [Google Scholar]
- 5.Jones CR, Hiley CR, Pelton JT, Mohr M. autoradiographic visualization of the binding sites for [125I] endothelin in rat and human brain. Neurosci Lett. 1989;97:276–279. doi: 10.1016/0304-3940(89)90610-1. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Jiang LG, Xu ZX, Rao ML. Apoptosis and delayed neuronal death in central nervous system of experimental mice with vascular dementia. Chinese journal of rehabilitation. 2005;9:133–135. [Google Scholar]
- 7.Li XL, Jia TM, Luan B, Liu T, Yuan Y. Effects of electric stimulation at the cerebellar fastigial nucleus on astrocytes in the the hippocampus of neonatal rats with hypoxic-ischemic brain damage. Chinese journal of Contemporary Pediatrics. 2011;13:317–320. [PubMed] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral Artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Morris R. Developments of a water maze producer for studying spatial learning in the rats. J Neurosci Methods 1984. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 10.Ni SJ, Qiu YH, Lu JH, Cao BB, Peng YP. Effect of cerebellar fastigial nuclear lesions on differentiation and function of thymocytes. J Neuroimmunol. 2010;222:40–47. doi: 10.1016/j.jneuroim.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. 4th Edition. San Diego: Academic Press; 2007. [Google Scholar]
- 12.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40:20–23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen XY, Wang H. Acupuncture and Moxibustion. 2nd Edition. BeijingPeople’s Health Publishing House; 2007. [Google Scholar]
- 14.Sui R, Zhang L. Cerebellar dysfunction may play an important role in vascular dementia. Med Hypotheses. 2012;78(1):162–5. doi: 10.1016/j.mehy.2011.10.017. doi:10.1016/j.mehy.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, Crawford J, Brodaty H, Sachdev P. Systemic inflammation is associated with MCI and its subtypes, the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;130:569–578. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Wu HQ, Zhang HX. Neurobiochemical Mechanisms of Vascular Dementia. Foreign medical sciences (cerebrovascular diseases) 2005;113:672–675. [Google Scholar]
- 17.Zhou F, Guo JC, Cheng JS, Wu GC, Xia Y. Electure increased cerebral blood flow and reduced ischemic brain injury, Dependence on stimulation intensity and frequency. J Appl Physiol. 2011;2011;111:1177–1187. doi: 10.1152/japplphysiol.00313.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuliani G, Ranging M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res. 2007;2007;41:686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]


