Abstract
Background:
Alpinia oxyphylla fruit (AOF, Yizhi in Chinese) is a well-known traditional Chinese medicine as an anti-diuretic agent and composed of two parts i.e. seed and shell. These two parts have different components, but the bioactivity differences of the two parts are not clear. This study aims to evaluate the different anti-diuretic effects of the seed and shell of AOF.
Materials and Methods:
The potential bioactive components were analyzed by UPLC-Q-TOF-MS. The diuretic and anti-diuretic activity was determined with saline-loads rats.
Results:
The results showed that the 200 mg/kg and 400mg/kg of SREAO displayed a short-time anti-diuretic activity 1h after administration and then a significant diuretic activity was being observed at 5-6 h in 400mg/kg group of SREAO. And the 400mg/kg doses of SREAO also showed a remarkable increase for electrolyte excretion of K+. Three sesquiterpene compounds, namely oxyphyllol A (1), oxyphyllol B (2), and nootkatone (3) were identified from the active SREAO fraction by UHPLC-ESI-Q-TOF/MS.
Conclusion:
The seed part of Alpinia oxyphylla possessed pronounced diuretic and anti-diuretic effect. The sesquiterpene components are the major constituents and possibly contributed the diuretic and anti-diuretic activity.
Keywords: Alpinia oxyphylla seed, Diuretic and anti-diuretic activities, sesquiterpene components, UHPLC-Q-TOF/MS
Introduction
Alpiniae oxyphyllae fructus (AOF, Yizhi in Chinese), the dried fruits of Alpinia oxyphylla Miq. (Zingiberaceae) is widely distributed in South-China. AOF is known traditionally to have anti-diuresis and anti-diarrhea action, and has been used for the treatment of diarrhea, enuresis and salivation for more than 1000 years (State Administration of traditional Chinese medicine “Chinese Maeria Medica” editorial board,1999). Phytochemical investigations showed that crude drug have several kinds of components such as sesquiterpenes, diphenylheptanes, flavones and volatiles (Luo et al., 2000; Zhang et al., 1997; Xie et al., 2014; Han et al., 2007; Bian et al., 2013). Modern pharmacological investigations have shown that its main compounds of nootkatone, yakuchinone A and tectochrysin have various bioactivities, such as anti-diarrhea, anti-inflammation, cardiac apoptosis protective effects, anti-ulcer (Zhang et al., 2012; Chang et al., 2013; Zhang et al., 2015; Shi et al., 2015; Xu et al., 2012, 2013; Yu et al., 2013; Chen P et al., 2014).
Our previous research showed the fruits of Alpinia oxyphylla can easily separate into two different parts by knocking i.e. the seed and shell. By the UFLC-MSMS analysis we found that the sesquiterpenes is mainly consisted in the seed, while the diphenylheptanes existed in the shell of the fruits (Chen F, 2014). Recent pharmacological studies have shown that the fruit can reduce the urine volume in the saline-loads rats (Li WB et al., 2013). But the bioactive fractions or the components responsible for the anti-diuretic activity and the anti-diuretic mechanism of AOF remain unclear. So the primary aim of this study was to evaluate the anti-diuretic effectiveness of two ethanol extracts respectively obtained from the seed (Sesquiterpenes-Rich Extraction, SREAO) and the shell (Diphenylheptanes-Rich Extraction, DREAO). In order to clarify the contribution of the different kind of compounds to the anti-diuretic activity of AOF, these two extracts were orally administrated to the rat. And then the urine excretion rate, pH and electrolyte excretion were measured in normal rats. The chemical constituents of the anti-diuretic SREAO fractions were analyzed by the method of UHPLC-Q-TOF/MS.
Materials and Methods
Materials
AOF was collected from Qiongzhong County, Hainan Province in June 2014, and was identified by Prof. Y.B. Li (Hainan Medical University, Haikou, Hainan, China). A voucher specimen (HY-20140602) was deposited at School of Pharmacy, Hainan Medical University.
Extracts Preparation
AOF (1000g) was broken by knock and separated to the seed part (628g) and the shell part (365g). The seed part of AOF was extracted with 95% ethanol under reflux, and then concentrated under reduced pressure to yield an ethanolic extract (50.4g). The ethanolic extract (40g) wad submitted to column chromatography over HP-20 macroporous resin eluted with 50% ethanol and 90% ethanol respectively. The 90% ethanol elution was concentrated under reduced pressure to yield brown oil (SREAO, 12.1g).
The shell part of AOF was extracted with 95% ethanol and yielded an extraction (30.7g). The 25g of extract was treated with HP-20 macroporous resin as the same process of seed. Then the 90% ethanol elution of shell was concentrated under reduced pressure and obtained a reddish brown extract (DREAO, 6.7g).
Chromatography Analysis
The nootkatone and yakuchinone A were the main compounds of sesquiterpenes and diphenylheptanes in fruit. So the content of nootkatone and yakuchinone A in the SREAO and DREAO were detected by HPLC-UV. The analysis was done as the previous method (Li YH et al., 2013) with minor modify. Briefly the LC analysis was carried out on a Shimadzu LC-20A HPLC system (Shimadzu Corp., Tokyo, Japan). A Phenomenex Luna C18 column (250 mm χ 4.6 mm, 5 μ m) was employed and the column temperature was maintained at 30°C. The mobile phase was composed of A (water) and B (acetonitrile) using a gradient elution: 70% A from 0 to 15.0 min, 70-56% from 15.0 to 45.0min, 56% from 45.0 to 55.0min, 56-30% from 55.0 to 60.0min with a flow rate set at 1.0 mL/min. The injection volume was 5 μ L. The Labsolution Analyst software packages were used to control the LC system.
For the analysis, 25mg of SREAO and DREAO were dissolved with 10ml of acetonitrile in an ultrasonic bath for 10 min. All solutions were filtered through a 0.45μm membrane filter before injection.
Diuretic and Anti-Diuretic Activity
This study was conducted in accordance with the Experimental Animal Administration regulations issue by the State Committee of Science and Technology of the People’s Republic of China. All procedures described here had prior approval from the Institutional Animal Care and Use Committee at the Hainan Medical University (Haikou, China). Male Wistar rats weighing 250-300 g were housed in standard conditions with free access to commercial chow and water. Animals were kept at a room temperature of 22±2°C with a light/dark cycle of 12/12 h. the experiments were conducted in accordance with internationally accepted standard procedures for the animal use.
The diuretic and anti-diuretic activity was determined according to the previous method (Kau et al., 1984) with some modifications. Brief, rats after overnight fasting and water ad libitum were divided into eight groups (n=6). Before treatment, all animals received isotonic saline (0.9% NaCl) with an oral dose of 5% body weight to impose a uniform water load. Thirty minutes later, the SREAO and DREAO extract were orally administered to the animals at doses of 100,200,400mg/kg BW respectively. Negative control group received the same amount of distilled water and positive control received 10mg/kg BW of furosemide. Each rat was individually placed in a metabolic cage, and the cumulative urine output was determined at hourly intervals for 6h. The electrolytes (Na+, K+ and Cl-) concentrations and pH were determined in the 6h urine samples from the rats.
Analytical Procedures
The urine Na+, K+ and Cl- concentrations were measured using flame photometry. The concentrations of Cl- were quantified by argentimetry (titration). The pH of fresh urine samples was determined using a pH-meter.
Statistical Analysis
The results were expressed as the mean ± S.E. (standard error of mean). The statistical differences were performed by analysis of variance (ANOVA) followed by Student’s t-test for multiple comparisons. A p value less than 0.05 were considered statistically significant.
Analysis of Active Fractions
The sample solution was prepared by dissolving 1.0 mg of fraction C in 10mL of methanol. UHPLC-DAD-Q-TOF-MS/MS analysis was performed with a Waters Acquity™ Ultra Performance LC system (Waters Corp., Milford, MA) equipped with HSS T3 column (2.1 mmx100mm, 1.8 μ m, UK). Mobile phase A was water with 0.1% formic acid, while mobile phase B was acetonitrile modified by the addition of 0.1% formic acid. A solvent gradient system was used: 95-70% A from 0 to 3.0 min, 70 45% A for 3.0 13.0 min, 45 5% A for 13.0 16.0 min, 5.0% A for 16.0 18.0 min, and 5.0 95% A for 18.0 20.0 min. The flow rate was 0.4 ml/min. The analytical column and auto-sampler were maintained at temperature of 35 °C and 4 °C, respectively. Every 2 μ l sample solution was injected for each run.
Results and Discussion
Phytochemical Analysis
The phytochemical analysis of two extracts showed that the contents of nootkatone in SREAO and DREAO were 18.2% and 5.34%, respectively. While the contents of yakuchinone A in SREAO and DREAO were 0.81% and 21.9%.
Urinary Excretion Volume
The data obtained after the oral administration of SREAO and DREAO are shown in Fig.1 significant decreasing in urinary excretion was observed for 200 mg/kg and 400mg/kg of SREAO for 1h (45.23% and 47.12%, p < 0.001), as well as for 3, 4h of 200 mg/kg (30.53% and 29.36%, p < 0.01). While the 400mg/kg dose of SREAO produced a remarkable increase in the urinary volume excretion at 5 and 6h (69.64% and 71.74% respectively, p < 0.01). From the result, it shows that the SREAO display a short-time anti-diuretic activity after the SREAO administrated, then the anti-diuretic activity decreased with the time extend, especially for 5 and 6h of the 400mg/kg administrated, a significant diuretic activity was observed.
Figure 1.
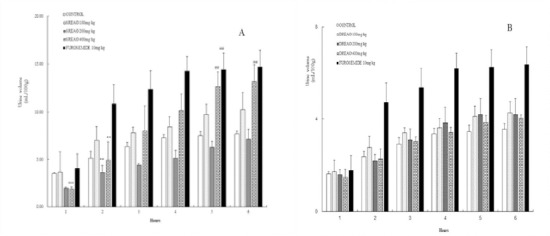
Time courses of diuretic and anti-diuretic activities of single oral doses (100, 200, 400mg/kg) of SREAO(A), (100, 200 and 400mg/kg) of DREAO(B), and the reference drug, furosemide (10mg/kg). The volume of excrete d urine was measured at 1,2,3,4,5 and 6h after administration. The cumulative values are reported as the mean ± S.E. for6 rats in each group.**P<0.01, ***P<0.001compared with the controls using Student’s t-test for anti-diuretic activity. ##P<0.01 compared with the controls using Student’s t-test for diuretic activity
Electrolyte Excretion
Table 1 shows the results of electrolyte excretion after oral administration of SREAO and DREAO. The 400mg/kg doses of SREAO showed are remarkable increase for urine volume and electrolyte excretion of K+ (12.4%, P<0.01 compared with the control group). The Na+/K+ ratio was also remarkable decreased by the 400mg/kg doses of SREAO (22.7%%, P<0.05 compared with the control group). The 100mg/kg doses of SREAO show significant increase of urine electrolyte excretion K+, but the results did not reach statistical significance. The 200 and 400 mg/kg doses of DREAO produced a slight decrease in Na+ (7.17% and 7.48%, respectively) and increase in K+ excretion(8.86% and 9.91%, respectively), which was in agreement with the total urine output.
Table 1.
Effect of acute oral administration of SREAO, DREAO, and furosemide on urinary electrolyte excretion
| Group | pH | Urine electrolytr concentration | Saluretic indexa | Na+/K+ | ||||
|---|---|---|---|---|---|---|---|---|
| Na+(mmol/L) | K+(mmol/L) | Cl- (mmol/L) | Na+ | K+ | Cl- | |||
| Control | 6.12±0.21 | 45.21±1.16 | 25.73±1.83 | 142.16±9.45 | 1.00 | 1.00 | 1.00 | 1.76±0.16 |
| Furosemide (10mg/kg) | 6.33±0.19 | 67.14±11.33* | 32.41±3.54* | 132.35±32.75 | 1.49 | 1.26 | 0.93 | 2.08±0.34 |
| SREAO (100mg/kg) | 6.15±0.14 | 44.83±16.37 | 30.76±16.08 | 149.02±32.26 | 0.99 | 1.20 | 1.05 | 1.61±1.34 |
| SREAO (200mg/kg) | 6.39±0.08 | 40.11±4.97 | 25.25±3.01 | 139.22±37.82 | 0.89 | 1.13 | 0.98 | 1.65±0.35 |
| SREAO (400mg/kg) | 6.25±0.24 | 41.05±4.60 | 28.95±1.23** | 113.73±31.45 | 0.91 | 0.98 | 0.80 | 1.33±0.29* |
| DREAO (100mg/kg) | 6.26±0.13 | 43.38±5.75 | 25.59±5.45 | 103.14±35.58 | 0.96 | 0.99 | 0.73 | 1.77±0.56 |
| DREAO (200mg/kg) | 6.19±0.08 | 41.97±2.81 | 28.01±5.15 | 129.41±55.10 | 0.93 | 1.09 | 0.91 | 1.50±0.15 |
| DREAO (400mg/kg) | 6.29±0.23 | 41.83±6.19 | 28.28±3.58 | 132.15±49.36 | 0.93 | 1.10 | 0.93 | 1.49±0.20 |
The pH values of the urine treated with SREAO and DREAO were higher than in the control group, but the results did not reached statistical significance.
Chemical Analysis
Initial UHPLC-DAD-Q-TOF-MS scanning of SREAO suggested that during the positive ion mode, the major peaks elicited good responses. Further experiments were therefore carried out in the positive mode. A typical BPI chromatogram was shown in Fig. 2. Through extensive MS and MS/MS analysis, three compounds (1-3) were identified in the fraction, and nootkatone was confirmed by comparing their retention times and MS data with those of the authentic compounds.
Figure 2.
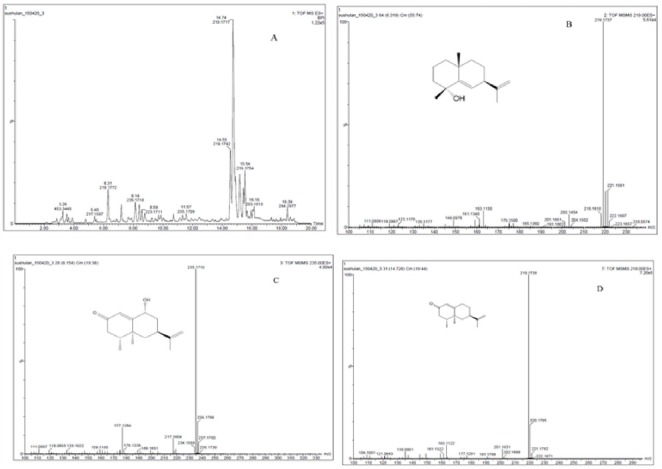
The BPI chromatogram of the SREAO fraction (A), and the MS2 spectrums for Oxyphyllol A (B), Oxyphyllol B (C) and Nootkatone (D).
Table 2.
Chromatographic and MS data of compounds identified in SREAO
| No. | tR (min) | Compound | [M+H]+(m/z) | Formula | Product ions (relative abundance %) |
|---|---|---|---|---|---|
| 1 | 6.31 | Oxyphyllol A | 221.1561 | C15H24O | MS2[221]: 221(18), 219(100), 203(6), 163(9) |
| 2 | 8.15 | Oxyphyllol B | 235.1710 | C15H22O2 | MS2[235]: 235(100), 217(8), 177(16) |
| 3 | 14.74 | Nootkatone | 219.1743 | C15H22O | MS2[219]: 219(100), 201(5),163(8) |
corresponded to the bicyclic moiety. By matching the MS and MS2 data with available reference (Xu et al., 2009), it was tentatively assigned as Oxyphyllol A, the possible fragmentation pattern was shown in Fig. 3.
Figure 3.
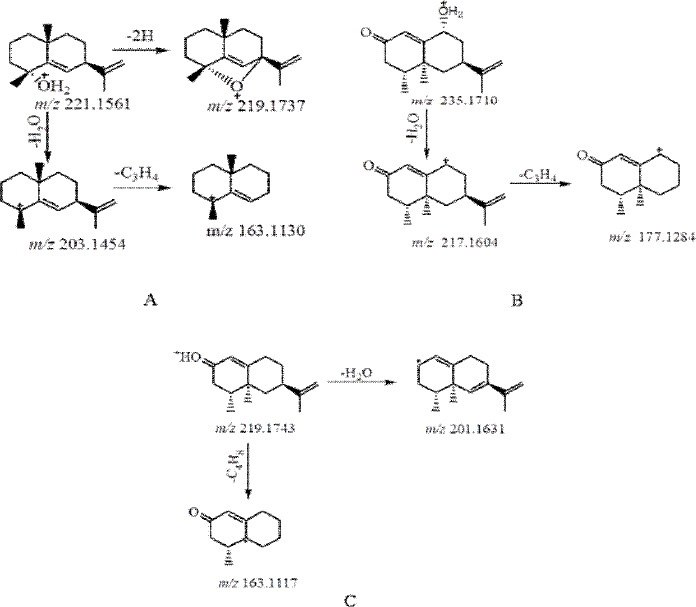
Fragmentation pathway of Oxyphyllol A (A), Oxyphyllol B (B) and Nootkatone (C)
Compound 2 showed an accurate mass of [M+H]+ ion at m/z 235.1710 corresponding to the molecular formula C15H22O2. In the MS/MS spectrum, signals for ion at m/z 217.1604 [M+H-18]+ via the loss of H2O and the characteristic ion at 177.1284 [M+H-18-40]+ that indicated loss of a isopropyl, suggesting compound 2 contain a hydroxyl and a isopropyl. According to its MS data and LC retention time with literature (Morikawa et al., 2002), compound 2 was deduced as Oxyphyllol B and the possible fragmentation pattern was shown in Fig.3.
Compound 3 was deduced as nootkatone compared with the authentic compound and its MS data. The possible fragmentation pattern of compounds 3 was shown in Fig.3.
From above results, we can infer that the main constituents of SREAO were sesquiterpene compounds. But beside these three compounds, there are still some peaks were unknown in the UPLC-QTOFMS chromatogram. It is possible that unknown anti-diuretic constituents are also present in SREAO fraction. The overall result indicated that the significant anti-diuretic activity of SREAO could be attributed to the presence of multiple compounds, but efforts are still needed to clarify exactly the active principles of the SREAO fraction. The results have provided evidence for further development and utilization of SREAO.
Conclusion
This study examined the diuretic and anti-diuretic activities of multiple doses of SREAO and DREAO in rats. The results showed the 200mg/kg and 400mg/kg doses of SREAO produced significant anti-diuretic activity within 1h (45.23% and 47.12%, respectively), and the urine volume increased with time extending, at 6h the 400mg/kg doses of SREAO produced a significant diuretic activity (71.74%). The 6h of electrolyte excretion show that 400mg/kg doses of SREAO can increase the K+ excretion consistent with the urine volume excretion. This result indicated that the SREAO possessed dual effect on renal function, including inhibiting diuretic activity at initial stage (1h) and promoting diuretic activity at later stage (5h and 6h), which is different than other diuretics in natural medicine. For the diuretic activity of DREAO, it did not present an obvious effect on the urine volume and electrolyte excretion. This indicated that the SREAO fraction was the active ingredient of Alpinia oxyphylla anti-diuretic activity. And the phytochemical study of SREAO revealed that the sesquiterpenes were the main compounds. So the sesquiterpene compounds contributed to the anti-diuretic active of Alpinia oxyphylla.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81560738, http://www.nsfc.gov.cn/,YHL), Technology Application Demonstration Project of Hainan Province, China (No.ZDXM2014071, http://nyinfo.hnkjonline.net/,YHL) and Hainan Provincial Important Projects, China (No. ZDZX 2013008-3, http://nyinfo.hnkjonline.net/,JQZ).
References
- 1.Bian QY, Wang SY, Xu LJ, Chi-On Chan, Mok DK, Chen SB. Two new antioxidant diarylheptanoids from the fruits of Alpinia oxyphylla. J Asian Nat Prod Res. 2013;15:9–10. doi: 10.1080/10286020.2013.816297. [DOI] [PubMed] [Google Scholar]
- 2.Chang YM, Tsai CT, Wang CC, Chen YS, Lin YM, Kuo CH, Tzang BS, Chen RJ, Tsai FJ, Huang CY. Alpinate oxyphyllae fructus (Alpinia Oxyphylla Miq) extracts inhibit angiotensin-II induced cardiac apoptosis in H9c2 cardiomyoblast cells. Biosci Biotechnol Biochem. 2013;77:229–234. doi: 10.1271/bbb.120541. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Li HL, Tan YF, Guan WW, Zhang JQ, Li YH, Zhao YS, Qin ZM. Different accumulation profiles of multiple components between pericarp and seed of Alpinia oxyphylla capsular fruit as determined by UFLC-MS/MS. Molecules. 2014;19:4510–4523. doi: 10.3390/molecules19044510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Wang PP, Jiao ZZ, Xiang L. Sesquiterpenoids from the Fruits of Alpinia oxyphylla and their Anti-Acetylcholinesterase Activity. Helvetica Chimica Acta. 2014;97:388–397. [Google Scholar]
- 5.Han JT, Lee SY, Lee YH, Beak NI. Antioxidative diarylheptanoids from the fruits of Alpinia oxyphylla. Food Sci Biotechnol. 2007;16:1060–1063. [Google Scholar]
- 6.Kau ST, Keddi JR, Andrews D. A method for screening diuretic agents in the rats. J Pharmacol Methods. 1984;11:67–75. doi: 10.1016/0160-5402(84)90054-8. [DOI] [PubMed] [Google Scholar]
- 7.Li YH, Lai WY, Huang SS, Wang J, Wang Y, Chen F, Song W, Zhang J. RP-HPLC determination of nootkatone and yakuchinone A in the fruits of Alpinia oxyphylla at different growth phases. Chin J Pharm Ana. 2013;33:808–811. [Google Scholar]
- 8.Li WB, Hu CJ, Wu SS, Gao Y, Yu LL. Study on urine reduction of fructus Alpiniae Oxyphyllae stir-frying with salt water in water-loading diuresis model rats. Chin J Exp Trad Med Formulae. 2013;19:261–264. [Google Scholar]
- 9.Luo XZ, Yu JG, Xu LZ, Li KM, Tan P, Feng JD. Studies on the chemical constituents of the fruits from Alpinia oxyphylla. Yao Xue Xue Bao. 2000;35:204–207. [Google Scholar]
- 10.Morikawa T, Matsuda H, Toguchida I, Ueda K, Yoshikawa M. Absolute stereostructures of three new sesquiterpenes from the fruit of Alpinia oxyphylla with inhibitory effects on nitric oxide production and degranulation in RBL-2H3 cells. J Nat Prod. 2002;65:1468–1474. doi: 10.1021/np020078o. [DOI] [PubMed] [Google Scholar]
- 11.Shi SH, Zhao X, Liu AJ, Liu B, Li H, Wu B, Bi KS, Jia Y. Protective effect of n-butanol extract from Alpinia oxyphylla on learning and memory impairments. Physiol Behav. 2015;139:13–20. doi: 10.1016/j.physbeh.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 12.State Administration of traditional Chinese medicine “Chinese Maeria Medica” editorial board. Chinese Materia Medica, Shanghai Science and Technology Press; Shanghai: 1999. p. 603. [Google Scholar]
- 13.Xie BB, Hou L, Guo BL, Huang WH, Yu GJ. The compounds from n-butanol fraction of Alpinia oxyphylla. Yao Xue Xue Bao. 2014;49:1569–1573. [PubMed] [Google Scholar]
- 14.Xu JJ, Ji CJ, Zhang YM, Su J, Li Y, Tan N. Inhibitory activity of eudesmane sesquiterpenes from Alpinia oxyphylla on production of nitric oxide. Bioorg Med Chem Lett. 2012;22:1660–1663. doi: 10.1016/j.bmcl.2011.12.114. [DOI] [PubMed] [Google Scholar]
- 15.Xu JJ, Su J, Li Y, Tan NH. Eremophilane-type sesquiterpenes from Alpinia oxyphylla with inhibitory activity against nitric oxide production. Chem Nat Compd. 2013;49:457–461. [Google Scholar]
- 16.Xu JJ, Tan NH, Xiong J, Adebayo AH, Han HJ, Zeng GZ, Ji CJ, Zjamg YM. Oxyphyllones A and B, novel sesquiterpenes with an unusual 4,5-secoeudesmane skeleton from Alpinia oxyphylla. Chin Chem Lett. 2009;20:945–948. [Google Scholar]
- 17.Yu XY, An LJ, Wang YQ, Zhao H, Gao C. Neuroprotective effect of Alpinia oxyphylla Miq. fruits against glutamate-induced apoptosis in cortical neurons. Toxicol Lett. 2013;144:205–212. doi: 10.1016/s0378-4274(03)00219-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JQ, Wang Y, Li YH, Lai WY, Li HL, Duan JA, Pei LX. Two new natural products from the fruits of A. oxyphylla with inhibitory effects on nitric oxide production in lipopolysaccharide - activated RAW264.7 macrophage cells. Arch Pharm Res. 2012;35:2143–2146. doi: 10.1007/s12272-012-1211-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Cui C, Chen CQ, Hu XL, Liu YH, Fan YH, Meng WH, Zhao QC. Anti-proliferative and pro-apoptotic activities of Alpinia oxyphylla on HepG2 cells through ROS-mediated signaling pathway. J Ethnopharmacol. 2015;169:99–108. doi: 10.1016/j.jep.2015.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QF, Luo SD, Wang HY. Studies on the chemical constituents of Yizhiren (Alpinia oxyphylla) Zhong Cao Yao. 1997;28:131–133. [Google Scholar]


