Abstract
Background:
Quorum sensing is the key regulator of virulence factors of Pseudomonas aeruginosa such as biofilm formation, motility, productions of proteases, hemolysin, pyocyanin, and toxins. The aim of this study was to explore the effect of the extracts from some medicinal plants on quorum sensing and related virulence factors of P. aeruginosa.
Material and Methods:
Quorum sensing inhibitory (OSI) effect of the alcohol extracts of 20 medicinal plants was evaluated by Chromobacterium violaceum reporter using agar cup diffusion method. The efficient QSI extracts were tested for their activity against biofilm synthesis, motility, and synthesis of pyocyanin from P. aeruginosa PA14
Results:
The extracts of Citrus sinensis, Laurus nobilis, Elettaria cardamomum, Allium cepa, and Coriandrum sativum exhibited potent quorum quenching effect. On the other hand, Psidium guajava and Mentha longifolia extracts showed lower QSI activity. These extracts exhibited significant elimination of pyocyanin formation and biofilm development of Pseudomonas aeruginosa PA14. In addition, they significantly inhibited twitching and swimming motilities of P. aeruginosa PA14.
Conclusion:
This study illustrated, for the first time, the importance of C. sinensis, L. nobilis, E. cardamomum, A. cepa, and C. sativum as quorum sensing inhibitors and virulence suppressors of P. aeruginosa. Thus, these plants could provide a natural source for the elimination of Pseudomonas pathogenesis.
Keywords: Quorum sensing inhibitory activity, P. aeruginosa, Chromobacterium violaceum, virulence factors
Introduction
Most medical applications of plants focused on their antimicrobial and antioxidant effects with low attention towards anti-pathogenic effects (Wallace, 2004). Recently, research efforts are focused on controlling bacterial infection through developing anti-pathogenic agents which manage bacterial diseases by inhibiting bacterial communication process called bacteria quorum sensing (QS). Quorum communication system regulates the release of Pseudomonas virulence factors such as protease, elastase, pyocyanin, alginate, biofilm formation, bacterial motility, and toxins production (Zhang and Dong 2004). Quorum sensing in P. aeruginosa is regulated by signaling molecules named N-acylated homoserine lactones (AHLs).
The concentration of these auto-inducers increases in relation to the increase in bacterial population till threshold, those signaling molecules return back to the bacteria to control bacterial pathogenicity (Fuqua and Greenberg, 2002). Therefore, elimination of QS represents an imperative advance to manage bacterial virulence and antimicrobial resistance (Hong et al., 2012). Plants are considered as a rich natural resource of quorum quenching agents (Mohamed et al., 2014; Koh and Tham 2011; Choo et al., 2006). Therefore, the current study investigated the QSI effect of some medicinal plants using the reporter Chromobacterium violaceum. Plant extracts that showed QSI activity were investigated for anti-pathogenic potential against P. aeruginosa PA14. For this instance, their influence on the virulence of P. aeruginosa was examined, including biofilm formation, pyocyanin production, and motility.
Materials and Methods
Collection of Plant Materials and Preparation of the Extracts
The fresh materials of Mentha longifolia, Senna italica, Valantia hispida, Tephrosia purpurea, Teucrium polium, Tribulus arabicus, and Commophora molmol were collected from Abyar Al-Mashy and Gabal Al-aquiq, Al Madinah Al Munawwarah, Saudi Arabia in March 2015. The other plant materials were purchased at the markets in Al-Madinah Al-Munawwarah, Saudi Arabia (Table 1). Authentication of the plant samples was established by Prof. Dr. A. Fayed (Professor of Plant Taxonomy, Faculty of Science, Assiut University, Egypt). The plants were shade dried for 7 days and ground to powder, using mortar and grinder of the electric mixer. Voucher specimens have been deposited at the Department of Pharmacognosy and Pharmaceutical Chemistry, Faculty of Pharmacy, Taibah University, Al Madinah Al Munawwarah, Saudi Arabia. Powdered samples (100 g each) were separately extracted using 95% methanol (5 x 250 mL). The extracts were concentrated under reduced pressure using rotary evaporator (Heidolph, Schwabach, Germany) and re-dissolved in dimethyl sulfoxide (DMSO 2.5%) for the assay of QSI activity. The solvent DMSO (1.5%) would not inhibit growth of the microorganisms (Zgoda and Porter 2001).
Table 1.
QSI potential of the tested plant extracts.
| Plant | Part used | Anti-QS zone (mm) | Anti- QS potential |
|---|---|---|---|
| Anethum graveolens | Fruits | - | - |
| Cucumis melo | Seeds | - | - |
| Carum carvi | Fruits | - | - |
| Citrus sinensis | Seeds | 20 | |
| Pimpinella anisum | Fruits | - | - |
| Foeniculum vulgrae | Fruits | - | - |
| Trigonella foenum graecum | Seeds | - | - |
| Coriandrum sativum | Fruits | 10 | ++ |
| Laurus nobilis | Leaves | 10 | ++ |
| Psidium guajava | Leaves | 3 | + |
| Allium cepa | Outer scales | 20 | ++ |
| Eugina aromatic | Flowers | - | - |
| Mentha longifolia | Aerial part | 5 | + |
| Elettaria cardamomum | Seeds | 10 | ++ |
| Senna italic | Aerial part | - | - |
| Valantia hispida | Aerial part | - | - |
| Tephrosia purpurea | Aerial part | - | - |
| Teucrium polium | Aerial part | - | - |
| Commophora molmol | Bark | - | - |
| Tribulus arabicus | Aerial part | - | - |
| Allium sativa (positive control) | Bulbs | 10 | ++ |
+: Moderate antiquorum sensing activity. ++: Potent antiquorum sensing activity
Bacterial Strains and Growth Conditions
The anti-quorum sensing assay was performed using Chromobacterium violaceum ATCC 12472. It was grown in Luria-Bertani (LB) media (1% peptone, 0.5% yeast extract (Bactoagar, BD Difco), and 1% NaCl (pH 7.4) solidified using 0.5 or 1.5% agar) (Bertani, 2004) and incubated at 28 °C for 48 h. C. violaceum ATCC 12472 was cultivated every 7 days on fresh LB agar slant and kept at 28 °C. P. aeruginosa PA14 was cultivated in LB media, incubated at 37 °C, and preserved as glycerol stocks at -20 °C.
Reporter Strain Assay of QSI Potential of Plant Extracts
Quorum sensing inhibitory effect of the tested extracts was estimated by agar cup diffusion assay using C. violaceum strain ATCC 12472 (McClean et al., 1997). Cultures were prepared by cultivating the bacteria in LB broth for 24 h at 28 °C. Luria-Bertani agar plates (1.5% agar) were prepared 15 mL/plate. C. violaceum was inoculated (100 μL/plate) in LB soft agar (0.5% agar) and poured on the solidified LB plates. Cups were made in LB agar medium of 10 mm diameter. A volume of 100 μL of each extract was placed in the corresponding cup and the assay plates were incubated at 28 °C for 48 h. Inhibition of quorum sensing was calculated using the equation (r2-r1) in mm; where r2 is the total growth and the QS inhibition zone radius and r1 is the clear zone radius. Quorum sensing inhibition zone <10 mm was considered moderate activity and when QSI zone >10 mm designated potent effect (Zaki et al., 2013).
Assay of Some Virulence Factors of P. aeruginosa PA14
Assay of Pyocyanin
Quantification of pyocyanin was carried out in triplicate via King A broth medium (peptone 2%, K2SO4 1.0%, and MgCl2 0.14%). An overnight P. aeruginosa PA14 culture (500 μ!) was inoculated into 5 mL of King A media with and without plant extracts (200 μΚ) and incubated for 24-48 h at 37°C (Essar et al., 1990). Pyocyanin was extracted using CHCl3 (3 mL), 1 mL HCl 0.2 N was added to the CHCl3 extract to have pink color and OD520 nm of the solution was measured. The concentration of pyocyanin was expressed as μ^η^ (OD520 χ 17.072) (Ra’oof and Latif, 2010).
Formation of Biofilm
The activity of various plant extracts on biofilm assembly by P. aeruginosa PA14 was measured using tube assay method (Christensen et al., 1982). Overnight culture of P. aeruginosa PA14 (500 μL) was inoculated into fresh LB broth (5 mL) with and without plant extracts (200 μL), then tubes were incubated overnight at 37 °C. Free unbound cells were discarded and biofilm layer was washed 3-4 times with water. The formed biofilm was stained by crystal violet (5% w/v) for 10 min, unbound stain was discarded. The tubes were washed and dried in opposite position. The formed biofilm on sides and bottom of the tubes was assigned.
Motility Assay
P. aeruginosa is capable of swimming on soft surfaces, twitching on hard surfaces, and swarming on semi-solid surfaces. The tested plants extracts were added to the motility plates. An overnight culture of PA14 was diluted to 0.1-0.2 nm at OD600. Twitching motility was performed through stab-inoculation 1% LB plates with and without plant extracts using 2 μL of the diluted Pseudomonas culture and inoculated at 37 °C for 48 h. The diameter of Pseudomonas twitching at the interface between plastic and agar was measured (Murray et al., 2010). Flagellum-dependent swimming was performed using swimming media (1% tryptone, 0.5% NaCl, and 0.5% agar). The plates were inoculated with 2 μL of diluted PA14 and incubated for 18 h at 37 °C. The diameter of the turbid zone (mm) was measured (Murray et al., 2010). Swarming motility was assayed using swarming media (0.5% agar, 0.5% peptone, 0.2% yeast extract, and 1.0 % glucose). Two micro-liters of the diluted PA14 culture were inoculated at the middle of the plate and the plates were incubated for 18 h at 37 °C (Krishnan et al., 2012). Motility assay was performed in triplicate and the mean of the diameter was assigned.
Statistical Analysis
The mean of three separate experiments ± standard deviation was calculated using Excel data sheet. Statistical analysis was estimated using GraphPad Instate software package (version3.05), Tukey-Kramer multiple-comparison test and the significant difference was assigned when P <0.05.
Results and Discussions
Quorum Sensing Inhibitory Effect
Assay of QSI activity of the alcohol extracts of 20 medicinal plants was performed using Chromobacterium violaceum ATCC12472 reporter strain (McClean et al., 1997). Most of the tested extracts exhibited potent antibacterial activity against the reporter strain. Inhibition of purple pigmentation of C. violaceum provided a readily and easily observable phenotype that simplified and facilitated screening for QSI effect. In the following work, seven of the screened extracts demonstrated QS antagonistic activity. The extracts of C. sinensis, L. nobilis, E. cardamomum, A. cepa, and C. sativum showed a pronounced quorum quenching effect with violacein inhibitory actions (Table 1). They showed clearly visible white halo in the violacin bioassay (20, 10, 10, 20, and 10 mm), respectively compared to garlic extract (A. sativa) (positive control). It was reported that A. sativa inhibited LuxR-based QSI in P. aeruginosa due to the presence of ajoene a sulfur-rich molecule (Jakobsen et al., 2012), while the extracts of P. guajava and M. longifolia showed moderate QSI effect.
Inhibition of Pyocyanin
P. aeruginosa produces green characterized pigment called pyocyanin after 24-48 h of growth. The disappearance of the green coloring of the P. aeruginosa PA14 culture indicated the lower produced levels of pyocyanin or no pyocyanin is present in the supernatant. The effect of C. sinensis, L. nobilis, E. cardamomum, A. cepa, and C. sativum extracts on the production pyocyanin was performed. In this study, pyocyanin level in Pseudomonas culture treated with these five plant extracts was significantly reduced (P<0.05) without affecting bacterial growth in contrast to the green pigment of untreated cultures (Figure 1). This could be explicated as quorum-control of pyocyanin pigment production (Dietrich et al., 2006). Moreover, quorum quenching agents have a great impact on pyocyanin release from P. aeruginosa (Morkunas et al., 2012; El-Mowafy et al., 2014).
Figure 1.

Influence of plant extracts on pyocyanin production by PA14: Extracts of C. sinensis, C. sativum, L. nobilis, A. cepa, and E. cardamomum significantly (*; P <.0.05) inhibited pyocyanin compared to control.
Effect on Biofilm Development
Furthermore, QS controls bacterial adhesion, biofilm formation, and production of alginates and polysaccharides required for biofilm development (Schuster and Greenberg, 2007). The first discovered natural products inhibited QS and biofilm maturation in Gram-negative bacteria are the halogenated furanones from Delisea pulchra (Givskov et al., 1997) and other QSI compounds such as cyclic sulfur derivatives obtained from garlic (Persson et al., 2005) and patulin produced by Penicillium sp. (O’Loughlin et al., 2013).
The extracts of C. sinensis, L. nobilis, E. cardamomum, A. cepa, and C. sativum were tested for Pseudomonas biofilm formation using tube assay method. All these plants extracts showed discriminative effect on biofilm formed by P. aeruginosa PA14 compared to the untreated control (Figure 2).
Figure 2.
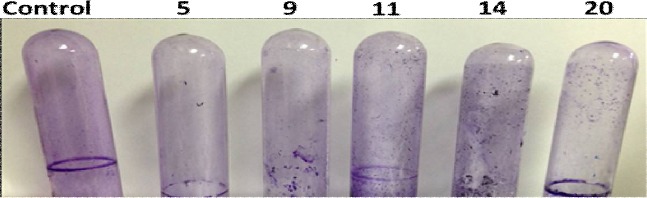
Effect of plant extracts on biofilm formation by PA14; Citrus sinensis, Coriandrum sativum, Laurus nobilis, Allium cepa, and Elettaria cardamomum (5, 9, 11, 14, and 20, respectively) compared to control untreated Pseudomonas PA14.
Motility Assay
Normal bacterial motility is important for proliferation of burn and colonization of wounds (Arora et al., 2005). P. aeruginosa has three types of bacterial motility: swimming, twitching, and swarming which are propagated by flagella and pili IV. The extracts of C. sinensis, L. nobilis, E. cardamomum, A. cepa, and C. sativum were tested for their influence on the motility of P. aeruginosa PA14. They manifested a distinct influence on swarming and twitching motility (Table 2). C. sinensis, L. nobilis, and A. cepa extracts reduced twitching in PA14 by 88, 77, and 85%, respectively (P<0.01). Previous studies verified that QS systems control Pseudomonas motilities and inhibition of QS signals affects the motilities (Glessner et al., 1999; Juhas et al., 2005). Although C. sativum and E. cardamomum extracts substantially inhibited biofilm and pyocyanin production in PA14, it was noticed that they did not significantly affect bacteria motility. It appears that these extracts contain compounds; some of them are agonist of the motility, while others may affect the different signaling pathways in P. aeruginosa PA14. Motility is a complex phenotype that involves various regulatory components (Overhage et al., 2008). Elimination of Pseudomonas motility confirmed the potential effect of C. sinensis, L. nobilis, and A. cepa on biofilm formation as modulation of bacterial motilities is associated with thin and dispersed biofilm (Shrout et al., 2006).
Table 2.
Effect of the tested plant extracts on Pseudomonas motility.
| Plant | Twitching diameter (mm) | Swimming diameter (mm) | Swarming diameter (mm) |
|---|---|---|---|
| Control/no plant extract | 45 ± 0.01 | 41 ± 0.01 | 50± 0.1 |
| Citrus sinensis | 10 ± 0.02 | 15 ± 0.00 | 15± 0.00 |
| Coriandrum sativum | 45 ± 0.01 | 33 ± 0.02 | 05 ± 0.00 |
| Laurus nobilis | 15 ± 0.01 | 05 ± 0.01 | 05 ± 0.03 |
| Allium cepa | 07 ± 0.00 | 06 ± 0.01 | 01 ± 0.00 |
| Elettaria cardamomum | 45 ± 0.01 | 35 ± 0.01 | 04 ± 0.00 |
Conclusion
P. aeruginosa is an opportunistic pathogen that causes serious human infections. The study of QSI activity of some medicinal plants and inhibition of various virulence factors among P. aeruginosa could play an important role in eliminating its pathogenicity. From current research, quorum inhibition appears to be a potential mode of action of some of tested extracts to control bacterial pathogenicity. Anti-QS could offer an alternative mode of action against opportunistic pathogenic bacteria. The extracts of C. sinensis, L. nobilis, E. cardamomum, A. cepa, and C. sativum exhibited strong anti-QS activity. Our findings highlight the importance of these medicinal plants as a rich source of compounds able to inhibit QS and QS-related virulence processes. These medicinal plants could manage Pseudomonas pathogenesis and hinder its dissemination. Further investigation of the nature of these QS inhibitor compounds and mechanism of action are still required.
Acknowledgements
This project was supported by grant No. 6781/1435 from Deanship of Scientific Research, Taibah University, Al Madinah Al Munawarah, Kingdom of Saudi Arabia. We gratefully acknowledge Prof. Bob McLean (Dept. Biology, Texas State University, San Marcos, TX 7866, USA) for providing the reporter of strain Chromobacterium violaceum strain ATCC 12472.
References
- 1.Arora S.K, Neely A.N, Blair B, Lory S, Ramphal R. Role of motility and flagellin glycosylation in the pathogenesis of P. aeruginosa burn wound infections. Infect. Immun. 2005;73:4395–4398. doi: 10.1128/IAI.73.7.4395-4398.2005. doi: 10.1128/IAI.73.7.4395-4398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choo J.H, Rukayadi Y, Hwang J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006;42:637–641. doi: 10.1111/j.1472-765X.2006.01928.x. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 4.Christensen G.D, Simpson W.A, Bisno A.L, Beachey E.H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immunol. 1982;37:318-326. doi, 0019–9567. doi: 10.1128/iai.37.1.318-326.1982. 82/070318-09$02.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich L.E.P, Price-Whelan A, Petersen A, Whiteley M, Newman D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of P. aeruginosa. Mol. Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. doi:10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Mowafy S.A, Abd El Galil K.H, El-Messery S.M, Shaaban M.I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microbiol. Pathog. 2014;1(74C):25–32. doi: 10.1016/j.micpath.2014.07.008. doi: 10.1016/j.micpath.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Essar D.W, Eberly L, Hadero A, Crawford I.P. Identification and characterization of genes for second anthranilate synthase in P. aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. doi:0021-9193-/90/020884-17$02.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuqua C, Greenberg E.P. Listening in on bacteria: acyl homoserine lactone signalling. Nat. Rev. Mol Cell. Biol. 2002;3:685–695. doi: 10.1038/nrm907. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 9.Givskov M, Eberl L, Molin S. Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol. Lett. 1997;148:115–122. doi: 10.1111/j.1574-6968.1997.tb10276.x. [Google Scholar]
- 10.Glessner A, Smith R.S, Iglewski B.H, Robinson J.B. Roles of P. aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J. Bacteriol. 1999;181:16231629. doi: 10.1128/jb.181.5.1623-1629.1999. doi: 0021-9193/99/$04.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong K.W, Koh C.L, Sam C.K, Yin W.F, Chan K.G. Quorum quenching revisited-From signal decays to signaling confusion. Sensors (Basel) 2012;12:4661–4696. doi: 10.3390/s120404661. doi:10.3390/s120404661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsen T.H, Van Gennip M, Phipps R.K, Shanmugham M.S, Christensen L.D, Alhede M, Skindersoe M.E, Rasmussen T.B, Friedrich K, Uthe F, Jensen P, Moser C, Nielsen K.F, Eberl L, Larsen T.O, Tanner D, H0iby N, Bjarnsholt T, Givskov M. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012;56:2314–2325. doi: 10.1128/AAC.05919-11. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhas M, Eberl L, Tummler B. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ. Microbiol. 2005;7:459–471. doi: 10.1111/j.1462-2920.2005.00769.x. doi:10.1111/j.1462-2920.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 14.Koh K.H, Tham F.Y. Screening of traditional chinese medicinal plants for quorum-sensing inhibitors activity. J. Microbiol. Immunol. Infect. 2011;44:144–148. doi: 10.1016/j.jmii.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan T, Yin W, Chan K. Inhibition of quorum sensing-controlled virulence factor production in P. aeruginosa PAO1 by Ayurveda spice clove (Syzygium aromaticum) Bud extracts. Sensors. 2012;12:4016–4030. doi: 10.3390/s120404016. doi: 10.3390/s120404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClean K.H, Winson M.K, Fish L, Taylor A, Chhabra S.R, Camara M, Daykin M, Lamb J.H, Swift S, Bycroft B.W, Stewart G.S, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiol. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed G.A, Ibrahim S.R.M, Shaaban M.I.A, Ross S.A. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia. 2014;98:215–221. doi: 10.1016/j.fitote.2014.08.014. doi: 10.1016/j.fitote.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Morkunas B, Galloway W.R, Wright M, Ibbeson B.M, Hodgkinson J.T, O’Connell K.M, Bartolucci N, Della V.M, Welch M, Spring D.R. Inhibition of the production of the P. aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics. Org. Biomol. Chem. 2012;42:8452–8464. doi: 10.1039/c2ob26501j. doi: 10.1039/c2ob26501j. [DOI] [PubMed] [Google Scholar]
- 19.Murray T.S, Ledizet M, Kazmierczak B.I. Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of P. aeruginosa clinical isolates. J. Med. Microbiol. 2010;59:511–520. doi: 10.1099/jmm.0.017715-0. doi:10.1099/jmm.0.017715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Loughlin C.T, Miller L.C, Siryaporn A, Drescher K, Semmelhack M.F, Bassler B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. PNAS. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. doi:10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overhage J, Bains M, Brazas M.D, Hancock R.E. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 2008;190:2671–2679. doi: 10.1128/JB.01659-07. doi:10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson T, Givskov M, Nielsen J. Quorum sensing inhibition: targeting chemical communication in gram-negative bacteria. Curr. Med. Chem. 2005;12:3103–3115. doi: 10.2174/092986705774933425. doi:10.2174/092986705774933425. [DOI] [PubMed] [Google Scholar]
- 23.Ra’oof W.M, Latif I.A. In vitro study of the swarming phenomena and antimicrobial activity of pyocyanin produced by P. aeruginosa isolated from different human infections. Eur. J. Sci. Res. 2010;47:405–421. [Google Scholar]
- 24.Schuster M, Greenberg E.P. Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genomics. 2007;8:287. doi: 10.1186/1471-2164-8-287. doi:10.1186/1471-2164-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrout J.D, Chopp D.L, Just C.L, Hentzer M, Givskov M, Parsek M.R. The impact of quorum sensing and swarming motility on P. aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. doi:10.1111/1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 26.Wallace R.J. Antimicrobial properties of plant secondary metabolites. Proceed. Nutri Soc. 2004;63:621–629. doi: 10.1079/pns2004393. [DOI] [PubMed] [Google Scholar]
- 27.Zaki A.A, Shaaban M.I, Hashish N.E, Amer M.A, Lahloub M.F. Assessment of anti-quorum sensing activity for some ornamental and medicinal plants native to Egypt. Sci. Pharm. 2013;81:251–258. doi: 10.3797/scipharm.1204-26. doi:10.3797/scipharm.1204-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zgoda J.R, Porter J. A convenient micro-dilution methods for screening natural products against bacteria and fungi. Pharmacol. Bio. 2001;39:221–225. doi: 10.1076/phbi.39.3.221.5934. [Google Scholar]
- 29.Zhang L.H, Dong Y.H. Quorum sensing and signal interference: diverse implications. Mol. Microbiol. 2004;53:1563–1567. doi: 10.1111/j.1365-2958.2004.04234.x. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]


