Abstract
Cyclophosphamide is a widely used medication and can cause oxidative stress. This study was conducted to investigate the effects of Vitamin C on reproductive organs' weight and the quality of sperm parameters in laboratory rats. In this experimental study, 40 rats were randomly assigned into five groups of eight each. Distilled water (DW) group received only food and water, Group 2 was administered with drug solvent (DW) by gavage, Group 3 intraperitoneally administered with 1.6 mg/kg cyclophosphamide, Group 4 gavaged Vitamin C at 0.88 mg/kg, and Group 5 administered with effective doses of Vitamin C and cyclophosphamide by gavage with 1-h intervals. Sperm parameters of the samples were taken from distal epididymis and tissues were studied, and the data were analyzed by SPSS version 22. The lowest weight of testicles and epididymis was seen in cyclophosphamide-exposed rats and the highest weight of testicles and epididymis in Vitamin C-exposed rats (P < 0.05). The highest motility, progression, viability, and count of sperm were seen in the Vitamin C-treated group and the lowest in the cyclophosphamide-exposed group. The highest proportion of sperm anomalies was seen in the cyclophosphamide-exposed group. Vitamin C, as an antioxidant, can be effective on some of the sperm parameters and can reduce cyclophosphamide-induced complications in animal model.
Key words: Ascorbic acid, cyclophosphamide, spermatozoa
INTRODUCTION
Cyclophosphamide is an alkylating drug and is a choice drug for cancer, multiple sclerosis, and lupus-induced glomerulonephritis, and can reduce cyclophosphamide-induced complications in an animal model.[1,2,3] Cyclophosphamide causes a decrease in luteinizing hormone, follicle-stimulating hormone, testosterone, and spermatogenesis.[4] This drug can disturb the reductive reactions in tissues through producing oxidative stress.[5,6,7] Oxidative stress can lead to a considerably decreased number of viable sperm through declining sperm motility and cell quality.[8] Sperm is enriched with certain small molecules that destroy free radicals, including carnitine, tyrosine, uric acid, and Vitamin C (Vitamin C) while passing through different parts of male reproductive system by means of the secretions of antioxidant enzymes such as glutathione peroxidase and pyridoxine.[9] Sperm extracellular antioxidants contribute significantly to protecting sperm membrane against oxidative stress.[10,11]
Vitamin C comprises approximately 65% of the antioxidant property of sperm plasma in fertile men.[12] Studies have demonstrated that Vitamin C is one of the most important treatments for male infertility.[13]
Reason for the induction of oxidative stress in sperm can be due to disequilibrium between the number of the produced free radicals and the inactivated free radicals by antioxidants.[14] Reason for this disequilibrium is the use of drugs. For example, a study on rats demonstrated that cyclophosphamide had adverse effects on sperm parameters and testicular antioxidants.[15]
Male infertility continues to be a global issue with heavy costs imposed on communities.[16,17] This study was conducted to investigate the role of Vitamin C in preventing cyclophosphamide-induced adverse effects on laboratory rats sperm through the mechanism of the production of free radicals and reactive oxygen species.
MATERIALS AND METHODS
For this experimental study on animal model, cyclophosphamide (Baxter Co., Germany) eosin 6, nigrosin (Pars Azma Co.), and Vitamin C (Darou Pakhsh Co., Iran) were used.
The rats were 40 male Wistar rats aged about 3 months and weighing 200–250 g. During treatment, the rats were given free access to food and water and maintained in similar conditions. Then, the rats were randomly assigned to five groups of eight each. Group 1 was considered control to remove gavage-induced stress and given only food and water.
Group 2 was administered with drug solvent (distilled water [DW]). Group 3 was intraperitoneally administered with 1.6 mg/kg cyclophosphamide and Group 4 gavaged with Vitamin C at 0.88 mg/kg. Group 5 was administered with Vitamin C and cyclophosphamide by gavage at the above-mentioned doses with 1-h interval.
At the completion of the treatment, the rats were anesthetized with a combination of xylazine (20 mg/kg) and acepromazine (0.2 mg/kg). After weighing of the bodies, the testicles were taken out for specimen taking and, the epididymis, after weighing, was placed in 5 mL Ham's FlO culture, dissected with scissors and scalpel, and smoothly shaken with a CO2-containing incubator at 37°C until the sperms were freed and became floating in the culture medium. To investigate the sperm viability, one drop of the suspension was placed on a microscopic lam and eosin-nigrosin dye (Pars Azma Co.) was introduced. In this technique, if the sperm is viable at the time of staining, the dye does not penetrate into head and trunk and hence appears as a light (white) color.
In contrast, if the sperm is not viable, the dye then penetrates into the sperm, and the sperm appears as pink. The proportion of survived sperm is calculated by calculating the ratio of undyed sperm to the total sperm. To investigate the appearance of the sperm, one drop of the suspension was placed on the lam and aniline blue staining was used. Untailed sperms and abnormal head and tail were some of the deformities of the sperm.
To investigate the sperm motility, the WHO[18] guideline was used. Briefly, 10 μl mixture of the culture and sperm on the lam was assigned to investigate the sperm motility. At least five microscopic fields were examined to investigate the motility of at least 200 sperm per specimen. Then, the percentage of three movements, progression, in situ, and immotility, were calculated.
Finally, the testicles and the testicular appendices were taken out for pathological examinations and fixed in formalin 10% (Merck, Germany). Then, they were fixed in paraffin, and 5 mm-thick slices were made using a microtome and stained with hematoxylin-eosin. The obtained lams were sent to a pathology laboratory for reading.
The data were first encoded and then analyzed by descriptive statistics (frequency, mean, and standard deviation) and analytical statistics (ANOVA and Chi-square test) in IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp software.
It is worth considering that all the steps of the experiments were supervised by a veterinarian and the rats sacrificed with a combination of ketamine and xylazine while they were intoxicated (without pain) and in compliance with the Guidelines of the Ethics Committee of the Shahrekord University of Medical Sciences.
RESULTS
There was a statistically significant difference in weight between the cyclophosphamide-exposed rats and other groups under study (P < 0.05). Moreover, there was a statistically significant difference in weight between the group administered with Vitamin C alone and the cyclophosphamide-exposed group (P = 0.001) [Table 1].
Table 1.
Comparison of mean body weight (g) among the five groups of the rats
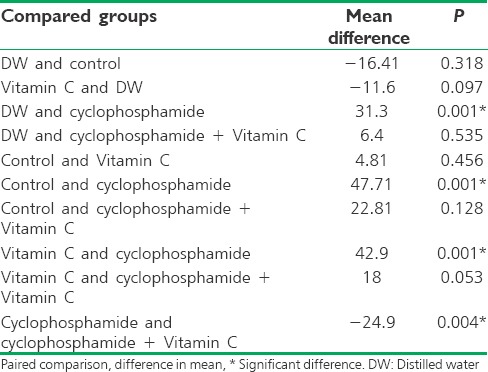
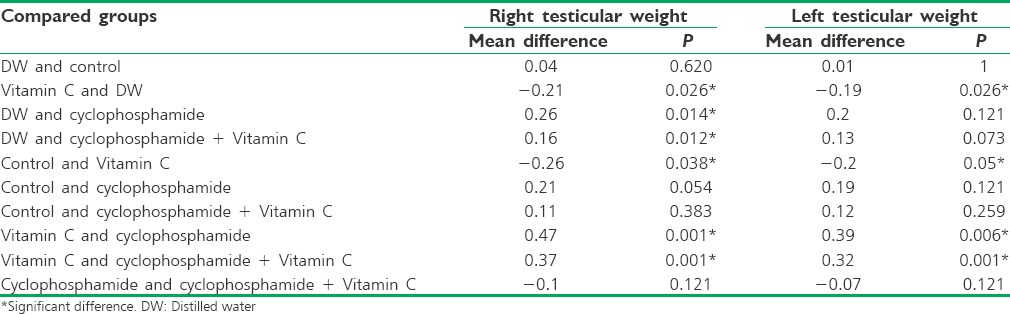
Regarding the testicular weight, the lowest mean weight was obtained in the cyclophosphamide-exposed group (mean weight of left and right testicles: 0.96 g and 0.83 g, respectively) and the highest mean weight in the Vitamin C-treated group (mean weight of left and right testicles: 1.35 g and 1.30 g, respectively).
The paired comparisons demonstrated that the difference in the mean weight was significant between the Vitamin C-treated group and other groups (P < 0.05). However, the addition of Vitamin C to cyclophosphamide had no considerable effect on the testicular weight with no significant difference from the cyclophosphamide-exposed group (P = 0.121) [Table 2].
Table 2.
Comparison of mean testicular weight (g) among the five groups of the rats
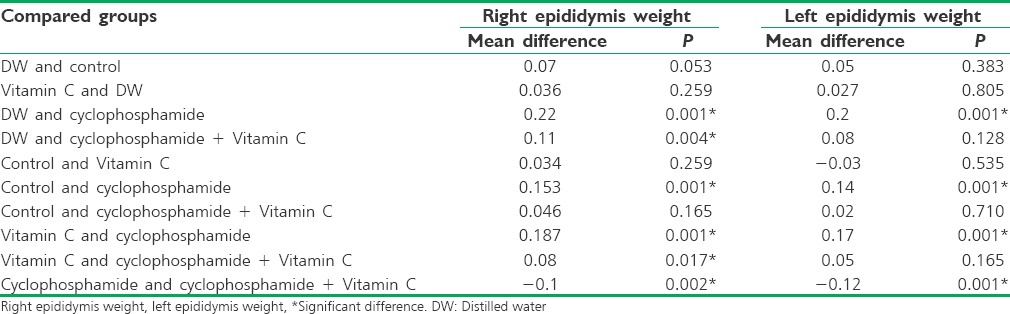
Regarding the epididymis weight, the lowest mean weight was seen in the cyclophosphamide-exposed group (mean weight of right and left epididymis: 0.14 and 0.17 g, respectively), and the highest mean weight in the DW group (mean weight of right and left epididymis: 0.36 g and 0.37 g, respectively) followed by the Vitamin C-treated group (mean weight of right and left epididymis: 0.33 g and 0.35 g, respectively).
Moreover, the epididymis weight was statistically significantly higher in the Vitamin C-treated, cyclophosphamide-exposed group than the cyclophosphamide-exposed group (P = 0.091) [Table 3].
Table 3.
Comparison of the mean weight of epididymis (g) among the five groups of the rats
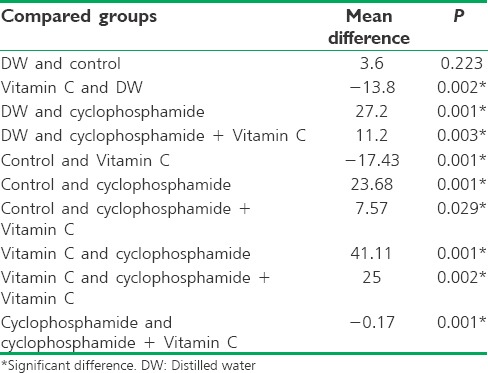
Regarding the cyclophosphamide effects on the sperm parameters, the cyclophosphamide-exposed group had a considerably lower motility (0.44%) compared to other groups. The highest motility (85%) was seen in the Vitamin C-treated group.
The paired comparisons indicated a significant difference in motility percentage between any pairs of the groups (P < 0.05), but no statistically significant difference was seen in motility percentage between the control and DW groups (P = 0.223) [Table 4].
Table 4.
Comparison of mean motility percentage among the five groups of the rats
The paired comparisons indicated a significant difference in the sperm progression percentage between any pairs of the groups (P < 0.05), but no statistically significant difference was seen in the sperm progression percentage between the control and DW groups (P = 0.059) [Table 5].
Table 5.
Comparison of mean percentage of sperm progression among the five groups of the rats
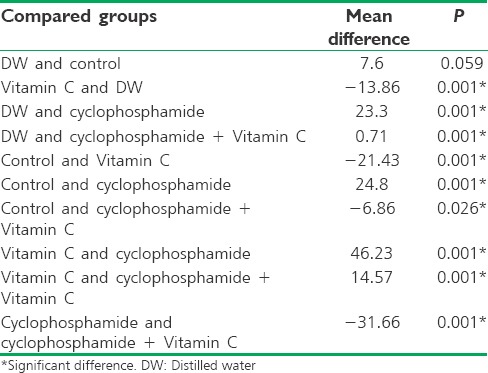
No statistically significant difference was observed in the sperm viability between the control and DW groups (P = 0.209), representing an approximately similar function of these two groups [Table 6].
Table 6.
Comparison of mean sperm viability among the five groups of the rats
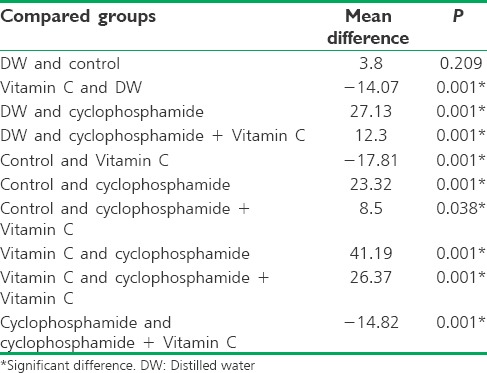
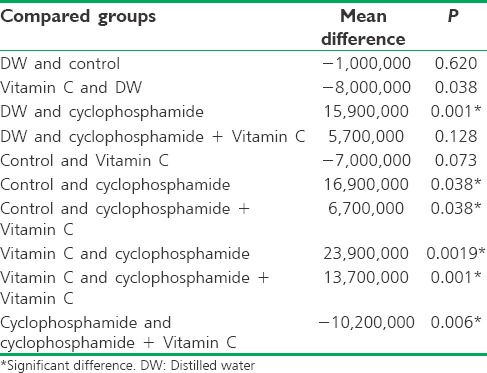
Regarding the sperm count, the cyclophosphamide-exposed group had a significantly low sperm count (1,650,000,000), and the Vitamin C-treated group had the highest sperm count (4,040,000,000).
No significant difference was seen in the sperm count between the DW and control groups, the control and the Vitamin C-treated, cyclophosphamide-exposed groups, and the control and the Vitamin C-treated groups (P > 0.05) [Table 7].
Table 7.
Comparison of mean sperm among the five groups of the study
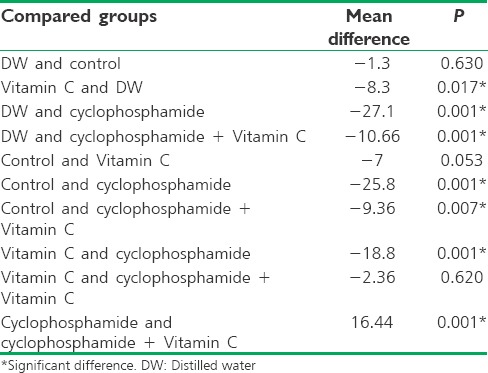
Moreover, the paired comparisons indicated a significant difference in the percentage of sperm anomalies between most pairs of the groups (P < 0.05), except between the control and the Vitamin C-treated groups and the Vitamin C-treated and the Vitamin C-treated, cyclophosphamide-exposed groups (P > 0.05) [Table 8].
Table 8.
Comparison of mean sperm anomalies among the five groups of the rats
The decreased count of germ cells in the seminiferous tubules [Figure 1], decreased tubular sperm count, congested vessels in the intertubular spaces, and in some tubules, maturation arrest were the histopathological changes observed in the cyclophosphamide-exposed group.
Figure 1.
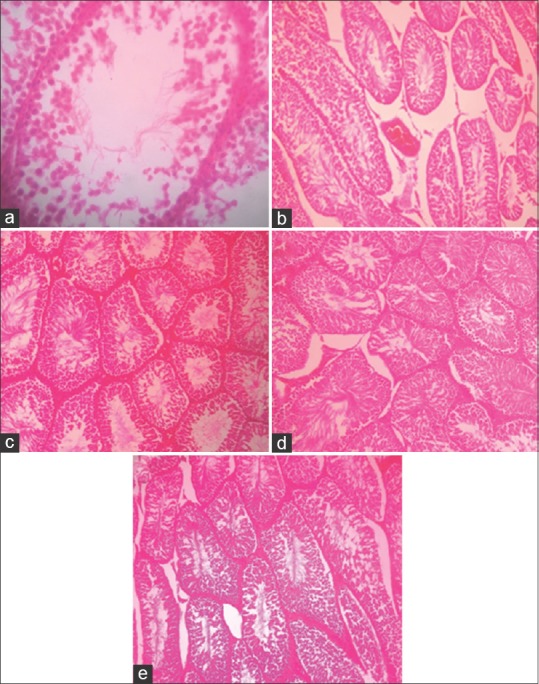
(a) Photomicrograph of testicular tissue in the cyclophosphamide-exposed group (decreased count of germ cells); (b) Photomicrograph of testicular tissue in cyclophosphamide-exposed group (vascular congestion); (c) Photomicrograph of testicular tissue in vitamin C-treated, cyclophosphamide-exposed group (decreased count of germ cells); (d) Photomicrograph of testicular tissue in Vitamin C-treated group; (e) Photomicrograph of testicular tissue in control group
Moreover, the decreased count of the germ cells and sperm, as well as tubular anomalies, was lower in the Vitamin C-treated, cyclophosphamide-exposed group than in the cyclophosphamide-exposed group. Maturation arrest was rarely seen in the tubules in the Vitamin C-treated, cyclophosphamide-exposed group.
DISCUSSION
The findings indicated that the cyclophosphamide-exposed rats had a lower weight than other groups. This finding is consistent with Kim et al. study.[19] Other studies also indicated that use of cyclophosphamide low doses in the long-term can decrease the weights of body and reproductive organs in male rats, cause atrophy in certain organs such as testicles and epididymis, and hence affect fertility rate.[15,19,20,21,22,23]
In some other studies, the sperm motility was found to be comparably low in the cyclophosphamide-treated rats.[19,22,23,24,25] The sperm cells capability of moving is considered a decisive and important factor for fertility and can ensure the process of insemination. The sperm motility is likely to decline when free radicals and the final products of lipids peroxidation increase in the culture medium.
The findings of this study are consistent with other studies.[22,23,24] The consistent findings of the present study and other research on the sperm motility and viability may be explained by the cyclophosphamide antioxidant property and decreased serum levels of luteinizing hormone, follicle stimulating hormone, and testosterone.
This study demonstrated that the sperm count in the cyclophosphamide-exposed rats was considerably low and in contrast, the Vitamin C-treated rats had the highest number of sperm count with a significant difference in most of the groups under study. Other studies have reported consistent findings.[8,23,24,26]
The loss of epithelial cells usually causes damage to Sertoli cells and destroys cytoplasmic bridges, and consequently, can cause a decrease in sperm count and increase in sperm deformities. The atrophy of toxic tubes of spermatogenic nephroCells can lead to increased morphological anomalies in sperm.[27]
Cyclophosphamide can prevent the activities of catalase and peroxidase and increase lipids peroxidation in testicular tissue. Because of having large amounts of unsaturated fatty acids, sperm is highly sensitive to lipids peroxidation. Because sperm cannot resynthesize its membrane compounds, this mechanism can cause disturbance of sperm functions through various free radicals.[25] Free radicals, at low concentrations, serve as a medium for the normal functions of sperm, while free radicals, if excessively produced, are highly toxic to cells.[9]
Besides that, cyclophosphamide can lead to decreased plasma testosterone and change in the count of germ cells. This affects steroid hormones and finally results in disturbed spermatogenesis process.[20] All the above-mentioned factors can influence the sperm parameters and cause a disturbance in male rats fertility. Other studies also reported consistent findings.
In this study, Vitamin C, an antioxidant, was found to exert positive effects on the reproductive system (testicles and epididymis) weight and the sperm parameters such as motility, progression, viability, count, and anomalies. Vani et al. investigated the effect of Vitamin C on infertile men and found a considerable decrease in the sperm with anomalous morphology.[28]
CONCLUSION
Vitamin C can affect sperm parameters and the complications due to cyclophosphamide in animal models. Therefore, use of an antioxidant, such as Vitamin C, and tissue cleansing during treatment seem necessary to decrease the cyclophosphamide-induced oxidative stress. The findings of this study should be cautiously generalized to humans. Therefore, randomized clinical trials should be done on this issue with the observance of the principles of research ethics. Furthermore, use of specific concentrations of other antioxidants is recommended in future studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The present study was derived from a research project funded by the Research and Technology Deputy of the Shahrekord University of Medical Sciences.
REFERENCES
- 1.Chrystal K, Cheong K, Harper P. Chemotherapy of small cell lung cancer: State of the art. Curr Opin Oncol. 2004;16:136–40. doi: 10.1097/00001622-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Makhani N, Gorman MP, Branson HM, Stazzone L, Banwell BL, Chitnis T. Cyclophosphamide therapy in pediatric multiple sclerosis. Neurology. 2009;72:2076–82. doi: 10.1212/WNL.0b013e3181a8164c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joy MS, La M, Wang J, Bridges AS, Hu Y, Hogan SL, et al. Cyclophosphamide and 4-hydroxycyclophosphamide pharmacokinetics in patients with glomerulonephritis secondary to lupus and small vessel vasculitis. Br J Clin Pharmacol. 2012;74:445–55. doi: 10.1111/j.1365-2125.2012.04223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johari H, Mahmoudinejad F, Amjad G. An evaluation of the effect of the hydroalcoholic extract of ginger on the hypothalamus-pituitary-gonadal axis in adult female rats (Rat) treated with cyclophosphamide. Pejoda. 2011;6:62–70. [Google Scholar]
- 5.Haque R, Bin-Hafeez B, Ahmad I, Parvez S, Pandey S, Raisuddin S. Protective effects of Emblica officinalis Gaertn. In cyclophosphamide-treated mice. Hum Exp Toxicol. 2001;20:643–50. doi: 10.1191/096032701718890568. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D, Das UB, Ghosh S, Mallick M, Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: A correlative study with testicular oxidative stress. Drug Chem Toxicol. 2002;25:281–92. doi: 10.1081/dct-120005891. [DOI] [PubMed] [Google Scholar]
- 7.Abraham P, Isaac B, Ramamoorthy H, Natarajan K. Oral glutamine attenuates cyclophosphamide-induced oxidative stress in the bladder but does not prevent hemorrhagic cystitis in rats. J Med Toxicol. 2011;7:118–24. doi: 10.1007/s13181-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comish PB, Drumond AL, Kinnell HL, Anderson RA, Matin A, Meistrich ML, et al. Fetal cyclophosphamide exposure induces testicular cancer and reduced spermatogenesis and ovarian follicle numbers in mice. PLoS One. 2014;9:e93311. doi: 10.1371/journal.pone.0093311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis SE, Sterling ES, Young IS, Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril. 1997;67:142–7. doi: 10.1016/s0015-0282(97)81871-7. [DOI] [PubMed] [Google Scholar]
- 10.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;12:CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, Lenzi A. The role of antioxidant therapy in the treatment of male infertility: An overview. Asian J Androl. 2011;13:690–7. doi: 10.1038/aja.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis SE, Boyle PM, McKinney KA, Young IS, Thompson W. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril. 1995;64:868–70. doi: 10.1016/s0015-0282(16)57870-4. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil (Camb) 2010;13:217–25. doi: 10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 14.Saleh RA, Agarwal A. Oxidative stress and male infertility: From research bench to clinical practice. J Androl. 2002;23:737–52. [PubMed] [Google Scholar]
- 15.Le XY, Luo P, Gu YP, Tao YX, Liu HZ. Interventional effects of squid ink polysaccharides on cyclophosphamide-associated testicular damage in mice. Bratisl Lek Listy. 2015;116:334–9. doi: 10.4149/bll_2015_063. [DOI] [PubMed] [Google Scholar]
- 16.Inhorn MC, Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–26. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 17.Kovac JR, Fantus J, Lipshultz LI, Fischer MA, Klinghoffer Z. Cost-effectiveness analysis reveals microsurgical varicocele repair is superior to percutaneous embolization in the treatment of male infertility. Can Urol Assoc J. 2014;8:E619–25. doi: 10.5489/cuaj.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organisation. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. United Kingdom: Cambridge University Press; 1999. [Google Scholar]
- 19.Kim SH, Lee IC, Baek HS, Moon C, Kim SH, Kim JC. Protective effect of diallyl disulfide on cyclophosphamide-induced testicular toxicity in rats. Lab Anim Res. 2013;29:204–11. doi: 10.5625/lar.2013.29.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das UB, Mallick M, Debnath JM, Ghosh D. Protective effect of ascorbic acid on cyclophosphamide- induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl. 2002;4:201–7. [PubMed] [Google Scholar]
- 21.Robaire B, Hales BF. Mechanisms of action of cyclophosphamide as a male-mediated developmental toxicant. Adv Exp Med Biol. 2003;518:169–80. doi: 10.1007/978-1-4419-9190-4_14. [DOI] [PubMed] [Google Scholar]
- 22.Oyagbemi AA, Omobowale TO, Saba AB, Adedara IA, Olowu ER, Akinrinde AS, et al. Gallic acid protects against cyclophosphamide-induced toxicity in testis and epididymis of rats. Andrologia. 2016;48:393–401. doi: 10.1111/and.12459. [DOI] [PubMed] [Google Scholar]
- 23.Lu WP, Mei XT, Wang Y, Zheng YP, Xue YF, Xu DH. Zn (II)-curcumin protects against oxidative stress, deleterious changes in sperm parameters and histological alterations in a male mouse model of cyclophosphamide-induced reproductive damage. Environ Toxicol Pharmacol. 2015;39:515–24. doi: 10.1016/j.etap.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Bakhtiary Z, Shahrooz R, Ahmadi A, Zarei L. Protective effects of ethyl pyruvate on sperm quality in cyclophosphamide treated mice. Iran J Reprod Med. 2015;13:291–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Nayak G, Vadinkar A, Nair S, Kalthur SG, D'Souza AS, Shetty PK, et al. Sperm abnormalities induced by pre-pubertal exposure to cyclophosphamide are effectively mitigated by Moringa oleifera leaf extract. Andrologia. 2016;48:125–36. doi: 10.1111/and.12422. [DOI] [PubMed] [Google Scholar]
- 26.Bakhtiary Z, Shahrooz R, Ahmadi A, Zarei L. Evaluation of antioxidant effects of crocin on sperm quality in cyclophosphamide treated adult mice. Vet Res Forum. 2014;5:213–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson D, Bishop JB, Garner RC, Ostrosky-Wegman P, Selby PB. Cyclophosphamide: Review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res. 1995;330:115–81. doi: 10.1016/0027-5107(95)00039-l. [DOI] [PubMed] [Google Scholar]
- 28.Vani K, Kurakula M, Syed R, Alharbi K. Clinical relevance of Vitamin C among lead-exposed infertile men. Genet Test Mol Biomarkers. 2012;16:1001–6. doi: 10.1089/gtmb.2012.0027. [DOI] [PubMed] [Google Scholar]


