Abstract
Adolescence is a critical period for brain maturation. Deciphering how disturbances to the central nervous system at this time affect structure, function and behavioural outputs is important to better understand any long-lasting effects. Hippocampal neurogenesis occurs during development and continues throughout life. In adulthood, integration of these new cells into the hippocampus is important for emotional behaviour, cognitive function and neural plasticity. During the adolescent period, maturation of the hippocampus and heightened levels of hippocampal neurogenesis are observed, making alterations to neurogenesis at this time particularly consequential. As stress negatively affects hippocampal neurogenesis, and adolescence is a particularly stressful time of life, it is important to investigate the impact of stressor exposure at this time on hippocampal neurogenesis and cognitive function. Adolescence may represent not only a time for which stress can have long-lasting effects, but is also a critical period during which interventions, such as exercise and diet, could ameliorate stress-induced changes to hippocampal function. In addition, intervention at this time may also promote life-long behavioural changes that would aid in fostering increased hippocampal neurogenesis and cognitive function. This review addresses both the acute and long-term stress-induced alterations to hippocampal neurogenesis and cognition during the adolescent period, as well as changes to the stress response and pubertal hormones at this time which may result in differential effects than are observed in adulthood. We hypothesise that adolescence may represent an optimal time for healthy lifestyle changes to have a positive and long-lasting impact on hippocampal neurogenesis, and to protect against stress-induced deficits. We conclude that future research into the mechanisms underlying the susceptibility of the adolescent hippocampus to stress, exercise and diet and the consequent effect on cognition may provide insight into why adolescence may be a vital period for correct conditioning of future hippocampal function.
Introduction
Adolescence represents a time of transition to independence during which significant lifestyle changes occur,1, 2 and it is believed to be a critical period for the programming of future adult behaviours.3 Although there are no definite markers for the adolescent period, in mice and rats adolescence is generally considered to be from post-natal day (PND) 21–60, and in humans from ages 12 to 18.4 Puberty, the maturation of the hypothalamic–pituitary–gonadal (HPG) axis occurs during the early adolescent period. In rodents, puberty typically occurs between PND28–42 in females and PND42–49 in males and is characterised by vaginal opening and preputial separation, respectively.5 In humans, puberty spans from ages 10 to 16 in girls and 11 to 17 in boys, and is characterised by the development of secondary sexual characteristics, and the onset of menses in girls5, 6 (please see Holder et al.5 for a pictorial representation of the relative adolescent period in rodents and humans). Significant changes in neuroendocrine, neurodevelopmental and behavioural systems occur during adolescence.4, 7, 8, 9, 10 For instance, there are changes in reward circuitry that render both rodent and human adolescents less sensitive to the aversive effects of drugs of abuse.11 Behaviourally, both rodents7, 12, 13 and humans7, 14, 15, 16 show increased risk-taking,16 social activity14, 17 and impulsivity12, 15 across the adolescent period. Cognitive changes have also been demonstrated at this time of the lifespan,18 especially in relation to executive function19 and cognitive control.20 This corresponds to neurodevelopmental changes in terms of maturation of the circuits related to learning and memory, including the hippocampus,21, 22 amygdala9 and prefrontal cortex.18, 22
The hippocampus is particularly altered during adolescent development, as an increased number of granule cells and overall increased volume of the hippocampal layers has been demonstrated in rodents during the adolescent period.23, 24 Notably, levels of hippocampal neurogenesis (the production, differentiation and integration of new neurons within the subgranular zone of the granular cell layer of the dentate gyrus (DG)),25 is up to four times higher during adolescence than during adulthood in rodents.21, 26, 27, 28, 29, 30 While the exact purpose of these newly born hippocampal cells is still controversial,31, 32 several lines of evidence from rodent studies have suggested that adult hippocampal neurogenesis is mandatory for stress susceptibility33 and optimal cognitive function,34, 35 at least during adulthood. Neurogenesis has been linked to cognitive behaviours that are hippocampal-dependent, and while some discrepancies are evident, overall it has been shown that tasks that require spatial memory, contextual memory or pattern separation are predominantly reliant on hippocampal neurogenesis.36, 37 More research is needed, however, to decipher the relative contribution of neurogenesis during adolescence to cognitive function, and especially to determine whether the types of behavioural tests that are reliant on hippocampal neurogenesis differ during this time period. While most studies that have examined the role of hippocampal neurogenesis in cognitive function have focused on the adult, it is important to consider that across the lifespan there may be differential effects. Likewise, most rodent studies examining the role of hippocampal neurogenesis in cognitive function have employed male rats; however, puberty may lead to the onset of sex differences in hippocampal neurogenesis.38, 39 Thus, there is still much to be explored in regards to possible sex differences in neurogenesis-associated behaviours, especially during the adolescent period.
Only a limited number of studies have examined the effects of blunted neurogenesis during adolescence on cognitive performance in later life. The results to date indicate that adolescent male rats and mice exposed to radiation, which ablates hippocampus neurogenesis, at PND21 or PND50 show a reduction in both the proliferation and survival of new neurons within the DG in adulthood,40, 41 as well as lasting deficits in performance in cognitive tasks such as the Morris water maze40 and fear-conditioning.41 This corresponds to findings in humans, which show that brain radiation for cancer treatment in children and adolescents produces long-term decreases in IQ scores along with other cognitive and behavioural changes.42 Thus, these data suggest that any changes to hippocampal neurogenesis during adolescence may have long-lasting effects on memory and learning. Altered hippocampal neurogenesis has also been linked to disorders that often emerge during adolescence or early adulthood, including depression,43, 44, 45, 46 schizophrenia,46, 47, 48 drug abuse49, 50 and impulse control disorders such as attention-deficit hyperactivity disorder,51, 52 all of which have significant cognitive as well as emotional symptoms. As such, alterations in neurogenesis during adolescence may be important in initiating the onset or development of disease.53
It is also important to consider other lifestyle and physiological changes that occur during the adolescent period, and how these could influence both neurogenesis and cognition. The transitional period of adolescence is a particularly stressful one,4, 54 with a majority of human adolescents reporting mood disturbances and anxiety.55 Stress has been shown to have a predominantly negative impact on hippocampal neurogenesis.56, 57 In particular, chronic exposure to stressful situations including psychosocial or physical stressors has been demonstrated to be detrimental to adult neurogenesis in the DG in several species including mice, rats, marmoset monkeys and tree shrews, in that it decreases cell proliferation, neuronal differentiation and cell survival.58, 59, 60 Related to this, chronic stress has been shown to impair hippocampal-dependent behaviours including learning and memory in rodents.61 As such, the stress associated with the adolescent period may produce alterations in hippocampal neurogenesis and cognitive function. Other extrinsic signals, such as physical exercise and diet, have also been identified as regulators of adult hippocampal neurogenesis in rodents.62 As adolescence is a vulnerable period for lifestyle influences, positive alterations in diet and exercise at this time could help to attenuate the negative impact of stressor exposure on neurogenesis and cognition.63, 64, 65, 66, 67
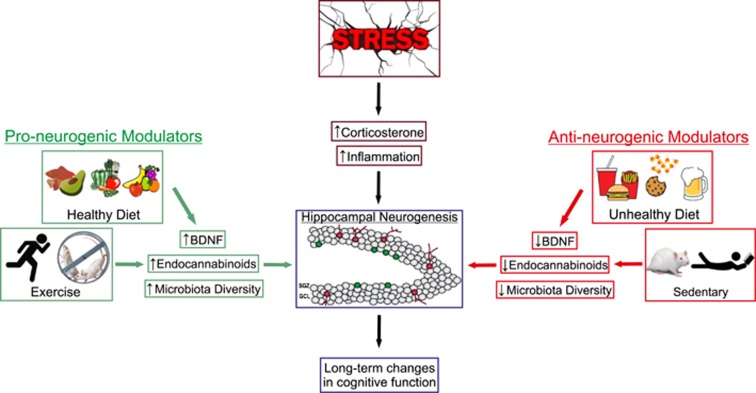
Not only are there basal differences in hippocampal neurogenesis during adolescence, but it is also a time period during which hormonal changes and alterations in lifestyle can affect neuronal proliferation and survival within the DG. Moreover, this period of the lifespan may be important in the programming of future hippocampal connectivity; thus, it is essential to better understand how potential modulators such as stress, diet and exercise could affect adolescent hippocampal neurogenesis, and subsequently have an impact upon cognitive function throughout the rest of the lifespan. This review will address the adolescent period as a critical window for the profound and lasting negative effects of stress, with a focus on how activation of the hypothalamic-pituitary-adrenal (HPA) axis and neuroinflammation may contribute to these effects. The role of puberty in producing differences between adolescent males and females in response to stress will also be discussed. Lastly, the review will consider the possible alleviation of chronic alterations to hippocampal neurogenesis and cognitive function through interventions with modifiable lifestyle factors such as exercise and diet (Figure 1).
Figure 1.
A schematic illustration of the impact of both negative and positive lifestyle factors on stress-induced alterations to neurogenesis in the dentate gyrus of the adolescent hippocampus. BDNF, brain-derived neurotrophic factor; GCL, granular cell layer; SGZ, subgranular zone.
The impact of stress during adolescence on hippocampal neurogenesis
Immediate effects of adolescent stress on neurogenesis
While it is well established that stress has a detrimental effect on neurogenesis in adulthood, the effect is relatively short-lived, as recovery can occur in as little as 10 days.68 The effects of stress during adolescence on hippocampal neurogenesis in rodent models remain relatively unexplored; however, what data there are indicate that stress during this time can produce much longer-lasting effects on neurogenesis and related cognitive function. Indeed, perhaps for pragmatic, logistical and timing reasons most experiments investigating the effects of stress in adolescence have utilised paradigms in which the final readout is during adulthood (see Table 1 for hippocampal cellular measures). The few studies in which neurogenesis measures were tested immediately while animals were still in the adolescent period have shown differing effects depending on the parameters used and examined. For example, male rats that had been exposed to social instability stress (PND30–45) had increased hippocampal cell proliferation and numbers of new neurons when examined at PND46,69 while female rats exposed to the same stress challenge had decreased hippocampal cell survival and no change in proliferation when examined at PND49.70 Male mice exposed to chronic isolation stress (5 weeks starting at PND24) exhibited a decrease in the survival of newly born cells, as well as a reduction in the proportion of newly born neurons in the cellular population when examined at PND53.71 Unlike the effects of social isolation in rats, no change in cell proliferation within the DG was observed.71 This could suggest that a longer stress challenge produces a differential response, or that the short-term effect of stress in adolescence is opposite to the effect at a later time point. In a non-human primate model, 8–10-month-old male and female marmoset monkeys showed a decrease in cell proliferation and in the number of new neurons within the DG immediately after either 1 or 3 weeks of social isolation stress.72 Chronic social or physical stress in male rats (4 weeks starting at PND28) did not result in changes to DG volume or neuronal density at PND56,73 while male rats at PND28 showed a reduced induction of long-term potentiation following elevated platform stress, suggesting impaired hippocampal function.74 However, DG volume, neuronal density and long-term potentiation are all indirect measures of neurogenesis, and as such may not be reflective of changes in hippocampal neurogenesis levels per se. Thus, from the few studies there are, it appears that the effect of stress in adolescence on neurogenesis can be either positive or negative depending on the timing of tissue collection in relation to the stress challenge and the sex of the animals tested.
Table 1. Summary of the effects of adolescent stress on hippocampal neurogenesis and neuroplasticity measures in rodent models.
| Stressor | Species | Sex |
Age (PND) at |
Effect on hippocampal neurogenesis and neural plasticity | References | ||
|---|---|---|---|---|---|---|---|
| Stress | BrdU (i.p.) | Tissue collection | |||||
| Elevated platform | Wistar rat | M | 28 | N/A | 28 | ↓ LTP in CA1 | Xiong et al.74 |
| 28 | ↑ LTD in CA1 | ||||||
| Chronic restraint | SD rat | M | 30–52 | 70 | 91 | ↔ Cell proliferation (Ki67) | Barha et al.75 |
| 91 | ↑ Cell survival (BrdU) | ||||||
| 91 | ↔ Neuronal differentiation (Brdu/NeuN); DG volume | ||||||
| Chronic restraint | SD rat | F | 30–52 | 70 | 91 | ↓ Cell proliferation (Ki67) | |
| 91 | ↓ Cell survival (BrdU) | ||||||
| 91 | ↔ Neuronal differentiation (Brdu/NeuN); DG volume | ||||||
| Chronic mild | SD rat | M | 30–58 | 72 | 77 | ↑ Number new cells (BrdU) | Toth et al.76 |
| 77 | ↔ Number of new neurons (BrdU/DCX) | ||||||
| 77 | ↑ BDNF protein in DG | ||||||
| Chronic physical | SD rat | M | 28–55 | N/A | 56 | ↑ Volume of CA1 | Isgor et al.73 |
| 56 | ↔ Volume; neuron number; neuronal soma size in CA3, DG | ||||||
| 76 | ↓ Volume of CA1, CA3, DG | ||||||
| 76 | ↑ Neuronal soma size in CA1, DG | ||||||
| 76 | ↔ Neuron number | ||||||
| Chronic social | SD rat | M | 28–55 | N/A | 56 | ↑ Volume of CA1 | |
| 56 | ↔ Volume; neuron number; neuronal soma size in CA3, DG | ||||||
| 76 | ↑ Neuronal soma size in CA1, DG | ||||||
| 76 | ↔ Volume; neuron number in CA1, CA3, DG | ||||||
| Chronic social | CD1 mouse | M | ~32–80 | N/A | ~450 | ↓ BDNF in CA1, CA3, DG | Sterlemann et al.77 |
| ~450 | ↓ DG synaptic density (synaptophysin protein) | ||||||
| ~450 | ↓ LTP in CA1 | ||||||
| Social instability | LE rat | M | 30–45 | N/A | 33 | ↑ Cell proliferation (Ki67) | McCormick et al.69 |
| 46 | ↔ Cell proliferation (Ki67) | ||||||
| 46 | ↑ Number of new neurons (DCX) | ||||||
| 74/75 | ↔ Cell proliferation (Ki67) | ||||||
| 74/75 | ↑ Number of new neurons (DCX) | ||||||
| Social instability | LE rat | F | 30–45 | 43–45 | 49 | ↔ Cell proliferation (Ki67) | McCormick et al.70 |
| 49 | ↓ Number of new cells (BrdU) | ||||||
| Social isolation | ICR mouse | M | 24–52 | 23 | 53 | ↓ Cell survival (BrdU); neuronal differentiation (BrdU/NeuN) | Ibi et al.71 |
| 52 | 53 | ↔ Cell proliferation (BrdU) | |||||
Abbreviations: BrdU, bromodeoxyuridine; BDNF, brain-derived neurotrophic factor; CA1, Cornus ammonis region 1 of the hippocampus; CA3, Cornus ammonis region 3 of the hippocampus; DCX, doublecortin; DG, dentate gyrus; F, female; ICR, Institute for Cancer Research; i.p., intraperitoneal; LE, long-Evans; LH, Lister Hooded; LTD, long-term depression; LTP, long-term potentiation; M, male; N/A, not applicable; NeuN, neuronal nuclei; PND, post-natal day; SD, Sprague–Dawley.
Lasting effects of adolescent stress on adult neurogenesis
The effect of stress during adolescence also appears to produce changes to hippocampal neurogenesis that last into adulthood. Male rats exposed to chronic variable physical stress for 4 weeks during adolescence (starting at PND28) showed a decrease in DG volume and an increase in DG neuronal density and DG granule cell soma size when measured 3 weeks following stressor cessation.73 Conversely, 4 weeks of social stress during the same period only increased DG soma size without affecting volume or neuronal density.73 When neurogenesis itself was measured in a similar experimental paradigm, social instability stress in male rats (PND30–45) resulted in increased numbers of immature neurons within the DG at PND74–75.69 In support, 4 weeks of chronic mild stress in adolescence (starting at PND30) resulted in an increase in hippocampal cell proliferation along with an increase in DG brain-derived neurotrophic factor (BDNF; a strong correlate of neurogenesis) in male rats.76 Interestingly, when the same stressor was applied for 4 weeks to adult male rats (starting at PND60), the opposite effect was found, with stress resulting in a decrease in cell proliferation and decreased BDNF expression.76 Similarly, 7 weeks of social stress starting during adolescence (PND31–33) and continuing into adulthood in male mice resulted in decreased mRNA expression of BDNF within the DG 12 months following stressor exposure.77 This suggests that stressor duration can have a differentially impact on neurogenesis, with the negative impact of adulthood stress potentially over-riding any pro-neurogenic effects of stress during adolescence. There also appears to be a sex difference in the impact of chronic stress on neurogenesis during adolescence, as chronic restraint stress (PND30–52) decreased hippocampal cell proliferation and survival in female rats, but increased cell survival in male rats.75 Thus, an overall trend for an increase in hippocampal neurogenesis in early adulthood is reported after adolescent stress exposure in males, while the opposite effect is observed in females and in males later in life. This highlights that factors such as stressor duration, the age at which it occurs, the sex of the species and the timing of tissue collection all warrant particular attention in the experimental design. As such, the factors influencing this differential response to stress-induced changes to neurogenesis across the lifespan deserves greater investigation.
Reconciling across-studies differences in stress-induced alterations in adolescent neurogenesis
When examining the results across these studies, there are many important confounds to keep in mind, which may underlie the discrepancies observed. First, not all measurements of hippocampal function may be reflective of neurogenesis. For instance, as stated earlier, non-direct measures such as hippocampal volume and BDNF levels do not always correlate with direct cell-counting measures. An example of this is that male rats had no alterations in BDNF mRNA expression within the dorsal hippocampus, but displayed increased numbers of newly born and immature neurons within the DG 2 weeks following chronic mild stress through mid to late adolescence (PND30–58).76 In addition, it has recently been suggested that the marker doublecortin, which is often used as a measurement of newly born neurons, may not be a necessary mediator of neurogenesis.78 Thus it is important to consider the type of measurement being made when interpreting data on neurogenesis.
In addition to the types of neurogenic measurements being used, the measurement of dividing cells with the thymidine analogue 5-bromo-2'-deoxyuridine (BrdU) can vary in terms of dosage, timing of injection and timing of tissue collection post injection. For instance, lower doses of BrdU (50–100 mg kg−1) result in fractional labelling of mitotic cells,79 which could explain some of the differential results between experiments. Varied lengths of time between BrdU injection and tissue collection to assess cell proliferation have also been employed in these adolescent neurogenesis studies. While some experiments have used a 24-h interval,71 others used a delay of ~5 days between BrdU injection and tissue collection.70 In adult neurogenesis studies in which BrdU is used to label cellular proliferation, the timing between injection and cull is usually 24 h or less,80, 81, 82, 83 with a number of studies using a 2-h interval,84, 85 as this has been demonstrated to represent the timing of one labelling cycle.86, 87 Thus, the longer delays used in the adolescent studies discussed here may more accurately represent short-term cell survival rather than proliferation.87 In addition, the timing of BrdU injection in relation to the stressor onset should be considered. In some studies BrdU was administered after the stress challenge to determine the levels of cell proliferation and survival in the post-stress environment,75, 76 while in others the BrdU injection was done during the stressor exposure in order to examine the direct effect of stress on neurogenesis.9, 71 As the effects of stress can sometimes be short-lived, it is thus important to bear in mind that these differences in BrdU injection schedules could result in differential outcomes in terms of cell proliferation or survival rates. Furthermore, there is some evidence that the blood–brain barrier permeability to BrdU may vary with age. For example, it appears that the foetal brain is more susceptible to BrdU permeability,79 which again would make comparisons of neurogenesis difficult across the lifespan, including between adolescence and adulthood. Ideally, a dose- and time-response curve at multiple ages of BrdU labelling would be helpful in determining the optimal dosage regime of BrdU across post-natal life.
Consideration should also be given to the duration and timing of the stress challenge itself when measuring hippocampal neurogenesis responses. One of the main factors that may influence stressor effects on neurogenesis is the length of the stress challenge. Although both acute and chronic stress have a detrimental effect on hippocampal neurogenesis, in adulthood the effects of acute stress are shorter-lived.60 However, in adolescence shorter stress challenges may be able to produce longer effects, as evidenced by a study in which adolescent male rats were subjected to a relatively short chronic stress paradigm (15 days) altered hippocampal neurogenesis up to 30 days later.69
The impact of stress during adolescence on hippocampal-dependent cognitive function
Immediate effects of adolescent stress on cognitive function
Stress in adulthood results in deficits in cognitive behaviours such as spatial learning88, 89 and novel object recognition,90, 91 which have been shown to be dependent on hippocampal neurogenesis.92, 93 During adolescence, stress produces varied changes to cognition (see Table 2 for cognitive behavioural measures in rodent models) depending on the type of stressor, the cognitive test employed and the timing of the stressor. For instance, eyeblink conditioning was increased following tailshock stress during mid-adolescence (PND35–40), but not early adolescence (PND25–29) in both male and female rats.94 A mixed stress challenge composed of daily exposure to predator odour and elevated platform stress (PND28–30) produced increased freezing in male rats exposed to auditory fear-conditioning (PND42), but reduced freezing in a contextual fear paradigm in females at the same time point,95 suggesting a decrease in hippocampal-related behavioural performance. Chronic stress challenges have also been found to produce a change in cognitive function when tested in adolescence. Social isolation in male rats (30 days starting at PND25–28) resulted in impaired object recognition memory on the last day of stressor exposure.96 Similarly, social isolation through adolescence (5 weeks starting at PND24) resulted in impaired spatial memory in the Morris water maze (PND53–59) in male mice.71 Neither chronic variable physical stress nor chronic variable social stress during adolescence (4 weeks starting PND28) affected spatial memory in the Morris water maze at the end of adolescence (PND56) in male rats.73 Together, these studies indicate that chronic stress during adolescence may produce a negative impact on cognitive function depending on the type of stressor imposed and the cognitive test employed.
Table 2. Summary of the effects of adolescent stress on hippocampal-related cognitive behaviours in rodent models.
| Stressor | Species | Sex |
Age (PND) at |
Cognitive test | Cognitive effect | Reference | |
|---|---|---|---|---|---|---|---|
| Stress | Testing | ||||||
| Elevated platform | Wistar rat | M | 26–28 | 60 | MWM | ↓ Learning | Avital et al.97 |
| 28 | 90 | MWM | ↑ Reversal | ||||
| Tailshock | SD rat | M | ~27 | ~27–28 | TEC | ↔ Responses | Hodes et al.94 |
| ~37 | ~37–38 | TEC | ↑ Responses | ||||
| Tailshock | SD rat | F | ~27 | ~27–28 | TEC | ↔ Responses | |
| ~37 | ~37–38 | TEC | ↑ Responses | ||||
| Chronic mild | SD rat | M | 30–70 | ~385 | RAM | ↔ Latency | Chaby et al.98 |
| Chronic mild | SD rat | M | ~43–63 | ~64–67 | TFC | ↑ Freezing | Reich et al.99 |
| ~64–67 | DFC | ↔ Freezing | |||||
| Chronic physical | SD rat | M | 28–55 | 56 | MWM | ↔ Learning | Isgor et al.73 |
| 28–55 | 76 | MWM | ↓ Learning | ||||
| Chronic social | SD rat | M | 28–55 | 56 | MWM | ↔ Learning | |
| 28–55 | 76 | MWM | ↔ Learning | ||||
| Chronic social | CD1 mouse | M | ~32–80 | ~270 | MWM | ↔ Learning | Sterlemann et al.77 |
| ~440 | MWM | ↓ Learning | |||||
| ~440 | Y-Maze | ↓ Learning | |||||
| ~440 | NOR | ↔ Recognition | |||||
| ~440 | SDL | ↔ Recognition | |||||
| Social instability | LE rat | F | 30–45 | 46–48 | SLR | ↔ Memory | McCormick et al.70 |
| 70–72 | SLR | ↓ Memory | |||||
| Social instability | LE rat | M | 30–45 | 46–49 | SLR | ↔ Memory | McCormick et al.69 |
| 46–49 | NOR | ↔ Recognition | |||||
| 70–73 | SLR | ↓ Memory | |||||
| 70–73 | NOR | ↔ Recognition | |||||
| Social isolation | ICR mouse | M | 24–59 | 53–59 | MWM | ↓ Learning | Ibi et al.71 |
| Social isolation | LH rat | M | ~28–58 | ~58 | NOR | ↓ Recognition | Bianchi et al.96 |
| Social isolation | LH rat | F | 28–70 | ~70 | NOR | ↓ Recognition | McLean et al.100 |
| ~70 | AS | ↓ Learning | |||||
| Variable | SD rat | M | 27–29 | 59–60 | TWS | ↓ Avoidance | Tsoory et al.101 |
| 33–35 | 59–60 | TWS | ↓ Avoidance | ||||
| Variable | SD rat | M | 27–29 | 60–61 | TWS | ↓ Avoidance | Ilin et al.102 |
| Variable | SD rat | M | 27–29 | 63 | TWS | ↓ Avoidance | Tsoory et al.103 |
| Variable | WH rat | M | 28–30 | 41–42 | CFC | ↔ Freezing | Toledo-Rodriguez et al.95 |
| 41–42 | AFC | ↑ Freezing | |||||
| 83–87 | CFC | ↔ Freezing | |||||
| 83–87 | AFC | ↑ Freezing | |||||
| Variable | WH rat | F | 28–30 | 41–42 | CFC | ↓ Freezing | |
| 41–42 | AFC | ↔ Freezing | |||||
| 83–87 | CFC | ↔ Freezing | |||||
| 83–87 | AFC | ↔ Freezing | |||||
Abbreviations: AFC, auditory fear-conditioning; AS, attentional set-shifting; CFC, contextual fear-conditioning; DFC, delay fear-conditioning; F, female; ICR, Institute for Cancer Research; LE, long-Evans; LH, lister hooded; M, male; MWM, Morris water maze; NOR, novel object recognition; PND, post-natal day; RAM, radial arm maze; SD, Sprague–Dawley; SDL, social discrimination learning; SLR, spatial location recognition; TEC, trace eyeblink conditioning; TFC, trace fear-conditioning; TWS, two-way shuttlebox; WH, Wistar Han.
While there is evidence from human studies that indicates that stress during adolescence affects behavioural outcomes such as risk-taking,104, 105 there is limited evidence reporting an effect of adolescent stress on cognition in humans. However, one study has suggested that adolescents may be more vulnerable to stress-induced cognitive impairment, as adolescents exhibited an increased deficit in response inhibition under stressful conditions when compared to adults in the same paradigm.106 Critically, many studies of adolescent human cognition have focused on cognitive behaviours associated with cortical function such as response inhibition106 and cognitive control107 as opposed to hippocampal-dependent behaviours, and thus cannot be directly used as a measure of hippocampal function. In addition, most studies of human adolescent cognitive function have focused on the impact of early-life stress, examples of which include neglect, maltreatment and placement into foster-care, rather than the effect of stress during adolescence itself, which limits the conclusions that can be drawn. For example, adolescents with a history of caregiver deprivation who had been put into adoptive placement (starting between ages 1 and 6) had decreased cognitive control in a ‘stop-signal' task when compared to controls.107 While studies like this one can inform future research into the effect of stress during the adolescent period on hippocampal-based cognitive function, caution should still be taken in the interpretation of how stress during adolescence may affect cognitive function. Overall, the immediate effect of stress during adolescence on cognitive function in humans (and rodent models) remains ripe for exploration.
Lasting effects of adolescent stress on adult cognitive function
Acute stressor exposure during adolescence can also have lasting effects on cognitive function, at least in rodent models. For example, 3 days of predator odour stress and elevated platform stress (PND28–30) increased freezing in an auditory fear-conditioning paradigm in male rats in adulthood, but produced no effects in females.95 This suggests that, like neurogenesis, there may be a sex difference in the cognitive response to stressor exposure during adolescence. There are also differences in the adult response depending on the timing of stressor exposure during adolescence, as variable stressor exposure in early adolescence (PND27–29) had a more pronounced negative effect in the two-way avoidance shuttle task in adulthood than did stressor exposure in mid-adolescence (PND33–35) in male rats.101, 103 In addition, it appears that adolescents may be more likely to develop long-term changes in cognitive function in response to stress, as adolescent male rats exposed to elevated platform stress (PND26–28) showed an increased impairment in spatial learning in the Morris water maze in adulthood when compared to rats exposed to the same stressor in adulthood.97 Furthermore, stressor exposure during the adolescent period may result in organisational changes that alter future responses to stress. This is evidenced by a study demonstrating that male rats exposed to elevated platform stress during adolescence, that were subsequently exposed to acute swim stress in adulthood, showed improved performance in the Morris water maze compared to either stress challenge alone.97 However, evidence of changes in neurogenesis in these studies has not been examined, and so to date we can only speculate on the role of acute stressor exposure during adolescence on neurogenesis and associated changes in cognition.
A larger number of studies have examined the effect of chronic stress during adolescence on adult cognitive behaviours. In contrast to acute stressor exposure, chronic mild stress for 3 weeks in mid-adolescence (starting between PND40 and 45) resulted in enhanced trace fear-conditioning in male rats, but had no effect on contextual conditioning when tested immediately following the end of stressor cessation in adulthood.99 Social isolation of female rats for 6 weeks (starting at PND28) impaired both object recognition and attentional set-shifting when tested at the beginning of adulthood.100 Similarly, chronic social instability stress during adolescence (PND30–45) resulted in impaired object location memory in both adult female70 and male69 rats. In contrast, no changes were observed when rats were tested in adolescence, suggesting that stress during adolescence may not affect behavioural outputs immediately. However, in a similar study, chronic variable physical stress, but not variable social stress, imposed for 4 weeks during adolescence (starting PND28) in male rats resulted in reduced spatial memory performance in the Morris water maze in adulthood.73 The reasons behind the discrepancy in these findings remains unclear, as all used social stress paradigms starting at a similar age, and utilised forms of spatial memory as the behavioural output. However, it may be that location recognition memory and spatial memory in the Morris water maze are differentially influenced upon by stress-induced deficits in hippocampal neurogenesis. The effect of chronic stress during adolescence on behaviour in aged animals has also been examined. While in one study male rats exposed to 5 weeks of chronic unpredictable stress during adolescence (from PND30 to 70) showed no changes in learning in the radial arm maze when tested 10 months later,98 in another, male mice exposed to 7 weeks of chronic social stress (starting approximately PND31–33) showed deficits in spatial memory in the Y-Maze and Morris water maze 12 months after the last stress exposure.77 Thus, the effect of stress during adolescence may not be apparent immediately, but may produce deficits throughout the lifespan, although future experiments are needed to better clarify these findings, and provide more conclusive support.
Collectively, these studies suggest that both acute and chronic stress exposure during adolescence results in long-term changes to performance in hippocampal-dependent cognitive behavioural tasks, potentially mediated through hippocampal neurogenesis. Unlike the recovery from stress observed in adulthood, which can occur in as little as 10 days,68 stress during adolescence can produce effects up to 12 months later. In addition, the adolescent period appears to be particularly vulnerable to certain types of stressors such as social isolation due to the evidence demonstrating that decreases in neurogenesis occurs during adolescence but not adulthood in response to this stressor.72, 108 However, more studies are needed to address the timing, stressor specificity and duration of these responses.
Reconciling differences across studies into the effects of adolescent stress on cognition
As with the measurement of neurogenesis, the divergent results obtained from adolescent stress and cognition studies most likely reflect differences in experimental design across experiments. Likewise, the stressors employed in these experiments are varied in terms of both duration and severity, and as such could produce differential outcomes. For instance, as stated above, in male rats a short-term variable stressor (3 days) did not affect contextual fear-conditioning in adulthood,95 while a longer-term variable stressor (3 weeks) produced an increase in freezing behaviour.99 It is hard to pinpoint whether in this case it was the difference in the duration of stress alone that was the significant factor, as the difference in behavioural performance could also be due to the types of varied stressor used, or due to the timing of stressor exposure.
The timing of the cognitive testing following stressor exposure could have an important role in mediating the effect on behavioural outcome. It has been shown that performance in cognitive tasks changes during developmental periods.9 For instance, chronic variable physical stress during adolescence produced deficits in spatial memory in the Morris water maze in late adolescence but not in adulthood in male rats,73 whereas social instability stress impaired object location recognition only in adulthood.69 In addition, the same stress challenge during adolescence resulted in disparate outcomes at different adult ages. Specifically, chronic social stress during adolescence in male mice produced no change in spatial memory in the Morris water maze 6 months post stress; however, at 12 months post-stress deficits in performance were observed.77 Therefore, it is important to test the effects of adolescent stress on cognitive performance at a range of ages later in life in order to get a greater understanding of the lasting cognitive deficits produced in response to stressor exposure.
The tests used to measure cognitive ability in response to adolescent stress differ across reports, and could contribute to some of the diverse findings. As the adolescent period is characterised by increased impulsivity109 and enhanced reward sensitivity,110 it is important to consider potential differences in responses in cognitive tests at this time compared to adulthood. For instance, adolescent rodents are more susceptible to fear-conditioning than adults,109 and both adolescent rodents and humans demonstrate impaired fear extinction,111 which could indicate that direct comparisons between adolescents and adults on fear-motivated tasks are not appropriate. It is also imperative to note that stressor exposure has been shown to impact locomotor activity,112 pain susceptibility113 and anxiety behaviours,114 and as such caution should be used when interpreting the results of behavioural studies for which these changes may be confounding.
The brain circuitry required to complete various cognitive tasks differs, and this may explain some of the discrepancies in the reports on cognitive function from the literature not just in studies using adolescent rodents but in adult rodent studies also. For instance, in adult rodents, spatial memory tasks such as the Morris water maze and radial arm maze, as well as tests requiring contextual memory such as contextual fear-conditioning, have been repeatedly demonstrated to require an intact hippocampus for successful completion.115, 116, 117, 118, 119, 120, 121 Similarly, humans with hippocampal damage show impairments in in a virtual Morris water maze task.122 In tests such as novel object recognition69, 77, 96, 100 and cued fear-conditioning,95, 99 hippocampal lesion studies in adult rodents have shown mixed results, with some indicating direct hippocampal involvement,93, 120 and others indicating that regions such as the peri-postrhinal cortex (novel object)118, 123 and amygdala (cued fear-conditioning)121 may have a role in correct completion of the task. In the studies examining the effect of adolescent stress on cognition, the majority of those indicating a stress-induced deficit in performance utilised hippocampal-dependent spatial navigation tasks like the Morris water maze,71, 73, 77, 97 spatial location recognition69, 70 or Y-maze.77 Following adolescent stress animals showed either no change69, 77 or a decrease96, 100 in performance in novel object recognition, a task that may be less reliant on the hippocampus. However, other tests that are believed to be dependent on hippocampal function such as context based fear-conditioning,95 and radial arm maze98 do not result in clear cut deficits following stressor exposure during adolescence.
One explanation for this discrepancy in performance in hippocampal-dependent tasks in response to adolescent stress is that even though these tasks require hippocampal-dependent types of memory, they may not be definitively associated with changes in hippocampal neurogenesis. Tasks that require spatial memory, contextual memory and pattern separation have been shown to be dependent on hippocampal neurogenesis, at least in adult rodents.124, 125, 126 For example, both drug- and ablation-induced reductions in adult hippocampal neurogenesis have been shown to impair performance in trace fear-conditioning,127 contextual fear-conditioning,119 spatial memory in both the Morris water maze37, 40 and Barnes maze,128 spatial pattern separation using a radial arm maze,36 place learning using the Morris water maze129 and long-term memory in a non-matching to sample task.130 Lentiviral-mediated inhibition of the WNT signalling pathways (which are necessary for neurogenesis) within the DG of rats resulted in impaired spatial memory and novel object recognition,131 and deficits in a modified spontaneous location recognition pattern separation task.132 However, it has also been demonstrated that drug-induced reduction in hippocampal neurogenesis did not affect spatial learning in the Morris water maze, elevated plus maze or contextual fear-conditioning.127 These discrepant findings may reflect recent research, which has indicated that hippocampal neurogenesis may be involved in the forgetting of old information rather than the addition of new memories.133 In support, stress in early life can decrease extinction in fear-conditioning,95 indicative of a decline in the ability to forget a previously learned connection. Thus, the involvement of neurogenesis in cognitive tasks may be reflective of which component of memory or learning is being tested, and the type of test being used. Because of the uncertainty still surrounding the association between hippocampal neurogenesis and different types of cognitive tasks, as well as the potential for this association to change throughout the lifespan, the best course of action to assess the effect of stress on adolescent neurogenesis and cognitive function would be to correlate cognitive performance in multiple tasks carried out at various times throughout the adolescent period with measures of hippocampal neurogenesis.
Factors that may contribute to stress-induced alternations in hippocampal neurogenesis and associated cognition during adolescence
HPA axis response to stress during adolescence
In response to stressor exposure, the HPA axis is activated, resulting in the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, which in turn stimulates the release of corticosterone (CORT) from the adrenal cortex into the bloodstream. In addition, ACTH and CORT production is also stimulated by increased central release of pro-inflammatory cytokines in response to stress, especially interleukin (IL)-1β.134 CORT in turn signals through glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) within the hippocampus to activate a negative feedback loop and clamp the HPA response.135 Chronically elevated CORT has long been shown to suppress neurogenesis in the adult rat DG,136 although both acute and chronic stress-induced increases in CORT levels by stress have also been associated with enhanced cell proliferation and survival, respectively, in male mice.137, 138 Moreover, the rate of hippocampal neurogenesis in the adult male rat is dependent upon circulating levels of CORT,139 suggesting a complex interplay between the type and duration of stressor exposure that induces CORT, as well as the critical period during the lifespan at which it occurs.61
Evidence suggests that during the adolescent period hippocampal negative feedback onto the HPA axis may be diminished. The HPA axis showed a longer latency to recover to baseline levels of circulating ACTH and CORT in response to acute restraint stress in adolescent (PND28) compared to adult male rats.140 Similarly, at PND25 male rats had a prolonged ACTH and CORT response to an ether exposure stress challenge, along with a slower rise to peak CORT concentrations compared to adult rats.141 Further evidence of this age difference in HPA activation comes from a study showing that adolescent male rats (PND28) did not habituate to 7 days of restraint stress, as indicated by an increased circulating CORT response, whereas adult male rats did habituate to this stress challenge.142 In adolescent male rats (PND31–33) exposed to the bacterial mimetic lipopolysaccharide, a blunted circulating CORT response was observed after 3 h.143 However, this may be reflective of a slower rise to peak concentrations of CORT similar to that which has been reported following ether stress.141 Interestingly, human male and female adolescents (age 13–17) also show increased HPA activation (as indicated by an increased cortisol response) in response to performance stressor exposure compared to male and female children (age 7–12) and adults.144, 145, 146
One of the mechanisms through which a differential HPA axis response is observed during adolescence may be a change in the corticosteroid receptor balance during this developmental period. For example, adolescent male rats exposed to variable physical stress or social stress throughout adolescence (4 weeks starting at PND28) showed reductions in GR mRNA expression within the DG.73 The effect of physical stress appeared more long-lasting however, as the reduction in GR mRNA within the DG was still evident in adulthood, 3 weeks after stressor cessation.73 As the beneficial effects of CORT on neurogenesis and cognition are believed to occur with low occupation of GR receptors and high occupation of MR,147, 148 alterations in the MR to GR ratio across adolescence may regulate the potential effects of CORT at this time. Indeed, both male and female rats show an increase in MR and GR gene expression within the hippocampus during the adolescent period; mRNA levels of both receptors increase between PND15 and PND35 and again from PND60.149 In marmoset monkeys the trend is reversed, with mRNA levels of MR and GR declining from infancy through adolescence to adulthood.150 While there are reported increases in GR mRNA expression in the prefrontal cortex in humans during adolescence (age 14–18), no changes have been reported in the hippocampus.151 Thus, although GR expression is also decreased by stress in adulthood,152 the altered basal MR/GR expression during adolescence could result in differential changes to the glucocorticoid system within the hippocampus following stress at this time. In addition, the alterations in MR and GR ratios may affect the negative feedback imposed by the hippocampus on the HPA axis in response to stress. Thus, the increased HPA and CORT response to stress in adolescence may be a mechanism through which stressor exposure at this time of life may exert long-lasting effects on both hippocampal neurogenesis and cognition.
Neuroinflammatory response to stress during adolescence
Stressor exposure also results in increased expression of pro-inflammatory cytokines such as IL-1β,153, 154 IL-6,155, 156, 157 and tumour necrosis factor α,158, 159, 160 which have detrimental effects on hippocampal neurogenesis.161 Specifically, exposure to these cytokines has been shown to decrease the proliferation,154, 155, 160 survival157, 159 and neuronal differentiation153, 156, 158 of new cells in the embryonic and adult hippocampus. In addition to the HPA axis regulation, CORT also signals through GR and MR in the hippocampus to negatively feedback on the inflammatory response, reducing IL-1β expression.162, 163, 164 Under conditions of severe and chronic stressor exposure however, not only does the HPA axis remain activated, but a neuroinflammatory milieu ensues within many brain structures including the hypothalamus and hippocampus.165, 166, 167, 168, 169, 170, 171, 172
There is some evidence to suggest that there may be differences in inflammatory signalling pathways responsible for regulating neurogenesis across the lifespan, especially during the adolescent period.173 For example, in vitro studies have shown that in neural progenitor cells (NPC) cultured from adolescent mouse hippocampus (PND21), administration of IL-1α promoted an increase in cell proliferation, while NPC cultured from adult murine hippocampus did not.155 However, cell proliferation was inhibited by IL-6 administration in NPCs prepared from both adolescent and adult hippocampi.174 Likewise, another report has indicated that, in vivo, the cellular response to IL-1β may change across the lifespan. Adult male mice (5 months) overexpressing the IL-1 receptor antagonist showed decreased hippocampal cell proliferation compared to wild types, yet this difference disappeared with ageing (22 months).175 However, it remains to be determined whether there are differential cytokine signalling pathways that have an impact upon neurogenesis during adolescence compared to adulthood, and indeed whether there are sex differences in the response to inflammatory-induced changes in neurogenesis and associated cognition.
Sex differences in the response to stress during adolescence
Sex is an important contributor to differences in basal levels of adult hippocampal neurogenesis,85, 176, 177, 178 for example, at PND35 male rats exhibit higher levels of cellular proliferation in the DG than females,179 while adult female rats have higher levels of proliferation than males.176 Moreover, as there are bi-directional interactions between the HPA and HPG axes starting during puberty, which allow for sex-dependent stress responses,180, 181, 182, 183 it is important to consider sex differences in the response to stress during adolescence in rodents, and that these responses may differ from those seen in adulthood. For example, in two reports from the same group, similar experimental design and tissue collection points were employed for both male and female rats. In one experiment, adolescent female rats showed decreased cell proliferation in the DG following social instability stress (PND30–45),70 while in another, male rats showed an increase in cell proliferation and numbers of new neurons within the DG at the same time point.69 This also corresponds with the finding that following chronic restraint stress in adolescents (PND30–52), female rats had decreased cell survival, whereas male rats showed increased cellular survival within the DG.75 Interestingly, these changes are opposite to those observed in adulthood, as adult male rats show a decrease in neuronal proliferation following acute stress184 as well as a decrease in survival of new neurons after chronic stress.185 In adult female rats, however, an increase in the survival of new neurons, and no change in proliferation, was observed following chronic stress.185 These results suggest that adolescent females may have an inherent vulnerability to chronic stress compared to adolescent males, while adult females appear to be resistant to the detrimental effects of chronic stress on hippocampal neurogenesis. The differences between males and females in the neurogenic response to stress may be due to alterations in circulating hormone levels,39, 186 as adult female rats show an increase in the number of proliferating cells during the pro-oestrus phase of the oestrus cycle,176 a time characterised by high oestrogen levels. In support, it has been reported that gonadectomised adult female rats treated with oestrogen show increased cell proliferation,85, 177 but decreased neuronal survival within the DG,177 while castrated adult male rats show decreased survival of newly born cells, an effect that is reversed with testosterone replacement as well as with the testosterone metabolite dihydrotestosterone.178 The mechanism underlying the effects of steroid hormones on neurogenesis is due to the prevalence of oestrogen,187 androgen188 and progesterone189 receptors on neural stem cells in the hippocampus.39, 186 Steroid hormones and their interaction with cognate receptors on neural stem cells are critical for the organisational programming of the brain during the pubertal period of adolescence.38, 190, 191 Thus, the impact of stress on the HPA axis during adolescence, particularly during the pubertal period in females, may produce lasting changes to the HPG system and consequently organisational changes to hippocampal structures.
Although there is limited evidence to show that HPG axis hormones can cause differences in cognitive function in adolescent rodents,192, 193, 194, 195, 196 the literature includes reports from studies using adult rodents. For example, gonadectomy in adult male rats resulted in impaired performance in an inhibitory-avoidance task, suggesting a role for testosterone in increasing hippocampal-dependent cognition.197 In female rodents, treatment with either progesterone or oestrogen following ovariectomy resulted in increased performance on a novel object task.198 Moreover, it has been demonstrated that hippocampal-dependent cognition, especially spatial learning and memory, can vary across the oestrus cycle, with performance waning during the oestrus phase in mice following the peak of oestrogen levels.199 Similarly, in human females, spatial cognitive ability was decreased during the follicular and luteal phases when oestrogen levels were low.200, 201 McCormick et al.69, 70 have attempted to explain the potential differences in neurogenesis-associated cognitive function between adolescent males and females in response to social instability stress. In their experiments, they showed that mid-adolescent male rats (PND46) had an increase in the number of new neurons within the DG in response to social instability stress,69 whereas females (PND49) had a reduction in the number of new cells at a similar time point,70 and neither sex had a change in cell proliferation.69, 70 However, both male and female adolescent rats showed no change in spatial location memory when tested during the adolescent period, but a decrease in memory performance when tested in adulthood.69, 70 While there were some discrepancies in the measures of neurogenesis used and the timing of tissue collection between these two studies, overall they suggest that, while males and females may have differences in the immediate response to stress, the lasting negative impact on cognitive function is not discrepant across sexes. In support, both male and female adolescent rats show an increase in trace eyeblink conditioning following footshock,94 indicating that the cognitive response to stress during adolescence may not differ between males and females. However, there is some evidence that suggests that females may be the more susceptible sex to the negative effects of stress on hippocampal-dependant cognition. Adolescent male rats showed an increase in auditory fear-conditioning following chronic variable stress, while females were impaired in a contextual fear-conditioning task,95 a task that is more reliant on hippocampal neurogenesis. Thus, it will be important in future studies of adolescent hippocampal neurogenesis and cognitive function to note the pubertal state of the animals and the phase of the oestrus cycle not only at the time of cognitive testing, but also at the time of stress challenge and tissue collection.
Potential interventions during adolescence that could ameliorate stress-induced impairments in hippocampal neurogenesis and cognition
Epidemiological studies have revealed that human adolescence is often associated with altered meal intake patterns and changes in physical activity levels.202 Studies have indicated that less than 50% of teens receive the daily-recommended intake of fruit, vegetable, dairy or protein, while intake of fats and sugars during adolescence is well above the recommended levels.203, 204 In addition, adolescents are more likely to engage in unhealthy behaviours such as dieting and weight-control behaviours,205 as well as increased alcohol and drug use.206, 207, 208 Along with the reduction in healthy eating behaviours during this period, adolescence has also been shown to be a time of reduced physical activity,209 especially moderate-to-vigorous activity.210 This is in parallel to an increase in sedentary behaviours, such as TV viewing, video game and computer activity, and increased homework and reading time.211 In addition, unhealthy diet, increased alcohol intake and decreased exercise levels have been associated with decreased hippocampal neurogenesis and cognitive performance in rodent models;66, 212, 213, 214, 215 thus, it is possible that the lifestyle changes often associated with adolescence may have a negative impact on hippocampal function. Adolescence is also a critical time for the programming of patterns of behaviour, such as diet intake and exercise, which will carry over to adulthood,3 and as such dietary and exercise interventions during this period have the potential to alter hippocampal function and cognition throughout the lifespan, not just in adolescence itself. It should be noted, however, that due to the current scarcity of evidence from human cohorts, data from rodent studies should be viewed as informative rather than strictly translational.
Exercise
In adult rodents, aerobic exercise increases synaptic strength and plasticity, learning and growth factors within the hippocampus,67, 216 and also increases neurogenesis within the DG.66, 80, 217 It has previously been shown that 1 week of forced low-intensity running during adolescence in male rats (starting approximately PND35) resulted in increased survival of BrdU-labelled cells, an increased percentage of new cells differentiating into neurons and increased hippocampal BDNF mRNA expression218 compared to sedentary rats. Similarly, male and female rats trained on a rotarod from PND35 to 38 showed increased cell survival at PND49 as measured by the number of BrdU-labelled cells.219 In support, 8 weeks of forced running in male rats (starting at PND22) resulted in increased performance in the Morris water maze and increased neuronal numbers measured with cresyl-violet staining throughout the hippocampus and DG, which may suggest increased neurogenesis.220 Male rats subjected to forced running for 5 weeks (from PND21 to PND60) also showed increased mossy fibre density and BDNF protein levels within the hippocampus, along with increased spatial memory in the Morris water maze.221 Voluntary exercise can also have an impact upon neurogenesis; 13 days of voluntary running during adolescence (PND30–42) by male rats produced increases in both cell proliferation and survival within the DG.222 Moreover, 4 weeks of voluntary access to a running wheel during adolescence (starting PND31) increased performance in the novel object recognition task in male rats, an effect that was still observed 4 weeks after the exposure to exercise ended.223 In the same paradigm during adulthood, the immediate positive impact of voluntary running on object recognition in male rats had diminished within 2 weeks of post-running wheel exposure.223 In humans, physical fitness during adolescence (~14 years of age) is positively correlated to cognitive performance, while an acute bout of exercise (20 min of graded cycling) produced no changes.224 It should be noted that both acute and chronic exercise have been linked to increased executive function,225, 226 although this most likely indicates prefrontal cortex rather than hippocampal changes. However, a positive correlation has also been observed between aerobic activity and both hippocampal size and cognitive performance on a virtual Morris water maze, indicating that the link between exercise and hippocampal-dependent tasks is also evident in human adolescents (~16 years of age).227
There is some evidence that exercise during adolescence ameliorates the deleterious effects of early-life stress on cognitive behaviours and neurogenesis. Voluntary running in adolescent male mice (PND21–52) reversed the negative cognitive effects of prenatal stress in the Morris water maze, and also increased granular cell dendritic length and intersections within the DG, which suggests a recovery of cell maturation.228 Environmental enrichment studies in adult rodents (in which they are housed in larger cages, in larger groups, with access to toys, tunnels, besting material and with opportunity to exercise on running wheels229 have demonstrated that exercise is the critical element of environmental enrichment-induced enhancement of hippocampal neurogenesis and cognitive performance.230, 231 While the effect of exposure to an enriched environment during adolescence on hippocampal neurogenesis has not been yet reported in the literature, it has been shown that environmental enrichment, including free access to a running wheel (starting at PND22), attenuated the increase in body weight gain and deficit in social behaviours observed following prenatal stress in male rats.232 In addition, enrichment with access to running wheels (starting PND30) immediately following stressor exposure during adolescence (PND27–29) attenuated the stress-induced anxiety-like behaviours, altered HPA axis tone and impaired avoidance learning in male rats.102 Although the beneficial effects of environmental enrichment in adulthood on hippocampal neurogenesis has been shown to be largely due to increased physical activity,230, 231 it remains to be determined whether the same holds true during the adolescent period. Taken together, despite the heterogeneity of types of exercise employed, these studies indicate a positive effect of exercise, especially aerobic exercise, on hippocampal neurogenesis and cognitive function in adolescence. This suggests that exercise could act to reverse the negative impact of adolescent stress. However, there is a lack of direct evidence for the benefit of exercise in modulating stress-induced effects in adolescence compared to adulthood, and as such future studies should investigate whether there is an age-dependent response, and through which mechanisms these changes are occurring.
Mechanisms underlying exercise-induced changes to hippocampal neurogenesis and cognition
Many mechanisms underlying the effects of exercise on hippocampal function, including its potent effect on hippocampal neurogenesis in adulthood, have been explored using rodent models; however, the mechanism in adolescence may be different. For example, it has been found that, in male rats, forced treadmill running during adolescence (PND21–60) decreases cannabinoid receptor expression throughout the brain, including within the hippocampus.233 This is opposite to the effect in adulthood, where voluntary running increased cannabinoid receptor expression within the hippocampus of male rats.234 As the endocannabinoid system has been demonstrated to modulate hippocampal neurogenesis,235 especially the proliferation and migration of neurons,235, 236, 237 it may be that alterations to this system during a period of brain remodelling such as adolescence can produce long-lasting alterations to hippocampal structure and function that are different than those seen in adulthood. Moreover, the endocannabinoid system has been linked to HPA axis function, in that it helps to maintain homeostasis and habituation of the HPA axis.238 Thus, the endocannabinoid system may be one mechanism through which exercise can alter the effects of stress on neurogenesis, especially during adolescence.
The BDNF system has also been shown to be a major mechanism underlying the positive effects of exercise on neurogenesis.239 Moreover, BDNF has been shown to be protective against the negative effects of stress on neurogenesis and cognition.240 Forced running during the late adolescent period (PND 41–50) in male rats produces heightened levels of BDNF in the hippocampus in response to high-intensity exercise.241 Because expression of the BDNF receptor TrkB decreases during the course of the lifespan,242 it could be that increased levels of this growth factor could have a greater effect during adolescence than in adulthood. Adolescence may thus represent a time during which exercise and the subsequent elevation of BDNF expression within the hippocampus could produce a more potent and longer-lasting attenuation of stress-induced decreases in neurogenesis and cognition compared to adulthood. In addition, there could be sex differences in the effect of exercise on BDNF levels, as the BDNF gene contains an oestrogen-response element243 and increased concentrations of oestrogen result in heightened levels of BDNF expression.244, 245 In support of this, a reduction in the exercise-induced increase in hippocampal BDNF expression in ovariectomised adult rats has previously been reported.246 As such, the changes in circulating oestrogens after puberty could contribute to a differential effect of exercise on BDNF levels, and consequently on hippocampal neurogenesis and associated cognition in adolescent males and females. However, more experiments are necessary to better elucidate the mechanisms underlying the amelioration of stress-induced effects by exercise, to further examine the differences between these mechanisms in adolescence in comparison to adulthood, and to determine whether there are sex differences in their actions.
Voluntary running has also been shown to alter the microbiota of adult male rats,247, 248 increasing the diversity of bacteria present. Recently, studies have begun to emerge that suggest a role for the microbiota in regulating both hippocampal neurogenesis249 and cognitive function.250 Interestingly, changes to the microbiota in adult female mice resulted in impaired hippocampal neurogenesis and decreased cognitive performance in a novel object recognition task, an effect that was rescued by 10 days of voluntary running.251 Thus, it appears that there is a relationship between exercise, the microbiota and neurogenesis, and as such microbiota changes may be one mechanism through which exercise can exert effects on hippocampal function and cognition in adolescence. In addition, it has also been demonstrated that adolescent humans have a differing microbiota diversity, with higher abundance of some genera, such as Bifidobacterium and Clostridium compared to adults.252 While in rodents early-life maternal separation stress results in changes to both the microbiota113 and deficits in cognitive performance,253 the effects during adolescence remain unclear. Interestingly, alterations of the microbiota during adolescence modifies hippocampal BDNF levels and decreases cognitive performance in the novel object recognition task in male mice.254 However, the role of the microbiota in mediating the effects of adolescent stress, as well as the potential role of exercise as a positive modulator during this critical window, is an exciting new mechanism to explore.
Dietary changes
Diet is one of the most easily modifiable lifestyle factors that is known to alter cognitive and behavioural performance, as well as neurogenesis.63, 64, 65 A growing body of evidence now points to the importance of adolescent diet for general well-being, including brain health.255, 256 Moreover, changes in the human diet over the past 50 years are potentially putting the adolescent brain in a more vulnerable state. ‘Western' diets high in processed foods, fats and sugars result in many problems including obesity,257, 258 diabetes259 and cognitive and emotional disorders.260, 261 On the other hand, healthy diets, such as a Mediterranean diet high in vegetables, fish, fruits and nuts provide many macronutrients such as omega-3 fatty acids, omega-6 polyunsaturated fatty acids and flavonoids,262 which promote both brain263, 264 and body health.265 Thus, the evidence to date suggests that not only is the nutritional status important for adolescent brain health, but so is the overall diet composition including macronutrients, fatty acids and vitamin levels.
To date, most studies examining the effect of diet during adolescence on cognition and neurogenesis have focused on the detrimental effect of high-fat diets (HFD) or high-sugar diets, rather than any beneficial effect of lower calorie or nutrient-supplemented diet. For example, in rodent models, male mice fed a HFD for 11 weeks throughout adolescence (starting at PND21) exhibited decreased cognitive performance in the radial arm maze, and decreased numbers of new neurons in the DG, while neither of these changes were observed when the diet was administered during adulthood.213 Similarly, a HFD fed to male rats during adolescence (for 11 weeks starting at PND21) decreased long-term memory performance in the Morris water maze, while a HFD during adulthood did not result in any of these changes.266 In addition, human adolescents consuming ‘Western' diets have decreased performance in visual-spatial learning and memory tasks, both of which are associated with hippocampal function.267 In support of the link between nutritional state and hippocampal function, consumption of diets lacking in Vitamin A and E resulted in decreased proliferation of new neurons in the DG268, 269 and reductions in spatial learning and memory268, 270 in adult male rats.
Some studies have examined the positive impact of nutrition on hippocampal neurogenesis and cognition in adulthood.63,64 Low-calorie diets have been shown to increase memory performance in aged human subjects.271 Findings from studies using adult male and female rodents suggest that low-calorie diets272, 273, 274, 275, 276 or diets with increased flavonoids,277, 278, 279 omega-3 fatty acids,280, 281, 282 polyphenols,283 magnesium284 or zinc285 can increase proliferation and survival of newly born neurons in the DG,274, 275, 276, 278, 279, 281, 282, 283, 285 as well as performance on hippocampal-related learning and memory tasks.272, 273, 277, 280, 284 There is also some evidence to suggest that nutritionally beneficial diets can protect against stress-induced impairment in hippocampal neurogenesis as well as cognitive deficits. For example, treatment of neuronal cultures with an omega-3 fatty acid reversed the CORT-induced prevention of neuronal differentiation.286 In addition, flavonoid-rich diets administered to rats (sex unspecified) that were subjected to chronic unpredictable stress reversed the stress-induced decrease in proliferating new neurons within the DG.287 Similarly, male and female mice subjected to 10 days of calorie restriction show attenuation of chronic social stress-induced depressive-like behaviours.288 Together, these results indicate that healthier diet interventions may have an important role in regulating hippocampal function. However, as with exercise, whether there is a differential effect of diet on stress-induced changes in hippocampal neurogenesis and cognition during adolescence compared to adulthood remains to be seen.
Mechanisms underlying diet-induced changes to hippocampal neurogenesis
BDNF also appears to be a critical mediator for the beneficial effects of diet on neurogenesis and cognitive function. For example, diets high in zinc have been shown to result in increased BDNF mRNA and protein expression within the hippocampus of adult male rats.289 In addition, the dietary restriction-related increase in the survival of newly born neurons within the DG in wild-type mice was attenuated in BDNF+/- adult male mice, suggesting a role for BDNF in mediating this effect.274 While there is no evidence in the literature to date on the potential role of BDNF in dietary-induced protection against changes to neurogenesis and cognition evoked by stress, there is some evidence to suggest that dietary changes may protect against brain damage through BDNF pathways. For instance, in adult male rats a diet high in omega-3 fatty acids or curcumin were protective against an injury-induced deficit in both hippocampal BDNF expression and performance in the Morris water maze.290, 291 While in these studies a physical brain injury stressor was used, the findings hint at a potential protective role of dietary-induced BDNF increases in response to other stress challenges, at least in adulthood. As BDNF-induced pro-neurogenic and pro-cognitive effects may be more pronounced during adolescence than adulthood,242 this suggests that a healthy diet, like exercise, may be especially beneficial for hippocampal development and function during this period of the lifespan than in later years. It may thus be the case that changes to diet during adolescence may produce longer-lasting effects than when a nutritional diet commences during adulthood.
It should also be noted that diet is the leading factor that induces changes in gut microbiota composition,292, 293, 294, 295 and the negative effect of stress on the microbiome in early life113, 296 and adulthood297 can be reversed by dietary changes. As with exercise, the effect of stress and diet on the microbiome has not yet been studied in adolescence, although it is known that there are changes to the gut microbiota structure and balance during the adolescent period.298 However, the influence of many of the lifestyle changes that begin during the adolescent period including stress, diet, alcohol and drug consumption has an impact on the microbiome, and thus it could be an important mechanism underlying hippocampal neurogenesis regulation and associated cognitive function during this time of the lifespan.299, 300
These findings suggest that, while adolescence may represent a period during which stress may have a more profound and long-lasting effect on hippocampal neurogenesis and cognitive function, it could also be a time during which positive changes such as healthier diet and increased exercise could not only combat these detrimental effects, but produce positive effects in their own right. Thus, future research into the potential synergistic effects of diet, exercise and stress are needed, and will help to determine which interventions during adolescence produce significant effects on cognition in later life.
Conclusions
Adolescence represents a unique period of the lifespan in which there is considerable re-organisation and growth of many brain structures, including the hippocampus. During this time there is increased hippocampal neurogenesis, and an altered HPA and inflammatory response to stress. As such, stress during this time of life may produce differential and long-lasting detrimental changes in the hippocampus that could last throughout adulthood. Specifically, stress-induced changes in hippocampal neurogenesis could lead to long-lasting deficits in cognitive function, especially in spatial and context related tasks. The pubertal onset of circulating HPG axis hormones leads to the onset of sex differences in the response to stress-induced impairments in hippocampal neurogenesis and cognition, with the scant data to date indicating that females are more vulnerable to stress. With growing evidence that the hippocampus is particularly responsive to environmental and lifestyle influences during adolescence, we hypothesise that intervention with positive factors such as a healthy diet and exercise may prevent the negative impact of stress during adolescence on hippocampal neurogenesis and cognitive function not only during the adolescent period itself, but throughout the rest of the lifespan. Moreover, maintaining a healthy diet and regular exercise during adolescence may help to programme these positive behaviours for the rest of the individual's lifespan.
However, much work is still needed to fully elucidate both the immediate and lasting impact of stress during adolescence on hippocampal neurogenesis and associated cognitive function, as well as the potential role for diet and exercise in attenuating these effects. Future research should address the adolescent period as a key window during which programming of hippocampal neurogenesis occurs, and focus on better understanding the mechanisms, such as the endocannabinoid system, BDNF and the gut microbiota, underlying stress-induced changes at this time. In addition, the effect of the onset of sex differences in the neurogenic and cognitive response to stress challenges should be examined, as well as the role of diet and exercise in modulating these changes. To better capture the effects of stress, diet and exercise, multiple measures of hippocampal neurogenesis and hippocampal-dependent cognitive behaviours should be investigated. Finally, it is important to clarify the translational impact of these findings through further study of hippocampal-dependent cognitive processes in the human adolescent in response to these modifiable lifestyle factors.
Acknowledgments
This work was supported by Science Foundation Ireland (grant number 12/IA/1537 to YMN and JFC). The authors would like to thank Ms. Erin Crowley for her helpful comments on the paper.
Footnotes
The authors declare no conflict of interest.
References
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci 2002; 22: 8647–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol 2000; 55: 469. [PubMed] [Google Scholar]
- Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC et al. Adolescence: a foundation for future health. Lancet 2012; 379: 1630–1640. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24: 417–463. [DOI] [PubMed] [Google Scholar]
- Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol 2014; 35: 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM. Growth at AdolescenceBlackwell Scientific Publications: Oxford, England, 1955. [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Current Direct Psychol Sci 2000; 9: 111–114. [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol 2010; 52: 244–253. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Progr Neuro-Psychopharmacol Biol Psychiatry 2010; 34: 756–765. [DOI] [PubMed] [Google Scholar]
- Green MR, McCormick CM. Effects of stressors in adolescence on learning and memory in rodent models. Hormones Behav 2013; 64: 364–379. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn 2010; 72: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci 2003; 117: 695. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 2004; 45: 153–162. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn 2010; 72: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev Psychobiol 2010; 52: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev 2008; 28: 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Achterberg EJM, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev 2016; 70: 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol 2007; 17: 251–257. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 2006; 47: 296–312. [DOI] [PubMed] [Google Scholar]
- Kuhn D. Do cognitive changes accompany developments in the adolescent brain? Perspect Psychol Sci 2006; 1: 59–67. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol Biochem Behav 2007; 86: 327–333. [DOI] [PubMed] [Google Scholar]
- Murty VP, Calabro F, Luna B. The role of experience in adolescent cognitive development: integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev 2016; 70: 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res 1998; 794: 199–210. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res 1982; 46: 315–323. [DOI] [PubMed] [Google Scholar]
- Ming G-l Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005; 28: 223–250. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 1996; 16: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013; 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010; 5: e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen DS, Takase LF, Fornal CA, Jacobs BL. Age-dependent decline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res 2008; 1228: 14–19. [DOI] [PubMed] [Google Scholar]
- Curlik DM, DiFeo G, Shors TJ. Preparing for adulthood: thousands upon thousands of new cells are born in the hippocampus during puberty, and most survive with effortful learning. Front Neurosci 2014; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol 2004; 14: 186–191. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 2002; 22: 635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Cryan JF, O'Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress 2015; 1: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008; 132: 645–660. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Marr RA. Of mice and men: neurogenesis, cognition and Alzheimer's disease. Front Aging Neurosci 2013; 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009; 325: 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience 2005; 130: 843–852. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 2005; 26: 163–174. [DOI] [PubMed] [Google Scholar]
- Galea LAM. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev 2008; 57: 332–341. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 2004; 188: 316–330. [DOI] [PubMed] [Google Scholar]
- Achanta P, Fuss M, Martinez JL Jr. Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci 2009; 123: 1036. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Trevino M, Zawaski JA, Gaber MW, Leasure JL. Neurogenesis, exercise, and cognitive late effects of pediatric radiotherapy. Neural Plast 2013; 2013; doi: 10.1155/2013/698528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci 2007; 10: 1110–1115. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Van Praag H, Gage F. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry 2000; 5: 262–269. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons?—Adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 2003; 54: 499–503. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry 2008; 21: 290–295. [DOI] [PubMed] [Google Scholar]
- Toro C, Deakin J. Adult neurogenesis and schizophrenia: a window on abnormal early brain development? Schizophr Res 2007; 90: 1–14. [DOI] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Lesch K-P. Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatr Clin Neurosci 2007; 257: 290–299. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatr Clin Neurosci 2007; 257: 261–270. [DOI] [PubMed] [Google Scholar]
- Nixon K, McClain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry 2010; 23: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Granados JL, Greene PL, Amsel A. Selective activity enhancement and persistence in weanling rats after hippocampal X-irradiation in infancy: possible relevance for ADHD. Behav Neural Biol 1994; 61: 251–259. [DOI] [PubMed] [Google Scholar]
- Lee TH, Lee CH, Kim IH, Yan BC, Park JH, Kwon S-H et al. Effects of ADHD therapeutic agents, methylphenidate and atomoxetine, on hippocampal neurogenesis in the adolescent mouse dentate gyrus. Neurosci Lett 2012; 524: 84–88. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 2008; 31: 183–191. [DOI] [PubMed] [Google Scholar]
- Dornbusch SM, Mont-Reynaud R, Ritter PL, Chen Z-y, Steinberg L. Stressful events and their correlates among adolescents of diverse backgrounds. In: Colten ME, Gore S (eds), Adolescent Stress: Causes and Consequences. Transaction Publishers: New Jersey, USA, 1991, pp 111–130.
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol 1999; 54: 317. [DOI] [PubMed] [Google Scholar]
- Cameron H, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience 1997; 82: 349–354. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Dev Brain Res 1997; 103: 91–93. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus 2006; 16: 233–238. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA 2010; 107: 4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci 2004; 19: 131–144. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol 2012; 233: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci 2015; 19: 151–161. [DOI] [PubMed] [Google Scholar]
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr 2009; 4: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainuddin MSA, Thuret S. Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull 2012; 103: 89–114. [DOI] [PubMed] [Google Scholar]
- Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast 2014; 2014; doi: 10.1155/2014/563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praag H. Neurogenesis and exercise: past and future directions. Neuromol Med 2008; 10: 128–140. [DOI] [PubMed] [Google Scholar]
- Wang Z, van Praag H. Exercise and the brain: neurogenesis, synaptic plasticity, spine density, and angiogenesis. In: Boecker H, Hillman CH, Scheef L, Struder HK (eds). Functional Neuroimaging in Exercise and Sport Sciences. Springer: New York, USA, 2012, pp 3–24. [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 1999; 113: 902. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Thomas CM, Sheridan CS, Nixon F, Flynn JA, Mathews IZ. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus 2012; 22: 1300–1312. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J. Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav Brain Res 2010; 208: 23–29. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem 2008; 105: 921–932. [DOI] [PubMed] [Google Scholar]
- Cinini SM, Barnabe GF, Galvão-Coelho N, de Medeiros MA, Perez-Mendes P, Sousa MB et al. Social isolation disrupts hippocampal neurogenesis in young non-human primatesFront Neurosci 2014;8; 100–108. [DOI] [PMC free article] [PubMed]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 2004; 14: 636–648. [DOI] [PubMed] [Google Scholar]
- Xiong W, Wei H, Xiang X, Cao J, Dong Z, Wang Y et al. The effect of acute stress on LTP and LTD induction in the hippocampal CA1 region of anesthetized rats at three different ages. Brain Res 2004; 1005: 187–192. [DOI] [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus 2011; 21: 1216–1227. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem 2008; 107: 522–532. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Rammes G, Wolf M, Liebl C, Ganea K, Müller MB et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus 2010; 20: 540–549. [DOI] [PubMed] [Google Scholar]
- Dhaliwal J, Xi Y, Bruel-Jungerman E, Germain J, Francis F, Lagace D. Doublecortin (DCX) is not essential for survival and differentiation of newborn neurons in the adult mouse dentate gyrus. Front Neurosci 2015; 9: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J Neurosci 2002; 22: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2: 266–270. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem 2000; 75: 1729–1734. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz J. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 2002; 115: 97–105. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 2003; 28: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LAM. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 2009; 30: 343–357. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Mckay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 2001; 435: 406–417. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev 2007; 53: 198–214. [DOI] [PubMed] [Google Scholar]
- Song L, Che W, Min-wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav 2006; 83: 186–193. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine treatment. Behav Neurosci 1996; 110: 1321. [DOI] [PubMed] [Google Scholar]
- Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, Del Rio J et al. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology 2008; 199: 1–14. [DOI] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object–place recognition memory. Neurobiol Learn Memory 2010; 93: 261–267. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012; 13: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 2004; 101: 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Hormones Behav 2005; 48: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast 2007; 2007: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Fone K, Azmi N, Heidbreder C, Hagan J, Marsden C. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci 2006; 24: 2894–2902. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol 2005; 8: 163–173. [DOI] [PubMed] [Google Scholar]
- Chaby LE, Sheriff MJ, Hirrlinger AM, Lim J, Fetherston TB, Braithwaite VA. Does chronic unpredictable stress during adolescence affect spatial cognition in adulthood? PLoS ONE 2015; 10: e0141908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Iskander AN, Weiss MS. Cannabinoid modulation of chronic mild stress-induced selective enhancement of trace fear conditioning in adolescent rats. J Psychopharmacol 2013; 27: 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S, Grayson B, Harris M, Protheroe C, Woolley M, Neill J. Isolation rearing impairs novel object recognition and attentional set shifting performance in female rats. J Psychopharmacol 2010; 24: 57–63. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by ‘juvenile'or ‘adolescent'stress. Int J Neuropsychopharmacol 2006; 9: 713–728. [DOI] [PubMed] [Google Scholar]
- Ilin Y, Richter-Levin G. Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS ONE 2009; 4: e4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol 2007; 17: 245–256. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Gorka SM, Matusiewicz A, Anderson K. Gender specific effect of psychological stress and cortisol reactivity on adolescent risk taking. J Abnorm Child Psychol 2013; 41: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Reynolds EK, Daughters SB, Wang F, Cassidy J, Mayes LC et al. Positive and negative reinforcement underlying risk behavior in early adolescents. Prev Sci 2010; 11: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahdar A, Galván A. The cognitive and neurobiological effects of daily stress in adolescents. Neuroimage 2014; 92: 267–273. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia 2010; 48: 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Philippens I, Manders E, Czéh B, Joels M, Krugers H et al. Distinct structural plasticity in the hippocampus and amygdala of the middle-aged common marmoset (Callithrix jacchus. Exp Neurol 2011; 230: 291–301. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Burk JA, Barnet RC. Adolescent transitions in reflexive and non-reflexive behavior: review of fear conditioning and impulse control in rodent models. Neurosci Biobehav Rev 2016; 70: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev 2016; 70: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Bisby MA, Richardson R. Impaired fear extinction in adolescent rodents: behavioural and neural analyses. Neurosci Biobehav Rev 2016; 70: 59–73. [DOI] [PubMed] [Google Scholar]
- Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacol Biochem Behav 1996; 54: 229–234. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EMM et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009; 65: 263–267. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Hormones Behav 2009; 55: 248–256. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol 1993; 60: 9–26. [DOI] [PubMed] [Google Scholar]
- Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res 1996; 716: 29–38. [DOI] [PubMed] [Google Scholar]
- Bannerman D, Deacon R, Offen S, Friswell J, Grubb M, Rawlins J. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci 2002; 116: 884. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 2004; 24: 5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 2006; 103: 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 1998; 8: 638–646. [DOI] [PubMed] [Google Scholar]
- Phillips R, LeDoux J. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106: 274. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res 2002; 132: 77–84. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Memory 2002; 9: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Oomen CA. Stress, hippocampal neurogenesis and cognition: functional correlations. Front Biol 2016; 11: 182–192. [Google Scholar]
- Hvoslef-Eide M, Oomen CA. Adult neurogenesis and pattern separation in rodents: a critical evaluation of data, tasks and interpretation. Front Biol 2016; 11: 168–181. [Google Scholar]
- Bowers M, Jessberger S. Linking adult hippocampal neurogenesis with human physiology and disease. Dev Dyn 2016; doi:10.1002/dvdy.24396. [DOI] [PubMed]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 2002; 12: 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 2004; 162: 39–47. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PEG, Bolwig TG, Wörtwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 2003; 119: 635–642. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 2006; 16: 296–304. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr., Consiglio A, Lie DC et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Memory 2009; 16: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM et al. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus 2014; 24: 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang H-L et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014; 344: 598–602. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 1999; 79: 1–71. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 1991; 12: 118–134. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci 1992; 12: 3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett 2004; 368: 7–10. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 2009; 19: 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 1994; 61: 203–209. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology 2004; 79: 125–132. [DOI] [PubMed] [Google Scholar]
- Vázquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res 1993; 34: 646–653. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 2006; 147: 1664–1674. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE, Deak T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol Behav 2015; 148: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol 2009; 21: 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT et al. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol 2009; 21: 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol 2009; 21: 1–6. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 1999; 22: 422–426. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition:implications for the field of brain and cognition. Brain Cogn 2007; 65: 209–237. [DOI] [PubMed] [Google Scholar]
- Bohn MC, Dean D, Hussain S, Giuliano R. Development of mRNAs for glucocorticoid and mineralocorticoid receptors in rat hippocampus. Dev Brain Res 1994; 77: 157–162. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C et al. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci 2005; 21: 1521–1535. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Herman MM, Kleinman JE, Weickert CS. Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol Aging 2007; 28: 447–458. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Karandrea D, Kittas C. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology 1999; 69: 331–338. [DOI] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane AK, Sullivan AM, O'Keeffe GW, Nolan YM. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci 2012; 49: 311–321. [DOI] [PubMed] [Google Scholar]
- Ryan SM, O'Keeffe GW, O'Connor C, Keeshan K, Nolan YM. Negative regulation of TLX by IL-1β correlates with an inhibition of adult hippocampal neural precursor cell proliferation. Brain Behav Immunity 2013; 33: 7–13. [DOI] [PubMed] [Google Scholar]
- McPherson CA, Aoyama M, Harry GJ. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: Differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immunity 2011; 25: 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O, Gong X, Rose-John S, Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell 2009; 20: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 2002; 22: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-α impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: role of Hes1. Mol Cell Neurosci 2010; 43: 127–135. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-α exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res 2005; 80: 789–797. [DOI] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav Immunity 2013; 30: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Nolan YM. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neurosci Biobehav Rev 2016; 61: 121–131. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M et al. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res 2000; 859: 193–201. [DOI] [PubMed] [Google Scholar]
- Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc Am Thorac Soc 2004; 1: 239–246. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Willis SA, McCracken GH Jr., Nisen PD. Protein synthesis-dependent induction of interleukin-1 beta by lipopolysaccharide is inhibited by dexamethasone via mRNA destabilization in human astroglial cells. J Clin Immunol 1995; 15: 199–204. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol Behav 2011; 104: 187–198. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic–pituitary–adrenal axis. Physiol Behav 2014; 124: 77–91. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor K, Watkins LR, Maier SF. The role of IL-1B in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience 2004; 127: 569–577. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF et al. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res 1999; 106: 109–118. [DOI] [PubMed] [Google Scholar]
- Blandino P Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immunity 2009; 23: 958–968. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Poulter MO, Anisman H. Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav Immunity 2011; 25: 468–482. [DOI] [PubMed] [Google Scholar]
- Kwon M-S, Seo Y-J, Lee J-K, Lee H-K, Jung J-S, Jang J-E et al. The repeated immobilization stress increases IL-1B immunoreactivities in only neuron, but not astrocyte or microglia in hippocampal CA1 region, striatum and paraventricular nucleus. Neurosci Lett 2008; 430: 258–263. [DOI] [PubMed] [Google Scholar]
- Arakawa K, Arakawa H, Hueston CM, Deak T. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology 2014; 100: 162–177. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Schwarz JM. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev 2016; 70: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson C, Aoyama M, Harry G. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immunity 2011; 25: 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber S, Oprica M, Bartfai T, Winblad B, Schultzberg M. Blunted neurogenesis and gliosis due to transgenic overexpression of human soluble IL-1ra in the mouse. Eur J Neurosci 2008; 27: 549–558. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 1999; 19: 5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LAM. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience 2008; 152: 888–902. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol 2007; 67: 1321–1333. [DOI] [PubMed] [Google Scholar]
- Perfilieva E, Risedal A, Nyberg J, Johansson BB, Eriksson PS. Gender and strain influence on neurogenesis in dentate gyrus of young rats. J Cereb Blood Flow Metab 2001; 21: 211–217. [DOI] [PubMed] [Google Scholar]
- Morale M, Gallo F, Tirolo C, Testa N, Caniglia S, Marletta N et al. Neuroendocrine-immune (NEI) circuitry from neuron-glial interactions to function: focus on gender and HPA-HPG interactions on early programming of the NEI system. Immunol Cell Biol 2001; 79: 400–417. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones Behav 1994; 28: 464–476. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol 2002; 14: 506–513. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Deak T. On the time course, generality, and regulation of plasma progesterone release in male rats by stress exposure. Endocrinology 2014; 155: 11. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LAM. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res 2003; 975: 22–36. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull 2004; 64: 303–308. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus 2006; 16: 225–232. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol 2000; 21: 95–101. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Hormones Behav 2002; 42: 327–336. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res 1992; 14: 239–249. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev 2016; 70: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck E, Lucassen P, Bakker J. Sex differences in adolescent depression: do sex hormones determine vulnerability? Journal of neuroendocrinology 2011; 23: 383–392. [DOI] [PubMed] [Google Scholar]
- Luine V. Sex steroids and cognitive function. J Neuroendocrinol 2008; 20: 866–872. [DOI] [PubMed] [Google Scholar]
- Miller DI, Halpern DF. The new science of cognitive sex differences. Trends Cogn Sci 2014; 18: 37–45. [DOI] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress 2002; 5: 205–216. [DOI] [PubMed] [Google Scholar]
- Bowman R, Ferguson D, Luine V. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience 2002; 113: 401–410. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology 1991; 16: 323–334. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5α-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology 2004; 29: 1019–1027. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Memory 2006; 86: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu LR, Germann J, Spring S, Alm C, Vousden DA, Palmert MR et al. Hippocampal volumes differ across the mouse estrous cycle, can change within 24 hours, and associate with cognitive strategies. NeuroImage 2013; 83: 593–598. [DOI] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 1990; 15: 97–111. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Teillon SM. Menstrual cycle variation in spatial ability: relation to salivary cortisol levels. Hormones Behav 2001; 39: 29–38. [DOI] [PubMed] [Google Scholar]
- Story M, Neumark-Sztainer D, French S. Individual and environmental influences on adolescent eating behaviors. J Am Diet Assoc 2002; 102: S40–S51. [DOI] [PubMed] [Google Scholar]
- Munoz KA, Krebs-Smith SM, Ballard-Barbash R, Cleveland LE. Food intakes of US children and adolescents compared with recommendations. Pediatrics 1997; 100(3 Pt 1): 323–329. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Story M, Resnick MD, Blum RW. Lessons learned about adolescent nutrition from the Minnesota adolescent health survey. J Am Diet Assoc 1998; 98: 1449–1456. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Wall M, Story M, Standish AR. Dieting and unhealthy weight control behaviors during adolescence: Associations with 10-year changes in body mass index. J Adolesc Health 2012; 50: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus D, Saint-Remy A, JeanJean M. Dietary habits during adolescence-results of the Belgian Adolux Study. Eur J Clin Nutr 2001; 55: 130–136. [DOI] [PubMed] [Google Scholar]
- Kosterman R, Hawkins JD, Guo J, Catalano RF, Abbott RD. The dynamics of alcohol and marijuana initiation: patterns and predictors of first use in adolescence. Am J Public Health 2000; 90: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry 2002; 41: 1495–1502. [DOI] [PubMed] [Google Scholar]
- Dumith SC, Gigante DP, Domingues MR, Kohl HW. Physical activity change during adolescence: a systematic review and a pooled analysis. Int J Epidemiol 2011; 40: 685–698. [DOI] [PubMed] [Google Scholar]
- Nader PR, Bradley RH, Houts RM, McRitchie SL, O'Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA 2008; 300: 295–305. [DOI] [PubMed] [Google Scholar]
- Hardy LL, Bass SL, Booth ML. Changes in sedentary behavior among adolescent girls: a 2.5-year prospective cohort study. J Adolesc Health 2007; 40: 158–165. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 2006; 13: 1385–1388. [DOI] [PubMed] [Google Scholar]
- Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012; 22: 2095–2100. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience 2006; 137: 437–445. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health 2003; 27: 197–203. [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007; 30: 464–472. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T Jr et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci 2003; 117: 1006. [DOI] [PubMed] [Google Scholar]
- Lou S-j Liu J-y, Chang H, Chen P-j. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res 2008; 1210: 48–55. [DOI] [PubMed] [Google Scholar]
- DiFeo G, Shors TJ. Mental and physical skill training increases neurogenesis via cell survival in the adolescent hippocampus. Brain Res 2017; 1654B: 95–101. [DOI] [PubMed] [Google Scholar]
- Uysal N, Tugyan K, Kayatekin BM, Acikgoz O, Bagriyanik HA, Gonenc S et al. The effects of regular aerobic exercise in adolescent period on hippocampal neuron density, apoptosis and spatial memory. Neurosci Lett 2005; 383: 241–245. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Unsain N, Mascó DH, Toscano-Silva M, de Amorim HA, Silva Araujo BH et al. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus 2012; 22: 347–358. [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res 2009; 1294: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience 2011; 194: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth S, Kubesch S, Dieterle K, Ruchsow M, Heim R, Kiefer M. Physical fitness, but not acute exercise modulates event-related potential indices for executive control in healthy adolescents. Brain Res 2009; 1269: 114–124. [DOI] [PubMed] [Google Scholar]
- Best JR. Effects of physical activity on children's executive function: contributions of experimental research on aerobic exercise. Dev Rev 2010; 30: 331–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburgh L, Königs M, Scherder EJ, Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br J Sports Med 2013; 48: 973–979. [DOI] [PubMed] [Google Scholar]
- Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav Brain Res 2012; 233: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C, Bilbao P, Contreras W, Martínez M, Mendoza A, Reyes Á et al. Effects of prenatal stress and exercise on dentate granule cells maturation and spatial memory in adolescent mice. Int J Dev Neurosci 2010; 28: 605–609. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nat Rev Neurosci 2000; 1: 191–198. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 2012; 219: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu Q-R, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Memory 2011; 18: 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci 2003; 18: 3367–3374. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Araujo BHS, Cossa AC, Scorza FA, Cavalheiro EA, MdG Naffah-Mazzacoratti et al. Physical exercise in adolescence changes CB1 cannabinoid receptor expression in the rat brain. Neurochem Int 2010; 57: 492–496. [DOI] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TTY, Gil-Mohapel J et al. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010; 20: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenderville JA, Kelly AM, Downer EJ. The role of cannabinoids in adult neurogenesis. Br J Pharmacol 2015; 172: 3950–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Berrendero F, Hernández ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci 2000; 23: 14–20. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA 2008; 105: 8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress 2011; 14: 384–397. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol 2011; 22: 536–542. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus 2005; 15: 246–253. [DOI] [PubMed] [Google Scholar]
- deAlmeida AA, Gomes da Silva S, Fernandes J, Peixinho-Pena LF, Scorza FA, Cavalheiro EA et al. Differential effects of exercise intensities in hippocampal BDNF, inflammatory cytokines and cell proliferation in rats during the postnatal brain development. Neurosci Lett 2013; 553: 1–6. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Dev Brain Res 2002; 139: 139–150. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol 2006; 27: 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci 2003; 23: 11641–11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology 2014; 76, Part C: 696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 2001; 14: 1992–2002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N et al. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem 2008; 72: 572–576. [DOI] [PubMed] [Google Scholar]
- Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics 2014; 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O'Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry 2015; 78: e7–e9. [DOI] [PubMed] [Google Scholar]
- Gareau MGMicrobiota-gut-brain axis and cognitive function. In: Lyte M, Cryan FJ (eds). Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Springer: New York, NY, 2014, pp 357–371. [Google Scholar]
- Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M et al. Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep 2016; 15: 1945–1956. [DOI] [PubMed] [Google Scholar]
- Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol 2011; 77: 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jiao J, Dulawa SC. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology 2011; 216: 207–218. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immunity 2015; 48: 165–173. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Taha AY, Tock JL, Totah NK, Cheon Y, Torres GE et al. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol Psychiatry 2014; 75: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RM, Cryan JF. Adolescent brain vulnerability and psychopathology through the generations: role of diet and dopamine. Biol Psychiatry 2014; 75: 4–6. [DOI] [PubMed] [Google Scholar]
- Astrup A, Buemann B, Western P, Toubro S, Raben A, Christensen N. Obesity as an adaptation to a high-fat diet: evidence from a cross-sectional study. Am J Clin Nutr 1994; 59: 350–355. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism 1996; 45: 1539–1546. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005; 81: 341–354. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry 2010; 167: 305–311. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 2011; 103: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995; 61: 1402S–1406S. [DOI] [PubMed] [Google Scholar]
- Féart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive function in older adults. Curr Opin Clin Nutr Metab Care 2010; 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Colacicco A, D'introno A, Capurso C, Torres F et al. Mediterranean diet and cognitive decline. Public Health Nutr 2004; 7: 959–963. [DOI] [PubMed] [Google Scholar]
- Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health. Biofactors 2013; 39: 335–342. [DOI] [PubMed] [Google Scholar]
- Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immunity 2014; 40: 9–17. [DOI] [PubMed] [Google Scholar]
- Nyaradi A, Foster JK, Hickling S, Li J, Ambrosini GL, Jacques A et al. Prospective associations between dietary patterns and cognitive performance during adolescence. J Child Psychol Psychiatry 2014; 55: 1017–1024. [DOI] [PubMed] [Google Scholar]
- Bonnet E, Touyarot K, Alfos S, Pallet V, Higueret P, Abrous DN. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS ONE 2008; 3: e3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppini R, Ciaroni S, Cecchini T, Ambrogini P, Ferri P, Cuppini C et al. Tocopherols enhance neurogenesis in dentate gyrus of adult rats. Int J Vitamin Nutr Res 2002; 72: 170–176. [DOI] [PubMed] [Google Scholar]
- Cocco S, Diaz G, Stancampiano R, Diana A, Carta M, Curreli R et al. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience 2002; 115: 475–482. [DOI] [PubMed] [Google Scholar]
- Witte A, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA 2009; 106: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol 1987; 42: 78–81. [DOI] [PubMed] [Google Scholar]
- Fontán-Lozano Á, Sáez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci 2007; 27: 10185–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 2002; 80: 539–547. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging 2004; 25: 333–340. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Dietary restriction increases hippocampal neurogenesis by molecular mechanisms independent of NMDA receptors. Neurosci Lett 2006; 393: 94–96. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang F, Yang YJ, Hu ZL, Long LH, Fu H et al. The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br J Pharmacol 2011; 162: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO, Han SM et al. (-)-epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res 2010; 24: 1065–1070. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim DH, Lee DH, Jeon SJ, Lee CH, Son KH et al. Oroxylin A, a flavonoid, stimulates adult neurogenesis in the hippocampal dentate gyrus region of mice. Neurochem Res 2010; 35: 1725–1732. [DOI] [PubMed] [Google Scholar]
- Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Effect of dietary n-3 polyunsaturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse.J Gerontol Series A 2008; 63: 1153–1160. [DOI] [PubMed] [Google Scholar]
- Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006; 139: 991–997. [DOI] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res 2010; 88: 2091–2102. [DOI] [PubMed] [Google Scholar]
- Valente T, Hidalgo J, Bolea I, Ramirez B, Anglés N, Reguant J et al. A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain. Journal of Alzheimer's disease 2009; 18: 849. [DOI] [PubMed] [Google Scholar]
- Abumaria N, Luo L, Ahn M, Liu G. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behavioural pharmacology 2013; 24: 255–263. [DOI] [PubMed] [Google Scholar]
- Levenson CW, Morris D. Zinc and neurogenesis: making new neurons from development to adulthood. Advances in Nutrition: An International Review Journal 2011; 2: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu MM, Nolan YM, Green HF, Robertson RC, Stanton C, Kelly P et al. The omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) reverses corticosterone-induced changes in cortical neurons. Int J Neuropsychopharmacol 2016; 19; doi:10.1093/ijnp/pyv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Zhang Y-Z, Yu N-J, Liu X-M, Zhao N, Yuan L et al. The total flavonoids extracted from Xiaobuxin-Tang up-regulate the decreased hippocampal neurogenesis and neurotrophic molecules expression in chronically stressed rats. Progr Neuro-Psychopharmacol Biol Psychiatry 2008; 32: 1484–1490. [DOI] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci 2008; 28: 3071–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa-Kućma M, Legutko B, Szewczyk B, Novak K, Znojek P, Poleszak E et al. Antidepressant-like activity of zinc: further behavioral and molecular evidence. J Neural Transm 2008; 115: 1621–1628. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol 2006; 197: 309–317. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 2004; 21: 1457–1467. [DOI] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn G, Roberfroid M. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J nutr 1995; 125: 1401–1412. [DOI] [PubMed] [Google Scholar]
- Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012; 61: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllan L, Skuse P, Cotter PD, O'Connor P, Cryan JF, Ross RP et al. Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice. PLoS ONE 2014; 9: e88904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology 2011; 214: 71–88. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immunity 2011; 25: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufeld K-A, Luczynski P, Seira Oriach C, Dinan TG, Cryan JF. What's bugging your teen?—The microbiota and adolescent mental health. Neurosci Biobehav Rev 2016; 70: 300–312. [DOI] [PubMed] [Google Scholar]
- McVey Neufeld K-A, Luczynski P, Dinan TG, Cryan JF. Reframing the teenage wasteland: adolescent microbiota-gut-brain axis. Can J Psychiatry 2016; 61: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13: 701–712. [DOI] [PubMed] [Google Scholar]