Abstract
Baclofen has been suggested as a potential pharmacotherapy for alcohol use disorder, but the clinical data are conflicting. Here we investigated the biobehavioral effects of baclofen in a sample of anxious alcohol-dependent individuals. This was a randomized, double-blind, placebo-controlled, human laboratory study in non-treatment seeking alcohol-dependent individuals with high trait anxiety (N=34). Participants received baclofen (30 mg per day) or placebo for at least 8 days, then performed an experimental session consisting of alcohol cue-reactivity followed by alcohol administration procedure (alcohol priming, then alcohol self-administration). Total amount of alcohol self-administered was the primary outcome; alcohol craving, subjective/physiological responses and mood/anxiety symptoms were also evaluated. There was no significant medication effect on the total amount of alcohol consumed during the alcohol self-administration (P=0.76). Baclofen blunted the positive association between maximum breath alcohol concentration during priming and the amount of alcohol consumption (significant interaction, P=0.03). Ratings of feeling intoxicated were significantly higher in the baclofen group after consuming the priming drink (P=0.006). During the self-administration session, baclofen significantly increased ratings of feeling high (P=0.01) and intoxicated (P=0.01). A significant reduction in heart rate (P<0.001) and a trend-level increase in diastolic blood pressure (P=0.06) were also detected in the baclofen group during the alcohol laboratory session. In conclusion, baclofen was shown to affect subjective and physiological responses to alcohol drinking in anxious alcohol-dependent individuals. These results do not support an anti-craving or anti-reinforcing effect of baclofen, but rather suggest that baclofen may act as a substitution medication for alcohol use disorder.
Introduction
Alcohol use disorder (AUD) is a chronic relapsing disorder with significant medical, social and economic consequences.1 Treatment of AUD may include psychosocial, behavioral and/or pharmacological interventions; however, therapeutic options are limited in number and efficacy.2 Increased understanding of the neurobiology of AUD has led to efforts focused on developing pharmacological treatments for this disorder. Prominent among these are medications that act on the GABAergic system. The GABAergic system modulates acute and chronic pharmacological and behavioral effects of alcohol and is involved in various stages of addiction.3 GABA receptors are widely expressed in brain regions involved in alcohol intake and reinforcement (for example, ventral tegmental area, amygdala, hippocampus, prefrontal cortex). In particular, the GABAB receptor has been investigated as a ‘druggable' target for AUD, using orthosteric agonists and positive allosteric modulators.4
The prototypic selective GABAB receptor agonist, baclofen, has been studied as a potential pharmacotherapy for AUD. While baclofen suppresses acquisition, maintenance and reinstatement of alcohol-seeking behavior in rodents,5, 6, 7, 8 results from human studies are conflicting. A few randomized clinical trials (RCTs) have reported that baclofen reduces alcohol drinking and craving,9, 10, 11 while others did not find positive effects on alcohol-related outcomes.12, 13 One plausible explanation for these conflicting findings may be the heterogeneity of AUD and individual variability in treatment response.2 Secondary analyses of RCTs indicate that baseline anxiety levels may predict response to baclofen treatment for AUD.14, 15 Also, in some of the previous RCTs, baclofen significantly reduced anxiety levels in alcoholic individuals.9, 12, 16 These observations are consistent with the role of the GABAB receptor in the neural circuits of anxiety17 and suggest that baclofen may be particularly efficacious in anxious alcoholic patients. However, this medication has not been tested in a study where alcoholic individuals with high anxiety levels were prospectively enrolled.
Further research is also warranted on the biobehavioral effects of baclofen to better understand how this medication works. Some of the previous RCTs indicate that baclofen may reduce naturalistic alcohol craving.9, 10 On the other hand, a pilot laboratory study suggested that baclofen may influence subjective responses to alcohol, with no effect on alcohol- or cue-induced craving.18 Given the limited number of studies, all with small sample sizes, characterizing baclofen's effects in relation to alcohol drinking is worth further investigation. Human laboratory studies conducted in well-controlled settings represent a cost-effective and ecologically valid approach to study the putative biobehavioral processes targeted by a specific medication.19, 20 The goal of this laboratory study was to investigate the biobehavioral effects of baclofen in anxious alcohol-dependent individuals.
Materials and methods
Study design and setting
This was a randomized, between-subject, double-blind, placebo-controlled, human laboratory study conducted at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD, from January 2013 to January 2016. The study protocol was approved by the NIH Addictions Institutional Review Board and registered at ClinicalTrials.gov (NCT01751386).
Participants
Eligible participants had a current diagnosis of alcohol dependence (Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID)21) and high trait anxiety (Spielberger State Trait Anxiety Inventory (STAI)22—trait version score ⩾40), and were not seeking treatment for alcohol dependence and/or anxiety. For the complete list of inclusion/exclusion criteria see Supplementary Information. Based on the previous human laboratory studies,18, 23 a Cohen's d effect size of ⩾0.5 was considered; with an 80% power and a two-tailed significance level of 0.05, a sample size of N=34 completers was calculated.
Study procedures
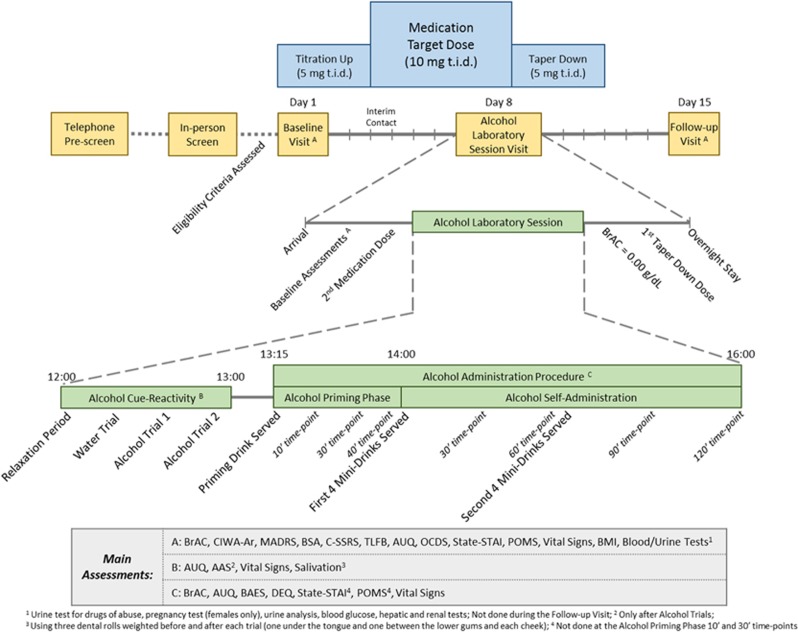
The study visits/procedures were performed in consecutive phases as outlined in Figure 1.
Figure 1.
Schematic outline of the study flow. AAS, Alcohol Attention Scale; AUQ, Alcohol Urge Questionnaire; BAES, Biphasic Alcohol Effects Scale; BMI, body mass index; BrAC, breath alcohol concentration; BSA, Brief Scale for Anxiety; CIWA-Ar, Clinical Institute Withdrawal Assessment for Alcohol-revised; C-SSRS, Columbia Suicide Severity Rating Scale; DEQ, Drug Effects Questionnaire; MADRS, Montgomery–Åsberg Depression Rating Scale; OCDS, Obsessive Compulsive Drinking Scale; POMS, Profile of Mood States; STAI, Spielberger State Trait Anxiety Inventory; t.i.d., three times a day; TLFB, Timeline Followback.
In-person screen
Baseline characteristics as well as the data needed to assess the eligibility criteria were collected.
Baseline/randomization visit
After having a verified breath alcohol concentration (BrAC)=0 g/dL, written informed consent was obtained and baseline assessments were performed (Figure 1). A personal preference log (choices of alcoholic beverage, drink mixer, television program and meals) was filled out for the next visit. Participants were randomized to receive baclofen or matched placebo (Day 1), with the initial dose of 5 mg three times a day (t.i.d.) for 3 days, followed by 10 mg t.i.d. (target dose) until the alcohol laboratory session (Supplementary Table S1). The baclofen dosage was chosen based on a number of previous studies with positive results.9, 10, 11, 18 The NIH Pharmacy Department prepared the study medications and was responsible for the randomization, allocation and blinding procedures (for details, see Supplementary Information).
Interim contact
A brief semi-structured telephone contact was performed on Day 4 (±1 day to allow flexibility) to assess the study medication adherence, any possible adverse event and/or new concomitant medication use.
Alcohol laboratory session visit
The alcohol laboratory session was scheduled on Day 8 (+1–8 days to allow flexibility), after taking the study medication at the target dose for at least 4 days. Participants were instructed to abstain from alcohol 24 h prior to this visit and take the first medication dose before coming to the clinic. Participants had to have BrAC=0 g/dL and Clinical Institute Withdrawal Assessment for Alcohol-revised (CIWA-Ar)24 score ⩽8 to start the visit. After blood sampling and a standardized breakfast, baseline assessments were performed (Figure 1). Participants took the second medication dose at 1100 hours and a standardized lunch was served (for the meal details see Supplementary Information). The alcohol laboratory session started at 1200 hours in a private bar-like laboratory room. This session included two paradigms: alcohol cue-reactivity followed by alcohol administration procedure, as previously piloted18 and described below.
Alcohol cue-reactivity
After a 3-minute relaxation period,25 the participant was exposed to visual, tactile, olfactory and proprioceptive stimuli associated with the beverage in a water trial followed by two alcohol trials. For the water trial, a tray containing a glass half full of water and a commercially-labeled bottle of water was placed on a table in front of the participant; these were replaced with the participant's preferred alcoholic beverage for the alcohol trials. The bottles were opened and the beverages were poured in front of the participant. During each 3-minute trial, participants were instructed via an audiotape to sniff the beverage upon hearing high tones and stop sniffing upon hearing low tones. After each trial, alcohol craving was assessed using Alcohol Urge Questionnaire (AUQ),26 which was accompanied by Alcohol Attention Scale (AAS)27 after the alcohol trials. Cue-induced physiological changes, including blood pressure (BP), heart rate (HR) and salivation were also measured.
Alcohol administration procedure
Participants' preferred beverage23 (alcohol and mixer) and television program were provided during this session, which consisted of two consecutive phases:
A. Alcohol priming phase: a priming drink was served and participants were instructed to consume it within 5 minutes. The grams of alcohol for this drink was calculated based on total body water, designed to raise blood alcohol concentration to 0.03 g/dL.28 At 10-, 30- and 40-minute time-points, BrAC and vital signs were taken and participants rated their alcohol craving (AUQ) and subjective responses to alcohol via the Biphasic Alcohol Effects Scale (BAES)29 and a modified version of the Drug Effects Questionnaire (DEQ).30, 31 Mood and anxiety symptoms were also assessed at the end, using the Profile of Mood States (POMS)32 and the state-STAI,22 respectively.
B. Alcohol self-administration: 40 minutes after consuming the priming drink, a sign stating ‘The Bar is Open' was displayed and a tray containing four mini-drinks was offered. Each mini-drink was half of the priming drink, designed to raise the blood alcohol concentration by 0.015 g/dL. Sixty minutes later, the first tray was removed and another tray of four mini-drinks was presented. The participant could choose to drink any or all glasses (0–8 mini-drinks); $3.00 was provided as an alternative reinforcer for each mini-drink not consumed. Every 30 minutes, BrAC and vital signs were taken and participants rated their alcohol craving (AUQ), subjective responses to alcohol (BAES, DEQ), mood (POMS) and anxiety (state-STAI).
After the alcohol laboratory session, participants were escorted to an inpatient unit, where they had dinner and were monitored until BrAC reached 0 g/dL. Participants were discharged the following morning.
Follow-up visit
One week after the alcohol laboratory session (−2/+10 days to allow flexibility), participants came back for a brief outpatient visit. In addition to the follow-up assessments, a counseling session was delivered during which the participant was informed of the diagnosis of alcohol dependence and recommendations to stop or at least reduce drinking were made by a health care provider.33
Outcomes
The primary outcome of this study was the total amount of alcohol consumed during the alcohol self-administration (ASA) session. Secondary outcomes included: (A) subjective and physiological responses to alcohol cues during the cue-reactivity session; and (B) subjective and physiological responses to alcohol drinking, and mood/anxiety during the alcohol administration procedure.
Statistical analysis
All data were examined for normal distribution using the Kolmogorov–Smirnov test; if not normally distributed, a square root transformation was applied. The study outcomes were analyzed using general linear mixed models in the PROC MIXED procedure (Statistical Analysis System (SAS) software, version 9.3, SAS Institute, Cary, NC, USA), with the medication group (baclofen/placebo) as the between-subjects factor and, if appropriate, time-points as the within-subjects factor. Post-hoc comparisons were performed using Tukey's honestly significant difference test. As recommended, the Kenward–Roger adjustment for denominator degrees of freedom was used in repeated measures analyses of covariance (ANCOVA).34, 35 Potential covariates were evaluated in the initial run of each model, including: age, gender, race, years of education, body mass index, smoking status (yes/no), average drinks per drinking days (based on alcohol Timeline Followback 90 days prior to the in-person screening visit), Alcohol Dependence Scale score, Self-Rating of Effects of Alcohol score, and family history density of problem drinking; covariates that were not at least at a trend level (P⩾0.10) were removed from the model analysis. The obsessive subscale score of the Obsessive Compulsive Drinking Scale was added as a covariate in all analyses due to a significant difference between the study groups at baseline (Table 1). The baseline value of each assessment (if collected) and the maximum BrAC (max-BrAC) during the priming phase were added as covariates to the alcohol administration procedure analyses. Body mass index was included as a covariate in the analysis of alcohol consumption (primary outcome); ASA analyses for secondary outcomes were also controlled for the number of drinks consumed during the first 30 minutes. Independent samples t-test and χ2-test were used to analyze the baseline characteristics, adverse events and medication adherence/guess data. Significance level was set at P<0.05 (two-tailed) for all analyses.
Table 1. Demographic characteristics and baseline data of the study sample.
| Variable | Baclofen (n=18) | Placebo (n=16) | X2 | t | P |
|---|---|---|---|---|---|
| Age, years,M(s.d.) | 46.4 (8.9) | 42.1 (10.8) | 1.30 | 0.20 | |
| Gender, males, n (%) | 14 (77.8) | 13 (81.3) | 0.06 | 0.80 | |
| Race, African Americans, n(%) | 11 (61.1) | 12 (75.0) | 0.93 | 0.63 | |
| Education, years,M(s.d.) | 12.4 (3.7) | 13.2 (3.4) | −0.62 | 0.54 | |
| BMI, kg/m2, M (s.d.) | 31.1 (8.5) | 28 (4.1) | 1.39 | 0.18 | |
| Cigarette smokers, n (%) | 12 (66.7) | 7 (43.8) | 1.80 | 0.18 | |
| FTND score, M(s.d.) | 3.5 (3.4) | 2.7 (1.7) | −0.61 | 0.54 | |
| Age of onset of alcohol dependencea, years,M(s.d.) | 30.6 (11.3) | 28.0 (10.3) | 0.68 | 0.50 | |
| Average drinks per drinking daysb, M(s.d.) | 8.6 (4.8) | 9.1 (6.5) | −0.26 | 0.80 | |
| Number of heavy drinking daysb,c, M (s.d.) | 56.3 (32.5) | 51.73 (25.02) | 0.45 | 0.64 | |
| Family history density of problem drinkingd, M(s.d.) | 0.1 (0.1) | 0.2 (0.2) | −1.13 | 0.27 | |
| ADS score, M(s.d.) | 12.5 (5.6) | 11.7 (4.7) | 0.46 | 0.65 | |
| SRE score, M(s.d.) | 10.6 (7.1) | 7.5 (2.5) | 1.72 | 0.10 | |
| AUQ score, M(s.d.) | 26.5 (12.4) | 28.1 (10.6) | −0.41 | 0.69 | |
| OCDS score,M (s.d.) | |||||
| Obsessive | 4.4 (3.0) | 6.8 (3.5) | −2.10 | 0.04 | |
| Compulsive | 9.3 (3.3) | 9.5 (2.4) | −0.17 | 0.87 | |
| Total | 13.8 (5.5) | 16.3 (5.2) | −1.34 | 0.19 | |
| MADRS score, M(s.d.) | 4.7 (7.5) | 4.3 (4.0) | 0.19 | 0.85 | |
| BSA score, M(s.d.) | 4.5 (5.2) | 3.6 (3.3) | 0.59 | 0.56 | |
| Trait-STAI score, M(s.d.) | 46.0 (5.9) | 48.8 (6.9) | −1.28 | 0.21 | |
| Mood disordersa,n(%) | |||||
| Current | 1 (5.6) | 0 (0) | 0.92 | 0.34 | |
| Lifetime | 2 (11.1) | 0 (0) | 1.89 | 0.17 | |
| Anxiety disordersa,n(%) | |||||
| Current | 4 (22.2) | 1 (6.3) | 1.72 | 0.19 | |
| Lifetime | 5 (27.8) | 1 (6.3) | 2.70 | 0.10 | |
| Substance use disordersa,e,n(%) | |||||
| Current | 1 (5.6)f | 1 (6.3)g | 0.01 | 0.93 | |
| Lifetime | 12 (66.7) | 8 (50.0) | 0.97 | 0.32 | |
Abbreviations: ADS, Alcohol Dependence Scale; AUQ, Alcohol Urge Questionnaire; BMI, body mass index; BSA, Brief Scale for Anxiety; FTND, Fagerström Test for Nicotine Dependence; M, mean; MADRS, Montgomery–Åsberg Depression Rating Scale; OCDS, Obsessive Compulsive Drinking Scale; SCID, Structured Clinical Interview for DSM-IV-TR Axis I Disorders; s.d., standard deviation; SRE, Self-Rating of Effects of Alcohol; STAI, Spielberger State Trait Anxiety Inventory; TLFB, Timeline Followback.
Based on SCID.
Based on alcohol TLFB 90 days prior to the in-person screening visit. Note: paired samples t-test showed a significant decrease in self-reported drinking measures of the whole sample from the in-person screening visit to the baseline/randomization visit based on TLFB 30 days prior to each visit (total drinks: t=2.50, P=0.02).
Heavy drinking day: ⩾4 and 5 drinks on a day for females and males, respectively.
Based on Family Tree Questionnaire: density of relatives (siblings, parents, grandparents) with definite problem drinking (self-reported).
Including substance abuse and/or dependence, other than alcohol and nicotine.
Current cannabis abuse.
Current cocaine abuse. Significant (<0.05) P values are shown in bold.
Results
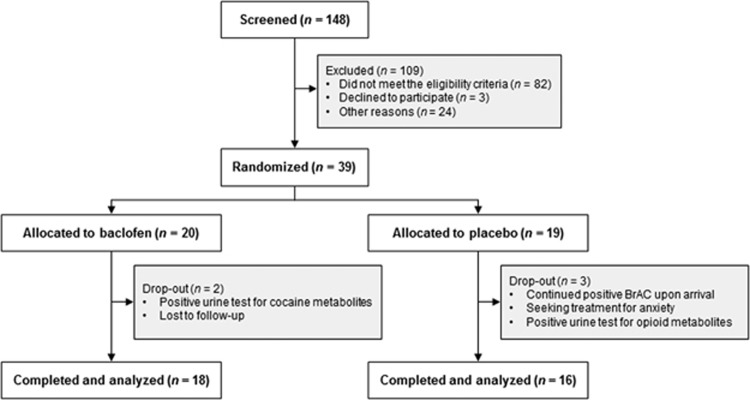
Figure 2 and Table 1 outline the flow diagram and baseline characteristics of the enrolled sample, respectively.
Figure 2.
Flow diagram of the study. BrAC, breath alcohol concentration.
No significant changes were found during the naturalistic phase of the study, that is, between the baseline/randomization visit (pre-medication) and the alcohol laboratory session visit (post medication but pre-laboratory session; Supplementary Table S2).
Alcohol cue-reactivity
No significant medication or time × medication interaction effects were found on the outcomes assessed, that is, AUQ, AAS, BP, HR and salivation (P's>0.05; details in Supplementary Table S3), except for a significant time × medication interaction (F1,81.9=4.08, P=0.009) showing higher diastolic BP after the second alcohol trial compared to the relaxation period in the placebo group.
Alcohol administration procedure
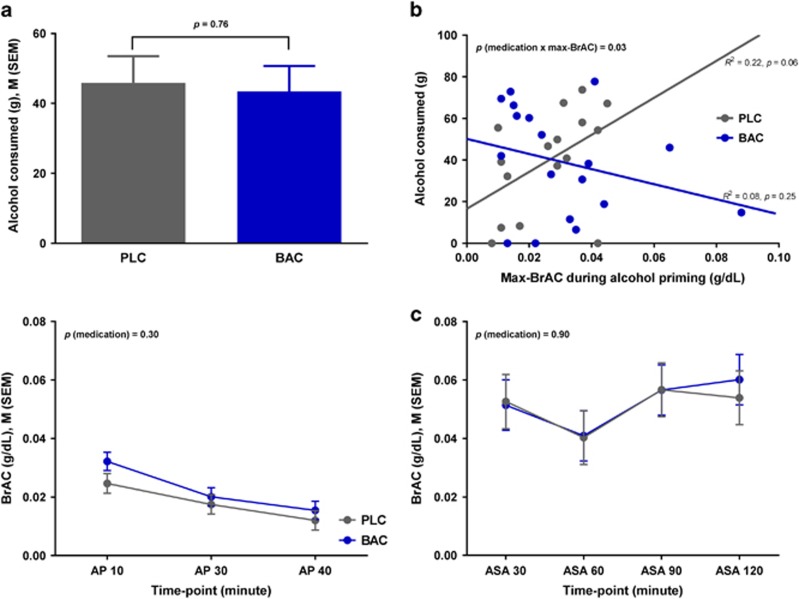
There was no significant effect of the medication group on the total amount (grams) of alcohol consumed during the ASA (mean±s.e.m.: 43.43±7.28 (baclofen) versus 45.90±7.62 (placebo); F1,26=0.09, P=0.76; Figure 3a), (number of mini-drinks consumed, mean±s.e.m.: 4.11±0.66 (baclofen) versus 4.50±0.72 (placebo); Supplementary Figure S1). However, there was a significant medication × max-BrAC interaction effect (F1,25=5.22, P=0.03); the positive association—observed in the placebo group—between max-BrAC during the priming phase and the amount of alcohol self-administered was absent in the baclofen group (Figure 3b). There was no significant medication effect on the BrAC measurements (P's>0.05) (Figure 3c).
Figure 3.
(a) Total amount of alcohol consumed during alcohol self-administration; (b) medication × max-BrAC interaction effect on the total amount of alcohol consumed during alcohol self-administration. Note: statistical analysis showed no outliers; the results remained significant after excluding the two subjects with high BrAC (far right); (c) breath alcohol concentrations during the priming (left) and ASA (right) phases. AP, alcohol priming; ASA, alcohol self-administration; BAC, baclofen; BrAC, breath alcohol concentration; M, mean; Max, maximum; PLC, placebo; SEM, standard error of the mean.
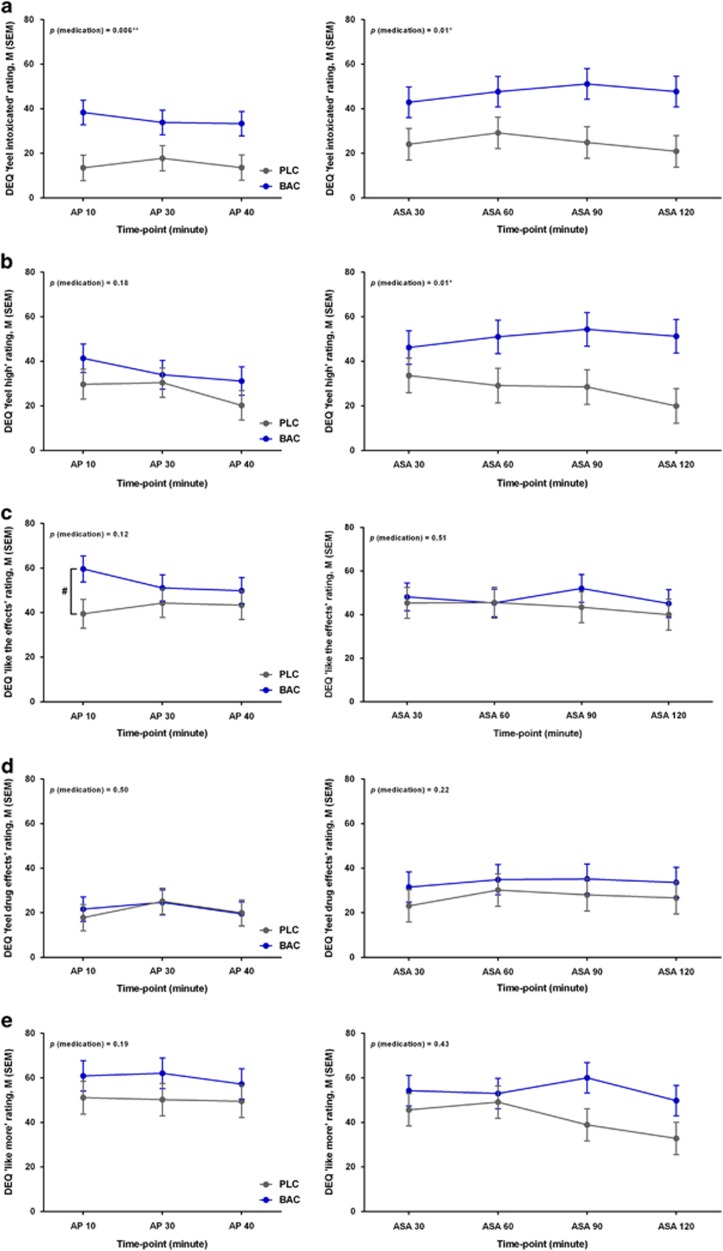
As for the subjective response to alcohol measured by DEQ (Figure 4), there were significant medication effects for ‘feeling intoxicated' during both alcohol priming (F1,27.4=8.68, P=0.006) and ASA (F1,27.4=7.05, P=0.01) phases (Figure 4a), as well as ‘feeling high' during the ASA (F1,29.8=4.48, P=0.01) (Figure 4b) with higher scores in the baclofen group. A significant time × medication interaction effect was also detected for ‘liking the effects' during the alcohol priming phase (F2, 62.2=5.78, P=0.005) (Figure 4c). No significant medication or time × medication interaction effects were found for the other DEQ questions, AUQ, BAES, POMS or state-STAI (P's>0.05; details in Supplementary Table S3).
Figure 4.
DEQ ratings during the alcohol priming (left) and ASA (right): (a) ‘Do you feel intoxicated?' (significant medication effect); (b) ‘Do you feel high?' (significant medication effect); (c) ‘Do you like the effects you are feeling now?' (significant time × medication interaction effect, #P=0.09 (post-hoc analysis)); (d) ‘Do you feel any drug effects?' (e) ‘Would you like more of what you received, right now?' *P<0.05, **P<0.01. AP, alcohol priming; ASA, alcohol self-administration; BAC, baclofen; BrAC, breath alcohol concentration; DEQ, Drug Effects Questionnaire; M, mean; PLC, placebo; SEM, standard error of the mean.
As for the physiological data, baclofen-treated individuals had significantly lower heart rate during both alcohol priming (F1,30.5=9.45, P=0.004) and ASA (F1,32.5=17, P<0.001) phases. Trend-level medication effects were also detected on mean arterial pressure (F1,26.8=4.12, P=0.05) and diastolic BP (F1,27.1=3.56, P=0.06) during the alcohol priming, that is, BP was higher in the baclofen group compared to the placebo group (Supplementary Table S3).
Information on comparative analyses between the alcohol laboratory session visit and the follow-up visit (Supplementary Table S4), safety outcomes (Supplementary Tables S5 and S6) and medication adherence/guess are summarized in the Supplementary Information.
Discussion
The main results of this study indicate that baclofen affects subjective responses to oral alcohol consumption. Baclofen-treated participants reported greater ratings of feeling high and intoxicated after alcohol intake. Notably, there was no medication effect on BrAC during the alcohol administration procedure, ruling out the possibility that the observed subjective effects might be due to changes in alcohol pharmacokinetics. Although baclofen did not reduce the amount of alcohol consumed in this study, it did blunt the positive association between blood alcohol levels during the priming phase and subsequent alcohol consumption during the ASA. These findings suggest a potential biobehavioral mechanism of how baclofen may affect alcohol intake. Hypothetically, amplification of subjective responses to alcohol after an initial drink may reduce the amount of subsequent alcohol drinking as the desired effects have been already achieved. This is in agreement with the previous preclinical reports on the efficacy of baclofen to prevent priming-induced reinstatement of drug-seeking behavior;36, 37 however, this hypothesis requires further investigation in the alcohol field7, 38 and does not explain how baclofen may promote abstinence, as shown in previous studies.9, 10
Contrary to some of the previous RCTs,9, 10, 11 baclofen did not reduce the amount of alcohol consumed in this study, for which different reasons may be hypothesized. First, the whole sample reported a significant reduction in alcohol drinking during the period between screening and randomization, that is, before the study medication was started. This potential floor effect may have obscured a difference between the two groups during the ASA session. Second, the sample of this study differed in important ways from patients enrolled in previous treatment trials where baclofen reduced alcohol drinking. Our participants were not seeking treatment and had less severe dependence, mild or no alcohol withdrawal symptoms, and no significant liver impairment. Noteworthy, the severity of alcohol dependence and withdrawal have been identified as possible predictors of better response to baclofen;14 also, baclofen seems to be particularly effective in alcohol-dependent patients with liver cirrhosis.10 Third, the ASA paradigm applies a behavioral economic approach where alcohol is considered a commodity whose reinforcing value is estimated by the cost that a consumer is willing to pay for. This approach evaluates a specific aspect of alcohol-related behavior, and may not capture other factors that contribute to excessive drinking behavior in alcohol-dependent individuals.
The central finding of this study, that is, baclofen affects alcohol-related subjective experiences, is of potential importance. Subjective response to alcohol plays an important role in shaping the pattern of alcohol drinking.39, 40 This is a multidimensional construct; an exploratory factor analysis by Ray and colleagues revealed a three-factor model for subjective responses to alcohol: (1) stimulation and other pleasant effects; (2) sedation and other unpleasant effects; (3) alleviation of tension and negative mood;41 an additional factor (craving/motivation) was also identified in another study.42 The present study applied a diverse set of assessments trying to investigate different dimensions of subjective response to alcohol. Several factors such as genetics, level of drinking and stage of alcoholism contribute to the individual variability in response to alcohol;43 in the present study, we controlled for this heterogeneity by adding the baseline Self-Rating of Effects of Alcohol score44 as a covariate in the initial run of each model. Notably, this variable was not found to be a significant covariate in any of the analyses.
Subjective response to alcohol is a potential biobehavioral target and a marker of early efficacy in medication development for AUD.45, 46 While decreasing the pleasant effects of alcohol and/or increasing its unpleasant effects have typically been considered the main mechanisms for reducing alcohol drinking, this dichotomous classification is too simplistic and unable to fully explain the effects of some medications. For example, responses like feeling ‘intoxicated' do not fall in either category. Also, the desired direction of change in responses like feeling ‘high' may not be clear-cut from a medication development perspective. In fact, the amplification of subjective responses to alcohol observed in this study is in line with a previously proposed notion that baclofen might act through alcohol mimicking properties, representing a substitution or complementarity therapy for alcohol use.47 Importantly, GABAB receptors, especially at the presynaptic sites, play a central role in responsiveness to alcohol by regulating the synaptic ethanol sensitivity on one hand,48 and modulating the release and effects of other key neurotransmitters in the brain on the other hand.49
Some of the previous treatment trials indicate that baclofen reduces alcohol craving.9, 10 In the present study, we did not find a medication effect on cue- or alcohol-induced craving. These conflicting findings may be due to differences in (1) methodologies: cue- and alcohol-induced craving assessed in real time versus non-elicited craving assessed retrospectively, (2) settings: experimental laboratory versus naturalistic environment, (3) populations: non-treatment versus treatment seekers. It is also important to note that the present study did not investigate all potential factors that may elicit craving for alcohol;19 for example, a stress manipulation procedure per se was not employed. While previous RCTs with baclofen in alcohol-dependent individuals suggest an anxiolytic effect,9, 12, 16 we did not find significant changes in anxiety, neither pre- to post randomization, nor during the alcohol laboratory session.
In spite of important methodological differences with the present study, previous human laboratory studies have also shown an effect of baclofen on subjective responses to alcohol, and no effect on cue- or alcohol-induced craving.18, 50 Specifically, one study tested acute effects of single doses of baclofen (0, 40 or 80 mg) administered before an alcoholic (0.75 g/kg) or placebo beverage in 18 non-dependent heavy drinkers and found increased ratings of ‘high' when baclofen and alcohol were co-administered. Baclofen alone dose-dependently increased ratings of ‘elevated mood', ‘drug strength', ‘good drug effects' and ‘bad drug effects' as well. There was also a trend-level increase in sedation when baclofen was combined with alcohol. Finally, no significant change in alcohol-induced craving was found in this study.50 Another pilot study was conducted in 14 alcohol-dependent heavy drinkers and used the same design and dosage as the present work. During the alcohol administration procedure, the baclofen group reported higher stimulation and sedation on the BAES. Neither cue- nor alcohol-induced craving showed a significant medication effect; no other measurements of subjective response to alcohol were performed.18
The present study did not find increased sedation with baclofen. In fact, lower rates of ‘sleepiness' were reported in the baclofen than the placebo group. Given the well-known sedative effects of baclofen, these findings are unexpected. A possible reason may be related to the specific population enrolled in this study. It is conceivable that alcohol-dependent individuals, particularly those with high anxiety, may have enhanced tolerance to the sedative effects of baclofen, mainly due to a possible cross-tolerance with the sedative effects of alcohol. This conclusion, albeit not driven directly from our findings, is consistent with the previous rodent experiments51 and clinical observations.52 While enhancement of subjective responses to alcohol might raise a concern about abuse liability of baclofen, this is unlikely. Importantly, baclofen did not affect the DEQ items that are more indicative of abuse liability (‘Do you like the effects you are feeling now?' and ‘Would you like more of what you received, right now?'). Moreover, baclofen has been used in several clinical trials for longer periods of time in populations addicted to different drugs of abuse, and none of these studies have reported cases of baclofen abuse.13, 53, 54
Individuals randomized to baclofen in this study had higher HR and lower BP than the placebo group at baseline (pre-medication). This pattern persisted after receiving baclofen/placebo for 1 week, but was reversed during the alcohol administration procedure: baclofen, combined with alcohol, decreased HR and increased diastolic BP and mean arterial pressure. These findings are inconsistent with Evans and Bisaga's study, where they found a significant increase in HR with both baclofen alone and in combination with alcohol, as well as a small increase in systolic BP with baclofen alone.50 Previous studies with baclofen have reported different and contrasting hemodynamic effects (for example, hypo/hypertension, brady/tachycardia), and the magnitude/direction of these effects remain unclear.55, 56 Sophisticated pharmacological and mathematical models57 are required to disentangle central versus peripheral and direct versus feedback mechanisms involved in the hemodynamic responses to baclofen itself and in combination with alcohol, a set of questions that is beyond the scope of this study.
This study should be seen in light of its strengths and limitations. To our knowledge, this is the first study investigating the biobehavioral effects of baclofen in anxious alcohol-dependent individuals and after the medication has been taken for a reasonable period of time to reach steady state. Combining the alcohol cue-reactivity, fixed-dose priming, and free-choice self-administration procedures in a well-controlled laboratory setting and using a comprehensive set of assessments/time-points provided a strong platform to study the potential effects of baclofen on several alcohol-related outcomes. Study limitations include: (1) only one dose of baclofen (30 mg per day) and one dose of alcohol were tested, and the findings cannot be generalized to other scenarios. Higher doses of baclofen and/or longer period of administration could be tested in future studies in combination with different doses of alcohol; (2) although oral administration of alcohol represents the natural route alcohol is consumed, it also results in high variability in blood alcohol concentrations,58 which may subsequently affect individuals' response to alcohol. We controlled for this variability by covarying for the max-BrAC of the priming phase in the analyses of the alcohol administration procedure; (3) due to safety reasons, the maximum number of mini-drinks was limited to eight and the session could not be continued if individuals wanted to drink more; (4) given the small number of females, possible sex differences in the effects of baclofen could not be investigated; nevertheless, gender was included among the covariates of our analyses; (5) the sample size of this study was relatively small; we had neither a less anxious control group, nor a non-alcoholic control drink. A logical follow-up would be a larger experiment with a fully factorial 2 (baclofen/placebo) × 2 (alcohol/placebo) × 2 (high/low anxiety) design.
In conclusion, the present human laboratory study showed that the GABAB receptor agonist baclofen affects subjective and physiological responses to alcohol drinking in anxious alcohol-dependent individuals. Future studies are needed to further investigate these biobehavioral effects and their potential implications in using baclofen as a medication for AUD.
Acknowledgments
We thank the clinical and research staff involved in data collection and support at the NIH Intramural Research Program, in particular staff in the joint NIAAA/NIDA CPN section, NIAAA clinical program of the Division of Intramural Clinical and Biological Research and NIH Clinical Center (Nursing, Nutrition and Pharmacy Departments). We also thank the NIH Fellows Editorial Board for editorial assistance, and Ms Karen Smith from the NIH Library for bibliographic assistance. Finally, we express our gratitude to the participants who took part in this study. This work was supported by: (1) NIH intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: LL), jointly supported by the NIAAA Division of Intramural Clinical and Biological Research and the NIDA Intramural Research Program; and (2) Brain and Behavior Research Foundation (BBRF; formerly NARSAD) grant number 17325 (PI: LL).
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015; 72: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Wilford BB, Falk DE, Ryan ML, Fertig JB. Potential medications for the treatment of alcohol use disorder: an evaluation of clinical efficacy and safety. Subst Abus 2016; 37: 286–298. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav 2013; 113: 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Reed C. Targeting GABAB receptors for anti-abuse drug discovery. Expert Opin Drug Discov 2014; 9: 1307–1317. [DOI] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S et al. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res 2004; 6: 403–414. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 2007; 31: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Bienkowski P, Carai MA, Gessa GL, Colombo G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend 2008; 95: 284–287. [DOI] [PubMed] [Google Scholar]
- Williams KL, Nickel MM, Bielak JT. Baclofen blocks yohimbine-induced increases in ethanol-reinforced responding in rats. Pharmacol Biochem Behav 2016; 144: 20–25. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol 2002; 37: 504–508. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet 2007; 370: 1915–1922. [DOI] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Edwards SM, Tidey JW, Swift RM, Kenna GA. A preliminary double-blind, placebo-controlled randomized study of baclofen effects in alcoholic smokers. Psychopharmacology 2015; 232: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 2010; 34: 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponizovsky AM, Rosca P, Aronovich E, Weizman A, Grinshpoon A. Baclofen as add-on to standard psychosocial treatment for alcohol dependence: a randomized, double-blind, placebo-controlled trial with 1 year follow-up. J Subst Abuse Treat 2015; 52: 24–30. [DOI] [PubMed] [Google Scholar]
- Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets 2010; 9: 33–44. [DOI] [PubMed] [Google Scholar]
- Morley KC, Baillie A, Leung S, Addolorato G, Leggio L, Haber PS. Baclofen for the treatment of alcohol dependence and possible role of comorbid anxiety. Alcohol Alcohol 2014; 49: 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, Krandashova GF, Lapin IP, Grinenko A et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug Alcohol Depend 1993; 33: 157–163. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 2005; 26: 36–43. [DOI] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR et al. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav 2013; 103: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M et al. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res 2012; 36: 972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Design 2010; 16: 2149–2158. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute: New York, 2002. [Google Scholar]
- Spielberg CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State and Trait Anxiety Inventory. Consulting Psychologist Press: Paolo Alto, CA, 1983. [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology 2002; 160: 19–29. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84: 1353–1357. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM et al. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res 1999; 23: 1386–1394. [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 1995; 19: 600–606. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol 1994; 62: 620–626. [DOI] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: updating the fundamentals. In: Crow K, Barr R (eds). Human Metabolism of Alcohol: Pharmcokinetics, Medicolegal Aspects, and General Interest, vol. 1. CRC Press: Boca Raton, FL, USA, 1989, pp 41–58.
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 1993; 17: 140–146. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) "Attitude" of opiate addicts toward opiate-like drugs. (B) a short-term "direct" addiction test. J Pharmacol Exp Therap 1961; 133: 371–387. [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci 2008; 28: 4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Services: San Diego, CA, 1971. [Google Scholar]
- National Institute on Alcohol Abuse and AlcoholismHelping Patients Who Drink Too Much: A Clinician's Guide. US Departmenet of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2005. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2nd Edition, SAS Institute Inc: Cary, NC, USA, 2006.
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997; 53: 983–997. [PubMed] [Google Scholar]
- Spano MS, Fattore L, Fratta W, Fadda P. The GABAB receptor agonist baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology 2007; 52: 1555–1562. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P. Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol 2009; 19: 487–498. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology 1998; 135: 169–174. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 1994; 151: 184–189. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res 2010; 34: 385–395. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res 2009; 33: 2154–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Roche DJ, Ray LA. Factor structure of subjective responses to alcohol in light and heavy drinkers. Alcohol Clin Exp Res 2015; 39: 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Substance Use Misuse 2010; 45: 1742–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol 1997; 58: 397–404. [DOI] [PubMed] [Google Scholar]
- Yardley MM, Ray LA. Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol 2016. (e-pub ahead of print;doi: 10.1111/adb.12349). [DOI] [PMC free article] [PubMed]
- Ray LA, Bujarski S, Roche DJ. Subjective response to alcohol as a research domain criterion. Alcohol Clin Exp Res 2016; 40: 6–17. [DOI] [PubMed] [Google Scholar]
- Chick J, Nutt DJ. Substitution therapy for alcoholism: time for a reappraisal? J Psychopharmacol 2012; 26: 205–212. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 2004; 24: 10679–10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 1995; 46: 423–462. [DOI] [PubMed] [Google Scholar]
- Evans SM, Bisaga A. Acute interaction of baclofen in combination with alcohol in heavy social drinkers. Alcohol Clin Exp Res 2009; 33: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology 2004; 174: 358–366. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, Caputo F, Gasbarrini G. Tolerance to baclofen's sedative effect in alcohol-addicted patients: no dissipation after a period of abstinence. Psychopharmacology 2005; 178: 351–352. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend 2009; 103: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R, Biswas K, Childress AR, Shoptaw S, Fudala PJ, Gorgon L et al. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend 2009; 103: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahl LA, Walker SB. The effect of baclofen on the cardiovascular system of the rat. Br J Pharmacol 1980; 69: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung NY, Whyte IM, Isbister GK. Baclofen overdose: defining the spectrum of toxicity. Emerg Med Australas 2006; 18: 77–82. [DOI] [PubMed] [Google Scholar]
- Kamendi H, Barthlow H, Lengel D, Beaudoin ME, Snow D, Mettetal JT et al. Quantitative PK/PD modeling of baclofen-mediated cardiovascular effects using blood pressure and heart rate in rats. Br J Pharmacol 2016; 173: 2845–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford NH. Clinical pharmacokinetics of ethanol. Clin Pharmacokinet 1987; 13: 273–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.