Abstract
Hyperbilirubinemia, caused by the accumulation of unconjugated bilirubin, is one of the most common clinical diagnoses in both premature and term newborns. Owing to the fact that bilirubin is metabolized solely through glucuronidation by UDP-glucuronosyltransferase (UGT) 1A1, it is now known that immaturity of UGT1A1, in combination with the overproduction of bilirubin during the developmental stage, acts as a bottleneck to bilirubin elimination and predisposes the infant to high total serum bilirubin levels. Although neonatal jaundice is mostly benign, excessively high levels of serum bilirubin in a small percentage of newborns can cause bilirubin-induced neurologic dysfunction, potentially leading to permanent brain damage, a condition known as kernicterus. Although a large portion of hyperbilirubinemia cases in newborns are associated with hemolytic diseases, we emphasize here the impaired ability of UGT1A1 to eliminate bilirubin that contributes to hyperbilirubinemia-induced neurotoxicity in the developmental stage. As a series of hereditary UGT1A1 mutations have been identified that are associated with UGT1A1 deficiency, new evidence has verified that delayed expression of UGT1A1 during the early stages of neonatal development is a tightly controlled event involving coordinated intrahepatic and extrahepatic regulation. This review recapitulates the progress that has been made in recent years in understanding the causes and physiopathology of severe hyperbilirubinemia, investigating molecular mechanisms underlying bilirubin-induced encephalopathy, and searching for potential therapies for treating pathologic hyperbilirubinemia. Several animal models have been developed to make it possible to examine bilirubin-induced neurotoxicity from multiple directions. Moreover, environmental factors that may alleviate or worsen the condition of hyperbilirubinemia are discussed.
Introduction
Newborn hyperbilirubinemia, characterized by elevated levels of total serum bilirubin (TSB) manifesting as jaundice, is one of the most common clinical diagnoses in neonates, especially in preterm infants. Hyperbilirubinemia in neonates is caused by elevated levels of bilirubin resulting from increased erythrocyte turnover immediately after birth and a delayed expression of hepatic UDP-glucuronosyltransferase 1A1 (UGT1A1) (Fig. 1), the sole enzyme responsible for bilirubin elimination through glucuronidation conjugation (Burchell et al., 1989; Fujiwara et al., 2015). More than 60% of otherwise healthy newborns develop hyperbilirubinemia during the first week of life, with most experiencing temporary, physiologic jaundice that has benign outcomes. A small portion of neonates, however, suffer dangerously high levels of unconjugated bilirubin (UCB) that are associated with bilirubin-induced neurologic dysfunction (BIND) (Bhutani and Johnson-Hamerman, 2015). For example, newborns with Crigler-Najjar syndrome type 1 (CNS1) inheriting a severe form of UGT1A1 deficiency manifest with critical nonhemolytic icterus within the first few days of life. Phototherapy is the most common treatment to reduce bilirubin levels in these infants; otherwise, they may succumb to kernicterus, an irreversible condition with an anatomic characteristic of yellow staining in certain regions of the brain, particularly the basal ganglia, hippocampus, cerebellum, and nuclei of the floor of the fourth ventricle (Shapiro et al., 2006; Bhutani and Johnson-Hamerman, 2015), exhibiting encephalopathy with symptoms of lethargy, ocular muscle paralysis, high-pitched crying, dystonia, seizures, mental retardation, and even death (Crigler and Najjar, 1952; Bhutani et al., 2013). To date, liver transplantation is deemed the only long-lasting therapeutic alternative to cure severe forms of hyperbilirubinemia, including CNS1, before the occurrence of neurologic damage (Schauer et al., 2003).
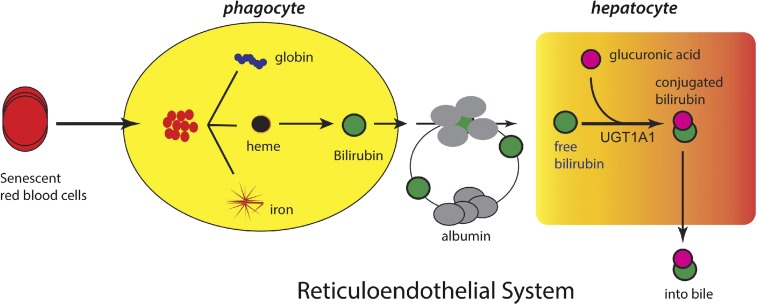
Fig. 1.
Release of bilirubin into blood and transport to the liver. The rapid increase in oxygen after birth stimulates red blood cell production and senescence, resulting in the release of heme from hemoglobin by the reticuloendothelial system. Heme undergoes metabolism by heme oxygenase and biliverdin reductase, resulting in the production of bilirubin, which is released into the blood and bound to serum proteins. After its uptake into the liver, bilirubin undergoes glucuronidation by UGT1A1, located in the endoplasmic reticulum. Bilirubin-glucuronide exits the hepatocyte where it works its way through the biliary canaliculi into the lumen of the intestines.
In preterm infants less than 30 weeks of gestational age, the incidence of kernicterus is about 1.8/1000 births (Morioka et al., 2015), and it is estimated that the current risk of chronic kernicterus is about one in seven infants with TSB levels >30 mg/dl (513 µM) (Bhutani and Johnson, 2009). In many parts of the world, however, especially in low-income countries, these ratios would underestimate the incidence of kernicterus because of major risk factors that induce hemolysis, such as rhesus disease and glucose-6-phosphate dehydrogenase deficiency. South Asia and sub-Saharan Africa have the highest incidence, with an incidence estimated at 3.9/1000 live births (Chime et al., 2011; Bhutani et al., 2013), and recent global estimates found the prevalence of extreme hyperbilirubinemia (>25 mg/dl) to be 4%, 32%, and 39% in Latin America, sub-Saharan Africa, and South Asia, respectively. At a country level, Nigeria was reported to have hyperbilirubinemia cases accounting for more than 35% of all hospital admissions, with 9% of newborns having developed kernicterus (Chime et al., 2011). The inability to clear bilirubin in cases of rapid accumulation in early phases of the neonatal window results from developmental delay or repression in expression of UGT1A1. We predict that improved understanding of the mechanisms behind this delay would become useful as a target toward developing treatment approaches that would accelerate bilirubin clearance and eliminate the prospects of neurologic toxicity.
We document in this review that the UGT1A1 activity is influenced by genetic polymorphism and is regulated at the transcriptional level through a number of mechanisms during the developmental stage; in addition to genetic elements of the UGT1A1 gene, we focused on the role of environmental factors in modulating UGT1A1 bilirubin conjugation capacity. Emphasis is also placed on recent data obtained from novel animal models that delineate cellular and molecular events occurring in the brain in response to bilirubin neurotoxicity. Finally, we present new evidence suggesting that bilirubin metabolism is accomplished by both hepatic and extrahepatic UGT1A1 activities.
UDP-Glucuronosyltransferase 1A1 is the Primary Enzyme Involving Bilirubin Encephalopathy
Under the normal physiologic conditions, bilirubin is poorly water-soluble and is therefore required to be metabolized to allow its disposition and excretion. UGTs are a family of member-bound enzymes that catalyze the conjugation of a wide array of xenobiotics and endogenous substrates with glucuronic acid (Tukey and Strassburg, 2000). Of all isoforms, only UGT1A1 has physiologic relevance to metabolize bilirubin (Bosma et al., 1994), which is the rate-limiting step for bilirubin biliary excretion and detoxification. Clinical data and animal experiments support the fact that regardless of the factors contributing to hyperbilirubinemia, bilirubin detoxification is predominantly determined by regulatory events that control expression of the UGT1A1 gene.
Inherited Mutations of the UGT1A1 Gene and other Contributing Factors to Hyperbilirubinemia.
Congenital inborn errors of the UGT1A1 gene are associated with altered UGT1A1 expression and thereby reduce or completely abolish bilirubin conjugating activity. More than 40 inherited mutations in the UGT1A1 gene are associated with hyperbilirubinemia, and the degree of deficiency of UGT1A1 activity primarily determines the severity of hyperbilirubinemia and encephalopathy (Tukey and Strassburg, 2000). Gilbert syndrome is a mild form of UGT1A1 genetic polymorphism that results in a slight reduction in UGT1A1 activity (Kadakol et al., 2000; Strassburg, 2008), whereas Crigler-Najjar (CN) syndrome exhibits complete abolishment (type 1) or severe reduction of UGT1A1 (type 2) (Ciotti et al., 1997). A few key mutations in the coding region and the promoter region of the UGT1A1 gene have been discovered in CN patients; these mutations are correlated with reduction or elimination of UGT1A1 activity (Kadakol et al., 2000; Fujiwara et al., 2015). Clinical data showed that untreated infants with CN type 1 rapidly develop high plasma levels of UCB (20–50 mg/dl), exposing them to the possibility of serious neurologic damage.
Mild forms of UGT1A1 mutations result in benign jaundice; however, when coupling other genetically determined traits, severe hyperbilirubinemia may take place. For example, infants who have hemolytic conditions caused by glucose-6-phosphate dehydrogenase deficiency and rhesus disease may be predisposed to severe hyperbilirubinemia (Huang et al., 2005; Bhutani et al., 2013). Expression of P-glycoprotein (P-gp) in the brain has also been reported to be associated with bilirubin neurotoxicity. P-gp is expressed abundantly in brain capillary endothelial cells and astrocytes of the blood-brain barriers and has the ability to transport bilirubin out of the brain across the blood-brain barrier by acting as a membrane efflux pump (Watchko et al., 1998, 2001). Compared with wild-type mice, Mdr1a (P-gp encoding gene) null mice had a higher brain bilirubin content, possibly by enhanced brain bilirubin influx, implying that Pgp expression in the blood-barrier plays a role in protecting the central nervous system against bilirubin neurotoxicity (Watchko et al., 1998, 2001). In addition to the aforementioned genetic factors, prematurity, concurrent illness, and interventions that impede bilirubin-albumin binding are also considered to be risk factors for severe hyperbilirubinemia (Bhutani and Johnson, 2009).
Experimental Models Established for Studying Neonatal Hyperbilirubinemia and Regulation of UGT1A1.
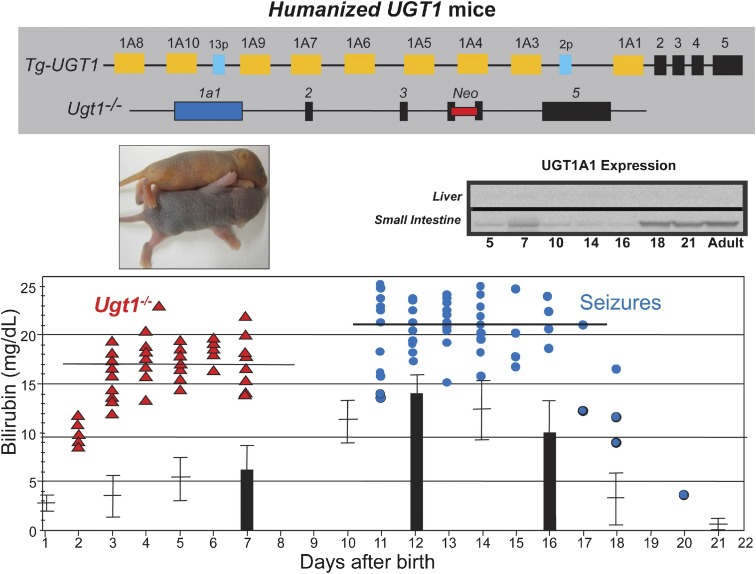
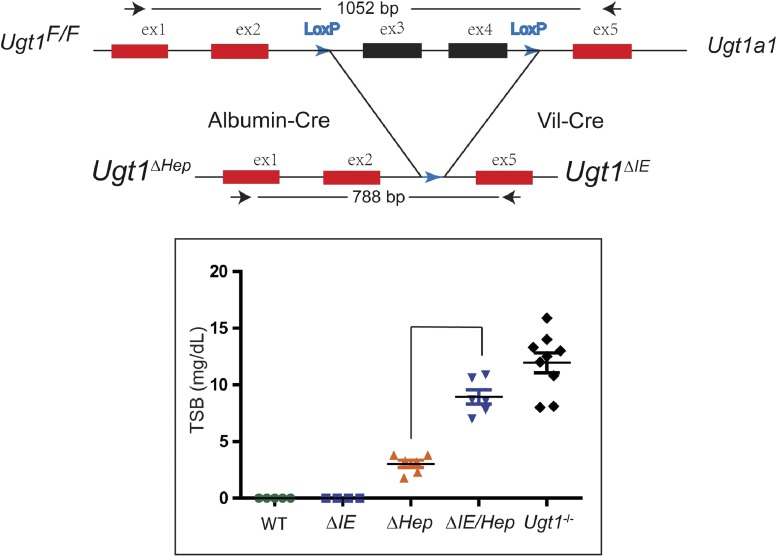
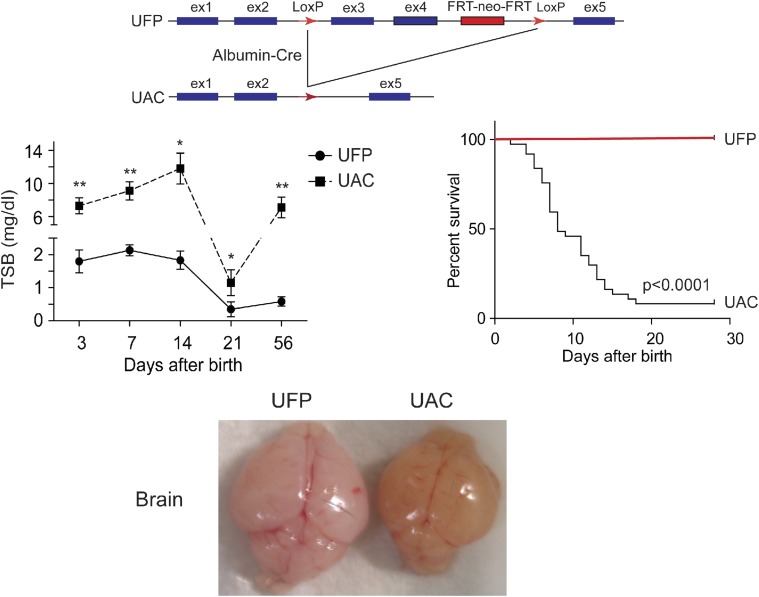
BIND is characterized by a wide range of neurologic deficits, and the underlying molecular mechanisms are only starting to emerge as a number of animal models producing the neonatal hyperbilirubinemia condition have been developed in the past few years. Most hyperbilirubinemia animal models harbor UGT1A1 mutations that occur naturally or are a result of genetic manipulations: 1) Gunn rats: Gunn (1938) discovered that mutant Wistar rats carrying a premature stop codon in the Ugt1a1 gene and exhibiting a very low UGT1A1 activity developed jaundice. These spontaneously jaundiced rats, termed Gunn rats, are deemed to be the first hyperbilirubinemia animal model, which mimics the condition of CN syndrome type 1. Since then, many studies have used Gunn rats in combination with administering sulfadimethoxine (displacing unconjugated bilirubin from albumin) or phenylhydrazine (inducing hemolysis) to examine acute bilirubin encephalopathy (Shapiro, 1988; Rice and Shapiro, 2008). 2) hUGT1A1*28 mice: To examine the role the human UGT1A1 gene in bilirubin metabolism, mice were humanized with the UGT1 locus encoding nine (−1A1, −1A3, −1A4, −1A5, −1A6, −1A7, −1A8, −1A9, and −1A10) functional UGT1A proteins. The generation of humanized UGT1 mice was accomplished in a few steps: A transgenic mouse line carrying the entire human UGT1 locus was first generated (Chen et al., 2005); the murine Ugt1 locus was inactivated by inserting a mutation into exon 4 to inactivate the nine mouse UGT1A proteins (Nguyen et al., 2008); and eventually Tg(UGT1) Ugt−−/− mice were generated by crossing heterozygous Ugt1+/− mice with TgUGT1 mice (Fujiwara et al., 2010). In conjunction with the fast breakdown of erythrocytes immediately after birth, these mice, which carried the genetic polymorphism of the (TA)7 dinucleotide repeat in the TATAA box promoter element of the UGT1A1 gene (termed hUGT1A1*28 mice), all developed neonatal hyperbilirubinemia with TSB peak levels exceeding 10 mg/dl. Approximately 10% of hUGT1A1*28 newborns experienced acute encephalopathy with TSB levels >17 mg/dl at 7–12 days after birth, imitating clinical kernicterus in newborn infants (Fig. 2). 3) UGT1F/F mice: Cre-Lox recombination sites have been positioned around common exons 2 and 3 of the Ugt1a1 gene. Breeding these mice with transgenic mice expressing tissue-specific Cre recombinase allows investigators to examine the tissue-specific impact of the Ugt1a1 gene and the other genes encoded by the Ugt1 locus. Although the liver has been assumed to be the primary target of neonatal bilirubin clearance by Ugt1a1, direct deletion of the Ugt1 locus in liver (Ugt1ΔHep) led to only modest levels of hyperbilirubinemia (Chen et al., 2013) (Fig. 3). Clearly, other tissues, such as the GI track, are capable of participating in the clearance of bilirubin during the neonatal window. When both the liver and intestinal epithelial cells of the gastrointestinal tract are targeted for deletion of the Ugt1 locus, Ugt1ΔIE/Hep mice develop high levels of TSB that are maintained in adult mice. 4) UFP/albumin-Cre (UAC) mice: Development of Ugt1F/F mice required the design of a targeting vector that contained the neomycin phosphotransferase (neor) gene to allow antibiotic selection after heterologous recombination in ES cells. The neor gene was flanked by flippase recombinase sites for easy removal of the neor gene after identification of the Ugt1LoxP/FRTneoFRT/LoxP mice. Deletion of the FRTneoFRT sequence was accomplished by crossing these mice with flippase recombinase transgenic mice, generating Ugt1F/F mice. If the Ugt1LoxP/FRTneoFRT/LoxP mice (UFP mice) are bred to homozygosity, however, insertion of the targeting construct along with the neor gene causes the mice to be hypomorphic for the Ugt1a1 gene, with all of the newborns and adults displaying hyperbilirubinemia (Fig. 4) resulting from poor expression of the Ugt1a1 gene in all tissues. By targeting the deletion of the Ugt1a1 gene in liver tissue using Cre/loxP recombination technology, UAC mice mice are created. Because of the preexisting hyperbilirubinemia in UFP mice, a robust kernicterus mouse model was developed in UAC mice with no mature hepatic Ugt1a1 gene or protein expression detected at 14 days after birth. The inability of the UAC mice to metabolize bilirubin results in TSB accumulation in the brain, causing severe CNS damage and leading to a 95% lethality rate (Barateiro et al., 2016). These mice serve as an excellent model to study the developmental impact of severe hyperbilirubinemia toward the onset of bilirubin-induced CNS toxicity. 5) Ugt1 mutant mice: The mouse Ugt1 locus was targeted, and a single base deletion was introduced in exon 4 of the Ugt1 gene, leading to a premature stop codon for generating Ugt1 mutant mice. These mice suffered severe neonatal hyperbilirubinemia within 5 days after birth (Bortolussi et al., 2015), very similar to the original Ugt−/− mice (Nguyen et al., 2008). The resulting bilirubin neurotoxicity is irreversible, and mice die shortly after birth.
Fig. 2.
The upper panel shows the genetic background of hUGT1A1*28 mice. The top diagram is a representation of the human UGT1A locus, which was inserted into the mouse genome (Tg-UGT1), and the bottom diagram shows the targeted disruption of the mouse Ugt1 locus with an insertion of the neoresistant gene into exon 4. Middle panel (left): The Ugt1−/− neonate exhibits the phenotypic trait of jaundice with a yellow skin color compared with in Ugt1+/− mice. Most of the Ugt1−/− mice die before day 7. Middle panel (right): Expression of UGT1A1 in neonatal liver and small intestine tissues in hUGT1A1*28 mice. The bottom diagram shows comparisons of TSB between the Ugt1−/− and hUGT1A1*28 mice during the developmental period.
Fig. 3.
Top: By using the Cre-loxP recombination technology, the hepatocyte- or intestinal enterocyte-specific deletion of the Ugt1a1 gene (Ugt1ΔHep or Ugt1ΔIE) was achieved. The bottom diagram shows the impact of the tissue-specific deletion of the Ugt1a1 gene on serum bilirubin levels.
Fig. 4.
Top: UFP mice carrying the target construct. Ugt1a1loxP[FRTneoFRT]loxP were bred into transgenic Albumin-Cre mice to generate UGT1a1F/F/Albumin-Cre mice (UAC mice). Middle (left): Comparisons of TSB levels between UFP and UAC mice during the developmental period. Middle (right): The Kaplan-Meier survival curves analyze the survival rates of UFP and UAC mice. Bottom: Excessively high levels of bilirubin penetrate to the brain of the 15-day-old UAC mouse.
Developmental Regulation of UGT1A1.
It is clear that UGT1A1 expression is a highly regulated event during development. By using hUGT1A1*28 mice, studies have shown that reduction of liver UGT1A1 gene expression in the developmental stage, which corresponds to the onset of hyperbilirubinemia and high levels of TSB, is actively regulated by pregnane X receptor (PXR). Reverse genetic experiments using PXR-deficient mice with the humanized UGT1 background demonstrated that in the absence of PXR, mice expressed significantly higher levels of UGT1A1 with a decrease in TSB levels, avoiding severe neonatal hyperbilirubinemia (Chen et al., 2012). These findings strongly indicate that PXR acts as a transcriptional repressor of the UGT1A1 gene during the neonatal period, and this is regulated as a developmental event since activation of the liver UGT1A1 gene in adult hUGT1/Pxr−/− mice has not been observed.
Bilirubin-Induced Neurotoxicity
In the normal state, more than 99.9% of UCB in blood is bound to plasma albumin, and only a small fraction of unbound bilirubin may diffuse into the brain and cerebrospinal fluid. With blood bilirubin exceeding a certain range (≥∼20 mg/dl) that saturates serum protein binding capacity, unbound UCB would dramatically rise and enter brain tissue (Ostrow et al., 1994). By comparing homozygous j/j Gun rats with nonjaundiced littermate heterozygous J/j controls with or without sulfadimethoxine exposure, studies have demonstrated that the levels of CNS bilirubin calculated correlate well with brain physiologic abnormalities and signs of neurotoxicity, attributable to the albumin-bilirubin binding constant and albumin concentrations in the brain (Daood and Watchko, 2006; Daood et al., 2009). The calculated neurotoxic bilirubin levels for Gunn rat pups are compatible with those in human neonates with extremely high levels of TSB (35 mg/dl) (Daood et al., 2009). Results derived from the Gunn rat model, along with in vitro brain cell line studies, provide evidence of the involvement of multiple mechanisms in bilirubin neurotoxicity, including central auditory processing abnormalities, mitochondrial impairment, prolonged hippocampal synaptic plasticity, and neuronal excitotoxicity, triggering downstream events, such as activation of apoptotic and necrotic cell death (Shapiro, 1988; Rice and Shapiro, 2008; Chang et al., 2009). High levels of UCB in the brain also resulted in chronic learning and memory deficits, as evidenced by the results of the Morris water maze test and place navigation ability test in hyperbilirubinemia rats (Song et al., 2014). In hUGT1A1*28 mice that develop signs of kernicterus, clear cognitive impairment can be observed because the mice have obvious balance issues, followed by grand mall seizures and dystonia (Fujiwara et al., 2010).
The involvement of innate immunity signaling in palliating bilirubin-induced neurotoxicity was illustrated using UGT1A1*28 mice by the study of kernicterus neonates (Yueh et al., 2014). The authors showed that despite the antioxidant property of bilirubin, excessively high levels of TSB produced systematic oxidative stress, as indicated by the decreased ratio of glutathione/glutathione disulfide and activation of the NADPH oxidase complex, as well as antioxidant response genes (e.g., heme-oxygenase 1) in the brain (Yueh et al., 2014). Whereas bilirubin escapes metabolism by UGT1A1 and continues to increase, accelerating TSB levels lead to cellular neuroinflammation, manifested as activation of microglia and astrocytes in the brain. It appears that the TLR2-medicated signaling pathway is critical in regulating gliosis, proinflammatory mediators, and oxidative stress and serves as an overall protective mechanism under the condition of severe hyperbilirubinemia as hUGT1A1*28/Tlr2−/− mice failed to activate glial cells, proinflammatory cytokines, and stress response genes, resulting in a significant drop in survival rates of hyperbilirubinemia neonates. One hypothesis that still needs to be proven is that toll-like receptor 2 signaling may have originated from ligands produced by the gut microbiota; that is, disturbed gut bacteria (dysbiosis) owing to high levels of serum bilirubin damage the intestinal barrier, leading to activation of innate immunity through TLR signaling.
To evaluate the role of oxidative stress in BIND, sulfadimethoxine-induced jaundiced Gunn rat neonates were administered antioxidants to alter their redox status, resulting in reduced lipid peroxidation; however, inhibition of oxidative stress did not lead to blockage of neurotoxicity as these pups still exhibited neurobehavioral abnormalities and bilirubin encephalopathy, resembling what was observed in control Gunn rat pups in the absence of antioxidants (Daood et al., 2012). One possible explanation of these results is that oxidative stress may occur in later rather than in initial stages of the disease, and reactive oxygen species are secondary manifestations of neuronal degeneration resulting from early events, such as inflammatory responses.
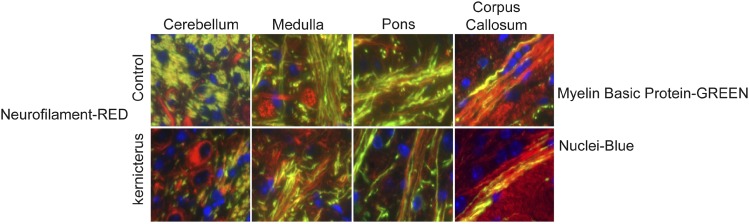
Results of experiments with Ugt1 mutant mice harboring a mutation in the Ugt1a1 gene showed that accumulated bilirubin in the brain leading to neurotoxicity is manifested as cerebellar abnormalities, hypoplasia, and neuronal cell death. Histologic studies have further demonstrated that neonates’ cerebellar architecture was significantly affected, together with reductions in Purkinje cell number and dendritic arborization (Bortolussi et al., 2015). Another mechanism of action that is linked to clinical features of kernicterus shown in UAC mice is axonal damage and myelin degeneration. These neonatal mice exhibited axonopathy as an early sign of BIND characterized by deficits in the myelin sheath formation in different brain regions, especially in the cerebellum (Fig. 5) (Barateiro et al., 2016). The connection between astroglial and microglial reactivity and myelination impairment in hyperbilirubinemia neonates has also been established based on histology and immunostaining studies, suggesting the occurrence of these events in the same brain regions. These results are consistent with previous findings using ganglia-oligodendrocyte cultures indicating that unconjugated bilirubin impairs oligodendrocyte differentiation and subsequent myelination (Barateiro et al., 2013). Indeed, clinical studies have also observed a decrease in the density of myelinated fibers and a reduction in cerebellar axons of a preterm infant with kernicterus (Brites, 2012).
Fig. 5.
Images from cerebellum, medulla, pons, and corpus callosum indicate reduced myelination, as shown by the reduction of the presence of myelin basic protein (MBP, green) in neurons (neurofilament, red).
Contribution of Environmental Factors to Hyperbilirubinemia
Breast Milk Jaundice and the Role of Extrahepatic UGT1A1.
The connection between breast milk and hyperbilirubinemia was first described by Arias and colleagues in 1964 (Arias et al., 1964). Early studies of breast milk jaundice showed a metabolite of progesterone, pregnane-3-20-dio, was present in breast milk and was implicated in the development of jaundice (Hargreaves and Piper, 1971), although eventually no scientific consensus was reached. To date, no specific component or combinations of components have been demonstrated to definitely contribute to breast milk jaundice.
In keeping with the concept that TSB levels are higher and last longer in infants fed on breast milk, experiments using the humanized UGT1A1*28 mouse model (Fujiwara et al., 2012) reiterated that neonatal hyperbilirubinemia that occurred after breast milk feeding disappeared when mice were fed formula. In contrast to conventional knowledge, however, this study further revealed that expression of extrahepatic UGT1A1, particularly intestinal UGT1A1, is subject to induction by formula feeding and is crucial for bilirubin metabolism and clearance during postnatal transition of hepatic UGT1A1 activity, which is present only later, at the end of the suckling period (Chen et al., 2012; Fujiwara et al., 2012). Conversely, breast milk contributes to the development of hyperbilirubinemia by suppressing UGT1A1 expression in the small intestine. Breast milk was found to suppress intestinal IĸB kinase α and β, resulting in inactivation of nuclear receptor NF-ĸB and nearly complete abolishment of intestinal UGT1A1 expression (Fujiwara et al., 2012).
UGT1A1 Activation by Xenobiotics through Nuclear Receptors.
Since UGT1A1 levels and activities determine bilirubin conjugation capacity, efforts have been made in seeking endogenous and exogenous compounds that have the ability to induce UGT1A1 expression. Several studies have demonstrated that UGT1A1 expression is modulated by a number of nuclear receptors, including PXR, constitutive androstane receptor, and peroxisome proliferator–activated receptor α, in a ligand-dependent fashion. Upon ligand binding, the nuclear receptor binds to the corresponding consensus response element in the UGT1A1 promoter region and activates UGT1A1 gene expression (Xie et al., 2003; Yueh et al., 2003; Chen et al., 2005; Senekeo-Effenberger et al., 2007; Yueh and Tukey, 2007). For example, phenobarbital, a constitutive androstane receptor agonist that induces UGT1A1 expression through interacting with a phenobarbital responsive element (PBREM), has been used clinically in conjunction with phototherapy for the treatment of severe jaundice in infants to enhance bilirubin metabolism, thus reducing the need for exchange transfusion (Valaes et al., 1980; Murki et al., 2005). PBREM involvement in controlling UGT1A1 expression is also supported by a study linking polymorphism of the UGT1A1 PBREM (T-3279G) to an increased risk of hyperbilirubinemia (Sugatani et al., 2002). Glucocorticoids have also been used to treat hyperbilirubinemia: Dexamethasone-treated infants experienced lower incidence of hyperbilirubinemia than untreated controls (Madarek and Najati, 2003). Studies in mice with neonatal hyperbilirubinemia indicated that PXR serves as a key regulator after glucocorticoid treatment by inducing liver UGT1A1 expression and reducing TSB levels (Chen et al., 2012). In addition to being regulated by the aforementioned nuclear receptors belonging to the nuclear hormone receptor superfamily, UGT1A1 is subject to regulation by the nuclear receptor aryl hydrocarbon receptor and nuclear factor erythroid 2–related factor, activation of which can transcriptionally induce UGT1A1, resulting in higher levels of bilirubin excretion (Yueh et al., 2003; Yueh and Tukey, 2007; Fujiwara et al., 2010). It is worth noting that bilirubin itself has been shown to activate aryl hydrocarbon receptor and induce UGT1A in a ligand-dependent manner (Togawa et al., 2008).
Modulation of UGT1A1 Expression by Environmental Chemicals.
When hyperbilirubinemia neonatal mice were exposed to the environmental chemicals arsenic and cadmium, their TSB levels unexpectedly decreased, correlated with elevated levels of intestinal UGT1A1 expression with no detectable changes in expression of hepatic UGT1A1. Gene expression profiling data and biochemical studies revealed that, as potent inducers of oxidative stress, arsenic and cadmium alter the redox state of the intestines, leading to induction of UGT1A1 and a dramatic reduction in TSB levels (Liu et al., 2016). These results suggest that modulation of intestinal UGT1A1 activity by initiating the oxidative stress signaling pathway may be an unconventional alternative for lowering TSB and improving hyperbilirubinemia.
Alternative Approaches to Treat Hyperbilirubinemia
Hepatocyte Transplantation and Gene-Transfer Therapy.
For CNS1 patients, phototherapy is often the first-line therapy, but it may transiently lower the serum bilirubin concentrations and gradually become ineffective beyond infancy. At present, liver transplantation is the curative treatment to prevent neurologic sequelae and kernicterus, but it often requires continuous immunosuppression with substantial risks (Schauer et al., 2003).
Evidence indicated that only ∼5% of normal UGT1A1 activity is adequate to significantly lower the plasma bilirubin concentration and eliminate the risk of kernicterus (Fox et al., 1998); therefore, alternative therapies intending to alleviate hyperbilirubinemia with persistent expression of the UGT1A1 enzyme are under way in the experimental stage. A recent study illustrated that the advantage of neonatal hepatocytes over adult hepatocytes lies in the fact that neonatal hepatocytes exhibit better engraftment and repopulation capacity after transplantation, thus resulting in better bilirubin clearance in icteric Gunn rats (Tolosa et al., 2015). Significant progress has also been made in past decades by means of gene therapy using adenovirus-based or similar techniques or the correction of UGT1A1 gene defects with the site-directed gene repair approach to treat hyperbilirubinemia animals (Li et al., 1998; Kren et al., 1999; Roy-Chowdhury et al., 2001; Bellodi-Privato et al., 2005). A gene therapy study showed that a single injection of a helper-dependent adenoviral vector expressing UGT1A1 that specifically targets the liver tissue can completely correct hereditary hyperbilirubinemia in Gunn rats with long-lasting effects and low chronic toxicity (Toietta et al., 2005).
Administration of Albumin.
As a result of the high affinity of albumin to bilirubin, in a normal state, UCB is bound to albumin after transportation through the circulation to the liver (Ostrow et al., 1994). When UCB levels exceed the capacity of albumin, free bilirubin is capable of crossing the blood-brain barrier and accumulating in the brain. Therefore, a potential approach to prevent bilirubin accumulation in the brain is to increase bilirubin-binding capacity by albumin supplementation. When hyperbilirubinemia neonatal mice carrying inherited mutations of Ugt1a1 were subject to daily albumin infusion, they were rescued from neurologic damage and lethality. By increasing plasma bilirubin-binding capacity, albumin mobilizes bilirubin from tissues to plasma and results in reduced systemic plasma bilirubin levels (Vodret et al., 2015).
Regardless of efficacy of these alternative treatments, they are still in the experimental stage, and clinical trials are apparently needed to evaluate the acute toxicity, immunogenic responses, and long-term safety profile before they can be applied in the market to humans.
Develop Therapeutics Targeted to Induce UGT1A1 Gene Expression.
The use of animal models, such as humanized UGT1A1*28 mice, helps define the mechanisms that control neonatal hyperbilirubinemia and provides an important venue to exploit the impact of safe and therapeutic chemicals to regulate the UGT1A1 gene and lower TSB levels. These noninvasive approaches could take advantage of drug delivery directly to newborns or, alternatively, by lactation after drug administration to nursing mothers. In vivo studies with humanized UGT1A1*28 mice can directly exploit tissue-specific contributions, such as the liver and gastrointestinal tract, that direct bilirubin clearance while also being able to examine pharmacokinetics parameters of the inducing agents.
Summary
Severe neonatal hyperbilirubinemia and prevention of bilirubin encephalopathy remain clinical concerns. Clinical data have indicated that whereas physiologic jaundice is a common benign condition observed in newborns, additional sources of hemolysis resulting in increased heme catabolism and severe UGT1A1 deficiency may lead to reversible or irreversible neuropathological conditions. Studies in animals have revealed that UGT1A1 levels can be regulated by environmental and dietary compounds through activation of nuclear receptors or alteration of oxidative-stress status. Whereas the complex cascade of molecular and cellular events leading to bilirubin-induced neurotoxicity and kernicterus remains incompletely delineated, emerging evidence indicates that high levels of TSB activate innate immunity and cause myelination impairment. As we have learned more about bilirubin metabolism and neurologic injury with the advent of novel toxicology models, some of the conventional knowledge regarding hyperbilirubinemia is now being challenged: Intestinal UGT1A1 is subject to the regulation of breast milk and environmental compounds and plays a critical role during the developmental stage when expression of hepatic UGT1A1 is delayed.
Abbreviations
- BIND
bilirubin-induced neurologic dysfunction
- CNS1
Crigler-Najjar syndrome type 1
- PBREM
phenobarbital responsive element
- P-gp
P-glycoprotein
- PXR
pregnane X receptor
- TSB
total serum bilirubin
- UAC
UFP/albumin-Cre
- UCB
unconjugated bilirubin
- UGT1A1
UDP-glucuronosyltransferase 1A1
Authorship Contributions
Conducted experiments: Yueh, Chen, Nguyen, Tukey.
Performed data analysis: Yueh, Chen, Nguyen, Tukey.
Wrote or contributed to the writing of the manuscript: Yueh, Tukey.
Footnotes
The writing of this review was supported in part by Public Health Service Grants [ES010337, GM086713, GM100481, R21ES024818, and R21ES023906].
References
- Arias IM, Gartner LM, Seifter S, Furman M. (1964) Prolonged neonatal unconjugated diol, in maternal milk that inhibits glucuronide formation in vitro. J Clin Invest 43:2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateiro A, Chen S, Yueh MF, Fernandes A, Domingues HS, Relvas J, Barbier O, Nguyen N, Tukey RH, Brites D. (2016) Reduced myelination and increased glia reactivity resulting from severe neonatal hyperbilirubinemia. Mol Pharmacol 89:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateiro A, Miron VE, Santos SD, Relvas JB, Fernandes A, Ffrench-Constant C, Brites D. (2013) Unconjugated bilirubin restricts oligodendrocyte differentiation and axonal myelination. Mol Neurobiol 47:632–644. [DOI] [PubMed] [Google Scholar]
- Bellodi-Privato M, Aubert D, Pichard V, Myara A, Trivin F, Ferry N. (2005) Successful gene therapy of the Gunn rat by in vivo neonatal hepatic gene transfer using murine oncoretroviral vectors. Hepatology 42:431–438. [DOI] [PubMed] [Google Scholar]
- Bhutani VK, Johnson L. (2009) Kernicterus in the 21st century: frequently asked questions. J Perinatol 29 (Suppl 1):S20–S24. [DOI] [PubMed] [Google Scholar]
- Bhutani VK, Zipursky A, Blencowe H, Khanna R, Sgro M, Ebbesen F, Bell J, Mori R, Slusher TM, Fahmy N, et al. (2013) Neonatal hyperbilirubinemia and rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res 74 (Suppl 1):86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani VK, Johnson-Hamerman L. (2015) The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med 20:6–13. [DOI] [PubMed] [Google Scholar]
- Bortolussi G, Codarin E, Antoniali G, Vascotto C, Vodret S, Arena S, Cesaratto L, Scaloni A, Tell G, Muro AF. (2015) Impairment of enzymatic antioxidant defenses is associated with bilirubin-induced neuronal cell death in the cerebellum of Ugt1 KO mice. Cell Death Dis 6:e1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL. (1994) Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem 269:17960–17964. [PubMed] [Google Scholar]
- Brites D. (2012) The evolving landscape of neurotoxicity by unconjugated bilirubin: role of glial cells and inflammation. Front Pharmacol 3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell B, Coughtrie M, Jackson M, Harding D, Fournel-Gigleux S, Leakey J, Hume R. (1989) Development of human liver UDP-glucuronosyltransferases. Dev Pharmacol Ther 13:70–77. [DOI] [PubMed] [Google Scholar]
- Chang FY, Lee CC, Huang CC, Hsu KS. (2009) Unconjugated bilirubin exposure impairs hippocampal long-term synaptic plasticity. PLoS One 4:e5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, Remmel RP, Trottier J, Barbier O, Ritter JK, et al. (2005) Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem 280:37547–37557. [DOI] [PubMed] [Google Scholar]
- Chen S, Yueh MF, Bigo C, Barbier O, Wang K, Karin M, Nguyen N, Tukey RH. (2013) Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11). Proc Natl Acad Sci USA 110:19143–19148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yueh MF, Evans RM, Tukey RH. (2012) Pregnane-x-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 mice. Hepatology 56:658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chime, H. E., J. A. Egenede, and J.E. Arute (2011) Prevalence of neonatal jaundice on Central Hospital, Warri, Delta State, Nigeria. Int J Health Res 123–126.
- Ciotti M, Obaray R, Martín MG, Owens IS. (1997) Genetic defects at the UGT1 locus associated with Crigler-Najjar type I disease, including a prenatal diagnosis. Am J Med Genet 68:173–178. [PubMed] [Google Scholar]
- Crigler JF, Jr, Najjar VA. (1952) Congenital familial nonhemolytic jaundice with kernicterus; a new clinical entity. AMA Am J Dis Child 83:259–260. [PubMed] [Google Scholar]
- Daood MJ, Hoyson M, Watchko JF. (2012) Lipid peroxidation is not the primary mechanism of bilirubin-induced neurologic dysfunction in jaundiced Gunn rat pups. Pediatr Res 72:455–459. [DOI] [PubMed] [Google Scholar]
- Daood MJ, McDonagh AF, Watchko JF. (2009) Calculated free bilirubin levels and neurotoxicity. J Perinatol 29 (Suppl 1):S14–S19. [DOI] [PubMed] [Google Scholar]
- Daood MJ, Watchko JF. (2006) Calculated in vivo free bilirubin levels in the central nervous system of Gunn rat pups. Pediatr Res 60:44–49. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. (1998) Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 338:1422–1426. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Chen S, Karin M, Tukey RH. (2012) Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia. Gastroenterology 142:109–118.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R, Maruo Y, Chen S, Tukey RH. (2015) Role of extrahepatic UDP-glucuronosyltransferase 1A1: advances in understanding breast milk-induced neonatal hyperbilirubinemia. Toxicol Appl Pharmacol 289:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R, Nguyen N, Chen S, Tukey RH. (2010) Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci USA 107:5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn CH. (1938) Hereditary acholuric jaundice in a new mutant strain of rats. J Hered 29:137–139. [Google Scholar]
- Hargreaves T, Piper RF. (1971) Breast milk jaundice: effect of inhibitory breast milk and 3 alpha, 20 abeta-pregnanediol on glucuronyl transferase. Arch Dis Child 46:195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Huang MJ, Lin MS, Yang SS, Teng HC, Tang KS. (2005) Genetic factors related to unconjugated hyperbilirubinemia amongst adults. Pharmacogenet Genomics 15:43–50. [DOI] [PubMed] [Google Scholar]
- Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR, Chowdhury NR. (2000) Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat 16:297–306. [DOI] [PubMed] [Google Scholar]
- Kren BT, Parashar B, Bandyopadhyay P, Chowdhury NR, Chowdhury JR, Steer CJ. (1999) Correction of the UDP-glucuronosyltransferase gene defect in the gunn rat model of crigler-najjar syndrome type I with a chimeric oligonucleotide. Proc Natl Acad Sci USA 96:10349–10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Murphree SS, Willer SS, Bolli R, French BA. (1998) Gene therapy with bilirubin-UDP-glucuronosyltransferase in the Gunn rat model of Crigler-Najjar syndrome type 1. Hum Gene Ther 9:497–505. [DOI] [PubMed] [Google Scholar]
- Liu M, Chen S, Yueh MF, Fujiwara R, Konopnicki C, Hao H, Tukey RH. (2016) Cadmium and arsenic override NF-κB developmental regulation of the intestinal UGT1A1 gene and control of hyperbilirubinemia. Biochem Pharmacol 110-111:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madarek EO, Najati N. (2003) The effect of glucocorticoid therapy in preventing early neonatal complications in preterm delivery. J Perinat Med 31:441–443. [DOI] [PubMed] [Google Scholar]
- Morioka I, Nakamura H, Koda T, Yokota T, Okada H, Katayama Y, Kunikata T, Kondo M, Nakamura M, Hosono S, et al. (2015) Current incidence of clinical kernicterus in preterm infants in Japan. Pediatr Int 57:494–497. [DOI] [PubMed] [Google Scholar]
- Murki S, Dutta S, Narang A, Sarkar U, Garewal G. (2005) A randomized, triple-blind, placebo-controlled trial of prophylactic oral phenobarbital to reduce the need for phototherapy in G6PD-deficient neonates. J Perinatol 25:325–330. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner MJ, Hardiman G, Bélanger A, Tukey RH. (2008) Disruption of the ugt1 locus in mice resembles human Crigler-Najjar type I disease. J Biol Chem 283:7901–7911. [DOI] [PubMed] [Google Scholar]
- Ostrow JD, Mukerjee P, Tiribelli C. (1994) Structure and binding of unconjugated bilirubin: relevance for physiological and pathophysiological function. J Lipid Res 35:1715–1737. [PubMed] [Google Scholar]
- Rice AC, Shapiro SM. (2008) A new animal model of hemolytic hyperbilirubinemia-induced bilirubin encephalopathy (kernicterus). Pediatr Res 64:265–269. [DOI] [PubMed] [Google Scholar]
- Roy-Chowdhury N., Kadakol A., Sappal B. S., Thummala N. R., Ghosh S. S., Lee S. W., Roy-Chowdhury J. (2001) Gene therapy for inherited hyperbilirubinemias. J Perinatol 21(Suppl 1):S114–S118. [DOI] [PubMed] [Google Scholar]
- Schauer R, Stangl M, Lang T, Zimmermann A, Chouker A, Gerbes AL, Schildberg FW, Rau HG. (2003) Treatment of Crigler-Najjar type 1 disease: relevance of early liver transplantation. J Pediatr Surg 38:1227–1231. [DOI] [PubMed] [Google Scholar]
- Senekeo-Effenberger K, Chen S, Brace-Sinnokrak E, Bonzo JA, Yueh MF, Argikar U, Kaeding J, Trottier J, Remmel RP, Ritter JK, et al. (2007) Expression of the human UGT1 locus in transgenic mice by 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid (WY-14643) and implications on drug metabolism through peroxisome proliferator-activated receptor α activation. Drug Metab Dispos 35:419–427. [DOI] [PubMed] [Google Scholar]
- Shapiro SM. (1988) Acute brainstem auditory evoked potential abnormalities in jaundiced Gunn rats given sulfonamide. Pediatr Res 23:306–310. [DOI] [PubMed] [Google Scholar]
- Shapiro SM, Bhutani VK, Johnson L. (2006) Hyperbilirubinemia and kernicterus. Clin Perinatol 33:387–410. [DOI] [PubMed] [Google Scholar]
- Song S, Hu Y, Gu X, Si F, Hua Z. (2014) A novel newborn rat kernicterus model created by injecting a bilirubin solution into the cisterna magna. PLoS One 9:e96171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburg CP. (2008) Pharmacogenetics of Gilbert’s syndrome. Pharmacogenomics 9:703–715. [DOI] [PubMed] [Google Scholar]
- Sugatani J, Yamakawa K, Yoshinari K, Machida T, Takagi H, Mori M, Kakizaki S, Sueyoshi T, Negishi M, Miwa M. (2002) Identification of a defect in the UGT1A1 gene promoter and its association with hyperbilirubinemia. Biochem Biophys Res Commun 292:492–497. [DOI] [PubMed] [Google Scholar]
- Togawa H, Shinkai S, Mizutani T. (2008) Induction of human UGT1A1 by bilirubin through AhR dependent pathway. Drug Metab Lett 2:231–237. [DOI] [PubMed] [Google Scholar]
- Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, Beaudet AL, Lee B. (2005) Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA 102:3930–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa L, López S, Pareja E, Donato MT, Myara A, Nguyen TH, Castell JV, Gómez-Lechón MJ. (2015) Human neonatal hepatocyte transplantation induces long-term rescue of unconjugated hyperbilirubinemia in the Gunn rat. Liver Transpl 21:801–811. [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616. [DOI] [PubMed] [Google Scholar]
- Valaes T, Kipouros K, Petmezaki S, Solman M, Doxiadis SA. (1980) Effectiveness and safety of prenatal phenobarbital for the prevention of neonatal jaundice. Pediatr Res 14:947–952. [DOI] [PubMed] [Google Scholar]
- Vodret S, Bortolussi G, Schreuder AB, Jašprová J, Vitek L, Verkade HJ, Muro AF. (2015) Albumin administration prevents neurological damage and death in a mouse model of severe neonatal hyperbilirubinemia. Sci Rep 5:16203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchko JF, Daood MJ, Hansen TW. (1998) Brain bilirubin content is increased in P-glycoprotein-deficient transgenic null mutant mice. Pediatr Res 44:763–766. [DOI] [PubMed] [Google Scholar]
- Watchko JF, Daood MJ, Mahmood B, Vats K, Hart C, Ahdab-Barmada M. (2001) P-glycoprotein and bilirubin disposition. J Perinatol 21 (Suppl 1):S43–S62. [DOI] [PubMed] [Google Scholar]
- Xie W, Yeuh MF, Radominska-Pandya A, Saini SPS, Negishi Y, Bottroff BS, Cabrera GY, Tukey RH, Evans RM. (2003) Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA 100:4150–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Chen S, Nguyen N, Tukey RH. (2014) Developmental onset of bilirubin-induced neurotoxicity involves Toll-like receptor 2-dependent signaling in humanized UDP-glucuronosyltransferase1 mice. J Biol Chem 289:4699–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. (2003) Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem 278:15001–15006. [DOI] [PubMed] [Google Scholar]
- Yueh MF, Tukey RH. (2007) Nrf2-Keap1 signaling pathway regulates human UGT1A1 expression in vitro and in transgenic UGT1 mice. J Biol Chem 282:8749–8758. [DOI] [PubMed] [Google Scholar]