Abstract
The uptake of iodide into the thyroid, an essential step in thyroid hormone synthesis, is an active process mediated by the sodium-iodide symporter (NIS). Despite its strong dependence on TSH, the master regulator of the thyroid, the NIS gene was also reported to be regulated by non-TSH signaling pathways. In the present study we provide evidence that the rat NIS gene is subject to regulation by sterol regulatory element-binding proteins (SREBPs), which were initially identified as master transcriptional regulators of lipid biosynthesis and uptake. Studies in FRTL-5 thyrocytes revealed that TSH stimulates expression and maturation of SREBPs and expression of classical SREBP target genes involved in lipid biosynthesis and uptake. Almost identical effects were observed when the cAMP agonist forskolin was used instead of TSH. In TSH receptor-deficient mice, in which TSH/cAMP-dependent gene regulation is blocked, the expression of SREBP isoforms in the thyroid was markedly reduced when compared with wild-type mice. Sterol-mediated inhibition of SREBP maturation and/or RNA interference-mediated knockdown of SREBPs reduced expression of NIS and NIS-specific iodide uptake in FRTL-5 cells. Conversely, overexpression of active SREBPs caused a strong activation of the 5′-flanking region of the rat NIS gene mediated by binding to a functional SREBP binding site located in the 5′-untranslated region of the rat NIS gene. These findings show that TSH acts as a regulator of SREBP expression and maturation in thyroid epithelial cells and that SREBPs are novel transcriptional regulators of NIS.
The most important function of the thyroid gland is to accumulate iodide for the biosynthesis of iodine-containing thyroid hormones, T4 and T3, which are essential for growth, development, and numerous pathways in intermediary metabolism (1). The uptake of iodide across the basolateral membrane of the thyroid follicular epithelial cells (thyrocytes) is an active process mediated by the sodium-iodide symporter (NIS), a glycoprotein located in the plasma membrane (2). The critical role for NIS in thyroid hormone synthesis is shown by the fact that iodide transport defects due to mutations in the NIS gene cause severe hypothyroidism (3–5). In addition, iodide uptake and, thus, thyroid hormone biosynthesis can be inhibited by thiocyanate and, more potently, by perchlorate (6, 7) due to competition of these anions with iodide for the transport by NIS.
All steps involved in thyroid hormone synthesis, including iodide uptake and NIS gene expression, as well as thyrocyte cell growth and differentiation, are primarily regulated by TSH (8). The effects of TSH are mediated by binding to the TSH receptor (TSHR), which leads to an activation of the cAMP pathway (9). Although the detailed mechanisms of NIS regulation by TSH are not completely understood, it is evident that NIS gene expression is stimulated by TSH through the cAMP pathway at both the mRNA and the protein level (10). However, despite its strong TSH/TSHR/cAMP dependence (8–10), the NIS gene is also subject to regulation by other signaling pathways, such as the nuclear factor-κB pathway (11), indicating that thyroid cells are also responsive to non-TSH signals by stimulating expression of key genes of thyroid hormone synthesis, which possibly modulates thyroid hormone homeostasis.
In addition to regulating thyroid-specific functions, TSH, albeit less well documented, also influences lipid biosynthetic pathways required for DNA synthesis and cell proliferation in thyrocytes; in this regard, studies in FRTL-5 thyrocytes, a well-established model for thyroid epithelial cells that are sensitive to TSH (12–18), like thyroid cells in vivo, revealed that TSH, but also the cAMP agonist forskolin, transcriptionally induce 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), the rate-limiting enzyme of cholesterol (Chol) biosynthesis, and malic enzyme, an enzyme involved in fatty acid synthesis, and increase Chol biosynthesis (13–15). Moreover, a more recent study demonstrated that TSH up-regulates HMGCR in hepatocytes, in which the TSHR, like in many other nonthyroidal tissues, is also expressed (19). Although the abovementioned studies provided clear evidence for the involvement of the cAMP pathway in mediating the stimulatory effect of TSH on HMGCR and malic enzyme gene transcription and Chol biosynthesis, it cannot be ruled out that other regulatory pathways, such as the sterol responsive element-binding protein (SREBP) pathway, contribute to this effect of TSH as well.
The SREBPs, from which the isoforms 1a, 1c, and 2 exist, were initially discovered as master transcriptional regulators coordinating the expression of a large set of genes encoding enzymes involved in lipid synthesis and uptake (20, 21). Whereas SREBP-1c activates preferentially genes required for fatty acid and triacylglycerol synthesis (eg, malic enzyme, fatty acid synthase [FAS], glycerolphosphate-acyltransferase [GPAT]), SREBP-2 stimulates mainly genes involved in Chol synthesis (eg, HMGCR) and uptake (eg, low-density lipoprotein [LDL] receptor) (22). Unlike the other SREBP isoforms, SREBP-1a stimulates genes of both the fatty acid and Chol biosynthetic pathways. SREBPs are synthesized as inactive 120-kDa precursors (pSREBP) integrated into the endoplasmic reticulum membrane and form a complex with the SREBP cleavage activating protein (SCAP). Under high-Chol conditions in the cell, the SCAP/SREBP complex is retained in the endoplasmic reticulum (23, 24), and SREBP-mediated gene transcription is prevented. In contrast, in cholesterol-depleted cells, SCAP escorts the SREBPs to the Golgi, where the SREBPs are processed by two sequential proteolytic cleavages releasing the mature N-terminal domain of the SREBPs (nSREBP), which translocates to the nucleus and binds to sterol-regulatory element (SRE) binding sites in the promoter of target genes, thereby activating their transcription (22, 25, 26). Because Chol and fatty acids are major and essential components of animal cells, it is not surprising that SREBPs are expressed in most tissues (22, 27). However, due to their prominent role in controlling lipid biosynthesis pathways, studies addressing the function of SREBPs have focused mainly on tissues in which lipid biosynthesis pathways play a major role, such as liver, white adipose tissue, intestine, and mammary gland (27–30), whereas tissues like the thyroid gland have not yet been considered in this regard. Nevertheless, using genome-wide screening techniques, such as gene expression microarrays and chromatin immunoprecipitation (ChIP)-on-chip, a great number of SREBP target genes, including genes not previously linked to lipid biosynthesis, have been identified (31, 32), suggesting novel and expanded roles for the SREBPs. Therefore, in light of the scarcity of knowledge regarding the functional role of SREBPs in the thyroid and the observed up-regulation of SREBP target genes by TSH in thyrocytes (13–15), we tested the hypothesis that SREBPs are activated by TSH in FRTL-5 cells through the TSHR/cAMP pathway. In addition, we tested the hypothesis that SREBPs play a specific role for thyroid function, such as NIS gene expression and iodide uptake, in addition to their classical action as regulators of lipid biosynthesis pathways.
Materials and Methods
Cell culture
HepG2 cells (DSMZ, Braunschweig, Germany), a human hepatoma cell line that is commonly used for transient transfection assays, were cultured in RPMI1640 supplemented with 10% fetal bovine serum and 0.5% gentamicin (all from Invitrogen, Karlsruhe, Germany) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Medium was changed every 2 days. After reaching a confluence of 70%–80%, the cells were either subcultivated or used for reporter gene experiments.
FRTL-5 cells, a strain of differentiated rat thyroid cells that are dependent on TSH (18), were obtained from CLS Cell Lines Service (Eppelheim, Germany) and grown at 37°C in a humidified atmosphere composed of 95% air and 5% CO2 in Ham's F12 medium supplemented with 5% newborn calf serum, 1% antibiotic-antimycotic-mixture, and a 6-hormone mixture (6H medium) of TSH (10 U/L), insulin (10 μg/mL), hydrocortisone (10 nmol/L), transferrin (5 μg/mL), somatostatin (10 ng/mL), and glycyl-l-histidyl-l-lysine acetate (10 ng/mL). Medium was changed every 2 days. After reaching a confluence of 70%–80%, the cells were either subcultivated or used for experiments.
Animals
Heterozygous Tshrtm1Rmar/J mice (TSHR−/+ genetic backround: C57BL/6 × 129/Sv) were obtained from The Jackson Laboratory (Bar Harbor, Maine) and intercrossed to produce homozygous mice (TSHR−/−). 129S1/SvImJ mice were used as wild-type control mice. From weaning, the homozygous TSHR−/− mice received a mouse chow supplemented with 100 ppm desiccated thyroid powder (MP Biomedicals, Eschwege, Germany) to get fertile animals. Mice were maintained in Macrolon cages in a room with controlled temperature (22 ± 2°C), relative humidity (50%–60%), and light (12-hour light, 12-hour dark cycle), and had free access to food and water. Mouse tail DNA was used for genotyping. All experimental procedures followed established guidelines for the care and handling of laboratory animals.
RNA isolation and real-time RT-PCR
FRTL-5 cells were seeded in 24-well plates at a density of 0.1 × 106 cells per well and incubated as indicated. After incubation, total RNA was isolated from cells using Trizol reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's protocol. Isolated RNA was preserved at −80°C until use. RNA concentration and purity were estimated from the optical density at 260 and 280 nm, respectively. cDNA was synthesized in less than 1 week after RNA extraction from 1.2 μg of total RNA using 100 pmol dT18 primer (Eurofins MWG Operon, Ebersberg, Germany), 1.25 μL 10 mmol/L deoxynucleotide triphosphate mix (GeneCraft, Lüdinghausen, Germany), 5 μL buffer (Fermentas, St. Leon-Rot, Deutschland), and 60 U M-MuLV Reverse Transcriptase (MBI Fermentas, St. Leon-Rot, Germany) at 42°C for 60 minutes, and a final inactivating step at 70°C for 10 minutes in a Biometra Thermal Cycler (Whatman Biometra, Göttingen, Germany). Subsequently, cDNA was stored in aliquots at −20°C. quantitative PCR (qPCR) was performed with a Rotorgene 2000 system (Corbett Research, Mortlake, Australia) using 2 μL cDNA combined with 18 μL of a mixture composed of 10 μL KAPA SYBR FAST qPCR Universal Mastermix (Peqlab, Erlangen, Germany), 0.4 μL each of 10 mM forward and reverse primers, and 7.2 μL DNase/RNase free water in 0.1-mL tubes (LTF Labortechnik, Wasserburg, Germany). Gene-specific primer pairs obtained from Eurofins MWG Operon were designed using Primer3 (version 0.4.0; Whitehead Institute for Biomedical Research, Cambridge, Massachusetts) and BLAST (National Center for Biotechnology Information). Features of primer pairs are listed in Table 1. The qPCR protocol was as follows: 3 minutes at 95°C, followed by 40 cycles of a 2-step PCR consisting of 5 seconds at 95°C (denaturation) and 20 seconds at 60°C (annealing and extension). Subsequently, melting curve analysis was performed from 50°C to 95°C to verify the presence of a single PCR product. In addition, the amplification of a single product of the expected size was confirmed using 2% agarose gel electrophoresis stained with GelRed nucleic acid gel stain (Biotium, Hayward, California). Ct-values of target genes and the reference gene (β-actin ) were obtained using Rotorgene Software 5.0 (Corbett Research). Relative quantification was performed using the 2-ΔΔCT-method (34). The mean of the control group was set to 1, and means and SD of the other treatment groups were scaled proportionally.
Table 1.
Characteristics of Gene-Secific Primers Used for qPCR Analysis
| Gene (NCBI Gene Bank) | Oligonucleotide Sequence Forward (5′-3′) Reverse (5′-3′) | PCR Product Size (bp) |
|---|---|---|
| Rat SREBP-1c | GGAGCCATGGATTGCACATT | 191 |
| (AF286470.2) | AGGAAGGCTTCCAGAGAGGA | |
| Rat SREBP-2 | CTGACCACAATGCCGGTAAT | 204 |
| (NM_001033694.1) | CTTGTGCATCTTGGCATCTG | |
| Rat NIS | GCTGTGGCATTGTCATGTTC | 219 |
| (NM_052983.2) | TGAGGTCTTCCACAGTCACA | |
| Rat FAS | AGGTGCTAGAGGCCCTGCTA | 281 |
| (X62888.1) | GTGCACAGACACCTTCCCAT | |
| Rat GPAT | CAGCGTGATTGCTACCTGAA | 194 |
| (U36771.2) | CTCTCCGTCCTGGTGAGAAG | |
| Rat HMGCR | CGAGCAAGTGATTACCCTGA | 223 |
| (NM_013134.2) | GTCTTGGTTCAGTCCTGGAT | |
| Rat LDLR | ACAGTGTCCTCCCAAGTCCAA | 221 |
| (NM_175762.2) | GCAAATGTGGATCTCGTCCTC | |
| Rat β-actin | GACCTCTATGCCAACACAGT | 154 |
| (NM_031144.2) | CACCAATCCACACAGAGTAC |
Preparation of cytosolic and nuclear extracts
FRTL-5 cells were seeded in 6-well plates at a density of 0.2 × 106 cells per well and incubated as indicated. After incubation, medium was aspirated and cytosolic and nuclear fractions were prepared by the Nuclear Extract Kit from Active Motif (La Hulpe, Belgium) according to the manufacturer's protocol. To prevent degradation of SREBPs, FRTL-5 cells were treated with 25 μg/mL of the calpain inhibitor N-acetyl-Leu-Leu-Norleucinal (ALLN) 3 hours before cell lysis according to Hua et al. (35). Protein concentrations of cytosolic and nuclear extracts were determined by the bicinchoninic acid protein assay kit (Interchim, Montluçon, France) with BSA as standard.
Immunoblotting
From the extracts, 10 μg (from the cytosolic fraction for NIS) and 20 μg protein (from both the cytosolic and nuclear fractions for precursor and nuclear SREBP-1 and SREBP-2) were separated on 7.5, 9, and 12.5% SDS-PAGE, respectively, and electrotransferred to a nitrocellulose membrane (Pall Corp, Pensacola, Florida). Loading of equal amounts of protein in each line was verified by Ponceau S (Carl Roth, Karlsruhe, Germany) staining. After incubating the membranes overnight at 4°C in blocking solution, membranes were incubated with primary antibodies against SREBP-1 (mouse monoclonal anti-SREBP-1; 1:100 dilution; Abcam, Cambridge, UK), SREBP-2 (rabbit polyclonal anti-SREBP-2; 1:200 dilution; Abcam), NIS (rabbit polyclonal high-affinity anti-NIS; 1:2000 dilution; kindly provided by Professor Nancy Carrasco, Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut), and β-actin (mouse monoclonal anti-β-actin; dilution 1:5000; Abcam) as a reference protein to control for adequate normalization at room temperature (RT). The membranes were washed, and then incubated with a horseradish peroxidase-conjugated secondary monoclonal antimouse-IgG antibody (Jackson ImmunoResearch, Suffolk, UK) for SREBP-1 and β-actin and polyclonal anti-rabbit-IgG antibody (Sigma-Aldrich, Taufkirchen, Germany) for SREBP-2 and NIS at RT. Afterward, blots were developed using ECL Plus (GE Healthcare, München, Germany). The signal intensities of specific bands were detected with a Bio-Imaging system (Syngene, Cambridge, UK ) and quantified using Syngene GeneTools software (nonlinear dynamics; Syngene).
Immunohistochemical analysis
The thyroid glands from male, 16- to 18-week-old homozygous TSHR−/− and wild-type mice (each n = 6) were excised and fixed in 4% paraformaldehyde (in 1×PBS) at 4°C for 2 hours, followed by cryoprotection in 30% sucrose solution (in 1× PBS). The fixed thyroid glands were embedded in Tissue-Tek (Hartenstein, Würzburg, Germany) and used for preparation of cryosections (20 μM thick slices). The cryosections were incubated with primary antibodies against NIS (rabbit polyclonal high-affinity anti-NIS; 1:500 dilution; kindly provided from Professor Nancy Carrasco), SREBP-1 (rabbit polyclonal anti-SREBP-1; 1:300 dilution; Santa Cruz Biotechnology, Heidelberg, Germany), SREBP-2 (rabbit polyclonal anti-SREBP-2; 1:300 dilution; Santa Cruz), and β-actin (mouse monoclonal anti-β-actin; 1:500 dilution; Abcam) overnight at 4°C in 0.5% Triton X-100 and 10% goat serum in 1× PBS. After washing, the sections were incubated with fluorescent-conjugated secondary IgG antibodies (Cy2-conjugated antimouse, 1:300 dilution; Cy3-conjugated antirabbit 1:300; both from Abcam) in 0.5% Triton X-100 and 10% goat serum in 1× PBS at RT for 1 hour. Sections were mounted in Aqua Polymount (Polyscience, Niles, Illinois) and analyzed using a TCS SP2 confocal microscope (Leica, Deerfield, Illinois).
Generation of plasmids
Using cDNA and genomic DNA sequences from NCBI GenBank (accession nos. NM_052983 and AABR06086855) a 3089-bp rat NIS promoter fragment denoted pGL4.10-rNIS-3089 (from −3002 to +87 relative to transcription start site) was PCR amplified from rat BAC clone CH230–277G13 (BACPAC Resources, Oakland, California) using the primer pair 5′-TCAAGATCTCATGGAGACAGGTGACTCG-3′ (forward) and 5′-ATCCTCGAGTACCGAATGCGAATCTTTGCA-3′ (reverse). The rat NIS promoter truncation constructs (pGL4.10-rNIS-1974, pGL4.10-rNIS-1109, pGL4.10-rNIS-472, and pGL4.10-rNIS-199) were PCR amplified from the parental pGL4.10-rNIS-3089 promoter construct using different 5′-primers and a common 3′-primer (Table 2). All generated PCR fragments were subcloned into BglII and XhoI sites of pGL4.10 [luc2] vector (Promega, Mannheim, Germany) upstream of the luciferase reporter gene. To further delineate the putative functional SRE, 2 additional NIS reporter gene constructs pGL4.23-rNIS_SRE-7 (from +16 to −57) and pGL4.23-rNIS_SRE+19/+37/+59 (from +83 to −2) were generated by PCR amplification and subcloning into BglII and XhoI sites of pGL4.23 [luc2/minP] vector (Promega) which contains the minimal promoter minP followed by the luciferase reporter gene luc2.
Table 2.
Oligonucleotides Used for PCR Amplification of Promoter Constructs from Rat NIS
| Oligonucleotide | Oligonucleotide Sequence (5′-3′) | PCR Product Size (bp) |
|---|---|---|
| rNIS_BglII-F | TCAAGATCTCATGGAGACAGGTGACTCG | |
| rNIS_XhoI-3089R | ATCCTCGAGTACCGAATGCGAATCTTTGCA | 3089 |
| rNIS_XhoI-1974R | ATCCTCGAGCATGAACATAATCCAGGCC | 1974 |
| rNIS_XhoI-1109R | ATCCTCGAGGCATGGCAATCCAGCCTAC | 1109 |
| rNIS_XhoI-472R | TATCTCGAGGGAGAAAGGTAGATGCTCTC | 472 |
| rNIS_XhoI-199R | TATCTCGAGCCAGGACCGAAAGGGTGCG | 199 |
| rNIS_Bglll-SRE-7-F | AAGAGATCTCAGCGCGAGTCACCGCT | |
| rNIS_XhoI-SRE-7-R | TATCTCGAGCTGACCCCGGAGTTCAA | 72 |
| rNIS_Bglll-SRE+19/+37/+59-F | TCAAGATCTGAGACAGGTGACTCGGTGA | |
| rNIS_XhoI-SRE+19/+37/+59-R | TATCTCGAGCAGCGGTGACTCGCGCT | 85 |
rNIS, rat NIS; F, forward; R, reverse.
The positive control vector pGL4.23–2x hLDLR-SRE (XhoI-TCGAGAAGACATTTGAAAATCACCCCACTGCAAATTGAAAATCACCCCACTGCAAACTCCTCCC and HindIII-AGCTGGGAGGAGTTTGCAGTGGGGTGATTTTCAATTTGCAGTGGGGTGATTTTCAAATGTCTTC) and pGL4.23–2x SRE+59 (XhoI-TCGAACGTCCTCCGCATCCTCTCCTCACCGAGTCCGCATCCTCTCCTCACCGAGTCACCTGTCT and HindIII-AGCTAGACAGGTGACTCGGTGAGGAGAGGATGCGGACTCGGTGAGGAGAGGATGCGGAGGACGT) and pGL4.23–2x SRE-481 (XhoI-TCGAGTGCCCTTGTACAGGTGGGGAGTCCTAAAGGTACAGGTGGGGAGTCCTAAAGCAGAAGAA and HindIII-AGCTTTCTTCTGCTTTAGGACTCCCCACCTGTACCTTTAGGACTCCCCACCTGTACAAGGGCAC) were prepared by annealing the oligonucleotides and subcloning into XhoI and HindIII sites of pGL4.23 [luc2] vector (Promega) upstream of the luciferase reporter gene.
cDNA expression plasmids of rat nuclear SREBP-1c and SREBP-2 were PCR amplified from FRTL-5 cDNA as template using the following primer: SREBP-1c (forward: 5′-TATAAGCTTATGGATTGCACATTTGAAGACA-3′, reverse: 5′-TAAGGATCCTCACATGCCTCGGCTATGTGAA-3′) and SREBP-2 (forward: 5′-TATAAGCTTATGGATGAGAACAGCGAGCT-3′, reverse: 5′-TATGGATCCTCAGTCCGGTTCATCCTTGACC-3′) and subcloned into the HindIII and BamHI digested pcDNA3.1 vector (Invitrogen). To confirm the integrity of all plasmids, the cloned DNA fragments were sequenced.
Site-directed mutagenesis of NIS promoter reporter constructs
Mutated NIS reporter gene constructs were generated by selectively introducing a mutation in the putative SRE using the QuikChangeXL Site-Directed Mutagenesis Kit from Stratagene Europe (Amsterdam, The Netherlands) according to the manufacturer's protocol using the following oligonucleotides: SRE+59mut (forward: 5′-ACAGGTGACTCGGTGATTAGAGGATGCGGAGGAC-3′, reverse: 5′-GTCCTCCGCATCCTCTAATCACCGAGTCACCTGT-3′), SRE+37mut (forward: 5′-GATGCGGAGGACGTCTGTGACTCTCGGTCAGTGG-3′,reverse: 5′-CCACTGACCGAGAGTCACAGACGTCCTCCG CATC-3′), SRE+19mut (forward: 5′-GACTCTCGGTCAGTAAGAGAGTCGCAGCGCGAGT-3′, reverse: 5′-ACTCGCGCTGCGACTCTCTTACTGACCGAGAGTC-3′). The underlined sequences indicate the mutated nucleotides. The mutant constructs were controlled for the intended mutations and the absence of any unexpected mutations by DNA sequencing.
Transient transfection
For transient transfection, HepG2 cells were seeded in 96-well culture plates at a density of 4–5 × 104 per well. After reaching a confluence of 70%–80%, cells were transiently transfected with 50 ng of the generated reporter gene constructs and cotransfected with 50 ng of either rat nuclear SREBP-1c or rat nuclear SREBP-2 expression plasmids or 50 ng of the empty vector (pcDNA3.1) using FuGENE 6 transfection reagent (Roche Diagnostics, Mannheim, Germany) for 12 hours according to the manufacturer's protocol. The transfection reagent-DNA ratio (microliters FuGENE 6 to micrograms plasmid DNA) was 3:1. As a positive control, a 2× hLDLR-SRE reporter gene containing 2 copies of the SRE from human LDL receptor in front of a luciferase reporter gene was also transfected. As negative controls, cells were transfected with 50 ng of either pGL4.10 [luc2] vector (Promega) or pGL4.23 [luc2/minP] vector (Promega). After transfection, cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) for 24 hours. Afterward, cells were washed with PBS and lysed with passive lysis buffer (Promega). Luciferase activities were determined with Beetle-Juice Firefly luciferase buffer (PJK, Kleinblittersdorf, Germany) according to the manufacturer's instructions using a Mithras LB940 luminometer (Berthold Technologies, Bad Wildbad, Germany).
For transient transfection of FRTL-5 cells, cells were seeded in 24-well culture plates at a density of 0.1 × 106 per well. At a confluence of 70%–80%, cells were transiently transfected with 250 ng of the generated reporter gene constructs and cotransfected with 250 ng expression plasmids using FuGENE 6 transfection reagent for 24 hours. After transfection, cells were cultured in 6H medium for an additional 24 hours. Afterward, cells were lysed with 100 μL passive lysis buffer (Promega), and 20 μL of each lysate was used for luciferase assay as described above.
Data were normalized for transfection efficiency by dividing the luciferase activity of the generated reporter constructs or control vectors by the protein concentration of cell lysates. Preliminary experiments revealed that a cotransfected Renilla luciferase plasmid as internal control was unsuitable for normalization of transfection efficiency because Renilla luciferase activity was influenced by rat nuclear SREBP-1c and SREBP-2, respectively, expression plasmids (10.5-fold and 11.3-fold for SREBP-1c and SREBP-2, respectively; data not shown), an observation that was also reported by Murphy et al. (36). Therefore, protein concentration of cell lysates as determined by the BCA assay was used for normalization as described previously (36). Normalized luciferase activities were calculated by dividing the luciferase activity of each construct by that of the corresponding empty vectors, pGL4.10 or pGL4.23 (37). Results are shown relative to cells transfected with the empty vector pcDNA3.1, which was set to 1.
EMSA
EMSA experiments were carried out as described recently (38) with minor modifications. Briefly, rat nuclear SREBP-1c and SREBP-2 proteins, respectively, were generated from the expression vectors by in vitro transcription/translation using TNT Quick Coupled Transcription/Translation Systems (Promega) according to the manufacturer's protocol. The following oligonucleotides were annealed with annealing buffer (10 mM Tris, 1 mM EDTA, 0.1 mM NaCl; pH 8.0): NIS-SRE+59 (forward: 5′-ACGTCCTCCGCATCCTCTCCTCACCGAGTCACCT-3′, reverse: 5′-AGACAGGTGACTCGGTGAGGAGAGGATGCGGAGG-3′), NIS-SRE+59mut (forward: 5′-ACGTCCTCCGCATCCTCTAATCACCGAGTCACCT-3′, reverse: 5′-AGACAGGTGACTCGGTGATT AGAGGATGCGGAGG-3′), human LDLR-SRE as specific control and for competition (forward: 5′-GTAGATTTTTGAAAATCACCCCACTGCAAACTCC-3′, reverse: 5′-GGGAGGAGTTTGCAGTGGGGTGATTTTCAAAAAT-3′), and mutated human LDLR-SRE as nonspecific control (forward: 5′-GTAGATTTTTGAAAGTCAAACCGTTGCAAACTCC-3′, reverse: 5′-GGGAGGAGTTTGCAACGGTTTGACTTTCAAAAAT-3′). After annealing, 1000 ng double-stranded DNA-probes were labeled with 0.05 mM digoxigenin (DIG)-ddUTP in 1× labeling buffer (0.2 M potassium cacodylate, 25 mM Tris-HCl, 0.25 ng/mL BSA; pH 6.6), 5 mM CoCl2, 20 U/μL terminal transferase (Roche) and incubated for 15 minutes at 37°C. Then 2 μL of either in vitro-translated rat nuclear SREBP-1c or rat nuclear SREBP-2 proteins were incubated with 8 ng DIG-labeled probes and 6.25-, 12.5- and 25-fold molar excess (SREBP-1c) or 3.13-, 6.25- and 12.5-fold molar excess (SREBP-2) of unlabeled specific probes (human LDLR-SRE) for competition in 1 μg poly d(I-C), 1 μL poly l-lysine, and EMSA binding buffer (10 mM Tris-HCl, 120 mM KCl, 0.5 mM EDTA, 0.1% Triton-X-100, 12.5% glycerol, 0.2 mM dithiothreitol) for 20 minutes at RT. The protein-DNA complexes were subjected to electrophoresis on 6% native polyacrylamide gels and transferred to a positive charged nylon membrane. The DIG-labeled DNA was detected by chemiluminescence using Anti-Digoxigenin-AP Conjugate and CSPD (disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro)tricyclo [3.3.1.13,7]decan}-4-yl)phenyl phosphate) (both from Roche), according to the manufacturer's protocol, and a Bio-Imaging system.
ChIP assay
ChIP was performed using the Magna ChIP G from Millipore (Schwalbach/Taunus, Germany) with minor modifications. For ChIP experiments, FRTL-5 cells were seeded at a density of 3 × 106 in 150-mm cell culture dishes and treated as indicated. After treatment of FRTL-5, formaldehyde was added at a final concentration of 1% for 10 minutes at RT to cross link proteins to DNA, and glycine was added subsequently at a final concentration of 125 mM for 5 minutes with agitation to quench unreacted formaldehyde. Cell lysis, sonication to shear DNA (five 15 sec pulses), collection of sheared cross-linked chromatin, and immunoprecipitation of cross-linked protein/DNA were performed according to the manufacturer's protocol (Millipore). For immunoprecipitations, 2 μg of antirabbit SREBP-1 or SREBP-2 antibodies (Santa Cruz) or preimmune rabbit IgG antibody (Santa Cruz) as negative control and 20 μL fully suspended protein G magnetic beads were used. Subsequently, protein-DNA complexes were eluted and cross links of protein-DNA complexes were reversed to free DNA according to the manufacturer's protocol; DNA was purified using QIAquick PCR purification kit (QIAGEN, Chatsworth, California). The purified samples were subjected to PCR amplification with a primer pair (forward, CATGGAGACAGGTGACTCG; reverse, GCTGACCCCGGAGTTCAAT) flanking a 144-bp fragment corresponding to the rat NIS 5′-untranslated region (UTR) containing the SRE+59. In addition, the same samples were subjected to PCR amplification using a control primer pair (forward, TGCTTGCATAGCACCAGGAA; reverse, GGAGAAAGCAGAGGACATCA) flanking a 168-bp fragment corresponding to a random DNA fragment of rat gDNA. PCR products were separated by 2% agarose gel electrophoresis. In addition, the same samples were also amplified by quantitative real-time RT-PCR analysis using 5 μL DNA, 10 μL KAPA SYBR FAST qPCR Universal Mastermix, 0.4 μL each of 10 mM forward and reverse primers (sequences as abovementioned) and 4.2 μL DNase/RNase-free water using the Rotorgene 2000 system as described above.
RNA interference
Knockdown variants of FRTL-5 cells expressing low levels of SREBP-1 and SREBP-2, respectively, were produced by small interfering (si) RNA-mediated gene knockdown. For this purpose, FRTL-5 cells were seeded in 6H medium in 6-well plates. At a confluence of 70%–80%, cells were transfected using the Lipofectamine 2000 Reagent (Invitrogen) and gene-specific Stealth RNA interference molecules (SREBP-1/-2 knockout small interfering RNA [siRNA]; Invitrogen) targeting rat SREBP-1 and SREBP-2, respectively, at a final concentration of 50 nM for 24 hours according to the manufacturer's protocol. After transfection, medium was replaced with fresh 6H medium, and cells were incubated for an additional 24 hours and 48 hours in 6H medium, before cytosolic and nuclear extracts were isolated as described above. Sequences were as follows (sense, antisense): SREBP-1 (5′-AGGCCAUCGACUACAUCCGCUUCUU-3′, 5′-AAGAAGCGGAUGUAGUCGAUGGCCU-3′), and SREBP-2 (5′-GGCUUCUUGGCUAGCUACUUCUUAA-3′, 5′-UUAAGAAGUAGCUAGCCAAGAAGCC-3′). To monitor for unspecific knockdown effects, control cells were transfected with BLOCK-iT Alexa Fluor Red Fluorescent Control (Control siRNA), which was not homologous to any mammalian gene sequence.
Iodide uptake
Iodide uptake was measured in FRTL-5 cells seeded into 6-well plates according to Tran et al. (17) with minor modifications. After treatment of the cells as indicated, the medium was removed and 2.0 mL of Hank's buffered salt solution containing Na125I (0.3 μCi/mL) were added to each well. For estimation of NIS-specific iodide uptake, potassium perchlorate was added to the incubation medium at a final concentration of 1 mM. Cells were incubated at 37°C for 60 min. The incubation was terminated by aspiration of the medium. After rinsing 2 times with ice-cold Hank's buffered salt solution, cells were lysed with 2 mL 0.5 M NaOH and incubated at room temperature for 30 minutes. The cell lysate from each well was transferred in a counter vial for 125I counting.
Statistical analysis
Numerical data were analyzed by one-way ANOVA using the Minitab Statistical Software Rel. 13.0 (Minitab, State College, Pennsylvania). Differences of P < .05 were considered to be significant.
Results
TSH stimulates expression of pSREBPs and nSREBPs and expression of SREBP target genes in FRTL-5 cells
Initial experiments in FRTL-5 cells incubated under growth conditions (6H medium) revealed that transcripts of SREBP-1c and SREBP-2, and premature and nuclear forms of SREBP-1 and SREBP-2, are detectable in FRTL-5 cells, indicating that SREBPs are expressed in this cell line. To study whether the master regulator of the thyroid, TSH, influences expression of SREBP-1c and -2, FRTL-5 cells were treated without (control) or with TSH (10 U/L) for 6, 12, and/or 24 hours. For analysis of protein levels of SREBP-1 and SREBP-2, FRTL-5 cells were treated during the final 3 hours with N-acetyl-Leu-Leu-Norleucinal (ALLN; 25 mg/L), a calpain inhibitor, to prevent degradation of SREBPs. As shown in Figure 1, A and B, the mRNA levels of SREBP-1c and SREBP-2 were increased approximately 3.0- and 1.5-fold, respectively, in response to TSH as early as 6 hours after beginning treatment. Although the effect of TSH on the mRNA level of SREBP-1c was even more pronounced at 12 and 24 hours of treatment, the mRNA level of SREBP-2 was not increased by TSH at 12 and 24 hours of treatment. The levels of pSREBP-1 and pSREBP-2 were increased by TSH approximately 4- and 1.2-fold, respectively (Figure 1, C and D). Levels of nSREBP-1 and nSREBP-2 were elevated by TSH approximately 32- and 1.8-fold, respectively. These findings strongly indicated that SREBPs are functional in FRTL-5 cells and that TSH stimulates expression of pSREBPs and nSREBPs. Whether the elevated levels of nSREBPs in response to TSH are due to an increased maturation of pSREBPs or a reduced degradation of nSREBPs in FRTL-5 cells remains to be demonstrated.
Figure 1.
TSH Stimulates Expression and Maturation of SREBPs in FRTL-5 Cells FRTL-5 cells were grown in 6H medium until 70%–80% confluent, then switched to 5H medium (without TSH) for 24 hours, and subsequently treated without (control) or with TSH (10 U/L) for 6, 12, and/or 24 hours. A and B, Relative mRNA levels of SREBP-1c and SREBP-2, respectively, at different time points. C and D, Relative protein levels of precursor (p; left) and nuclear (n; right) forms of SREBP-1 and SREBP-2 determined in cytosolic and nuclear extracts, respectively, at 24 hours. Representative immunoblots are shown at the top, and results from densitometric analysis are given below. E, Relative mRNA levels of FAS, LDLR, GPAT, and HMGCR at different time points. A–E, Bars represent means ± SD from 3 independent experiments and are expressed as fold of control (−TSH). *, Different from control, P < .05.
To study whether the activation of SREBP-1c and SREBP-2 causes up-regulation of classical cholesterogenic and lipogenic genes in FRTL-5 thyrocytes, like in hepatocytes, FRTL-5 cells were treated without (control) or with TSH (10 U/L) for 6, 12, and 24 hours. In agreement with the TSH-mediated increase of nuclear SREBP-1, we observed about 2-fold increases in the mRNA levels of classical SREBP-1c target genes, such as FAS and GPAT, as early as after 6 hours of TSH treatment (Figure 1E). In addition, we observed elevations in the mRNA levels of SREBP-2 target genes (LDLR, HMGCR), which were stronger (LDLR, 5-fold; HMGCR, 3-fold) than those of the SREBP-1c target genes. The temporal pattern of TSH-mediated induction of SREBP-1c target genes was clearly different from that of SREBP-2 target genes. Whereas the mRNA levels of FAS and GPAT were increased only at 6 hours of TSH treatment and decreased from there, the mRNA levels of LDLR and HMGCR remained elevated from 6–24 hours of TSH treatment (Figure 1E). Thus, our results indicated that TSH, by activating SREBP-1c and SREBP-2, stimulates classical lipogenic and cholesterogenic pathways, which are known to be regulated by SREBP-1c and SREBP-2, respectively, in major lipid-synthesizing tissues.
Sterols regulate expression and maturation of SREBPs in FRTL-5 cells
To further investigate whether SREBP maturation in FRTL-5 cells is regulated similarly as in liver cells, FRTL-5 cells were treated with 25-hydroxycholesterol (25-HC; 5 μmol/L) or Chol (5 μmol/L) in the absence or presence of TSH (10 U/L) for 24 hours. Both, 25-HC and, to a lesser extent, Chol block the movement of the SCAP-SREBP complex to the Golgi by binding to Insig and SCAP, respectively, thereby inhibiting proteolytic processing of pSREBPs in the Golgi, which leads to a lowering of nSREBP levels. During the last 3 hours of treatment, FRTL-5 cells were treated with ALLN (25 mg/L) to prevent degradation of SREBPs (Figure 2, A–F). As shown in Figure 2, C and F, nSREBP-1 and nSREBP-2 in FRTL-5 cells were reduced by treatment with 25-HC (by ∼80%) and, less pronounced, with Chol (by ∼25%), both in the presence and absence of TSH. In contrast, the protein levels of pSREBP-1 and pSREBP-2 were increased by 25-HC by about 2.5- and 1.5-fold, respectively (Figure 2, B and E).
Figure 2.

Sterols Regulate Expression and Maturation of SREBPs in FRTL-5 Cells FRTL-5 cells were grown in 6H medium until 70%–80% confluent, and subsequently treated with 25-HC (5 μmol/L) or Chol (5 μmol/L) in the absence or presence of TSH (10 U/L) for 24 hours. A–F, Relative protein levels of precursor (p) and nuclear (n) SREBP-1 (A–C) and SREBP-2 (D–F) determined in cytosolic and nuclear extracts, respectively. Representative immunoblots are shown in panels A and D, and results from densitometric analysis for precursor and nuclear forms are given in panels B and E and C and F, respectively. Bars represent means ± SD from 3 independent experiments and are expressed as fold of control (−TSH −Chol). Bars with different lowercase letters differ, P < .05.
The cAMP agonist forskolin mimics the effects of TSH on expression of SREBPs and expression of SREBP target genes in FRTL-5 cells
TSH is well known to mediate its stimulatory effect on expression of genes encoding proteins important for thyroid function, by activating the adenylate cyclase/cAMP pathway. To explore whether the increased expression of SREBP-1c and SREBP-2 by TSH is also mediated by the adenylate cyclase/cAMP pathway, we investigated the effect of the synthetic cAMP agonist forskolin on expression of SREBP-1c and SREBP-2 in FRTL-5 cells. For this, FRTL-5 cells were treated without (control) or with forskolin (10 μmol/L) for 6, 12, and/or 24 hours. As shown in Figure 3, A and B, forskolin markedly increased the mRNA level of SREBP-1c (5-fold) and slightly that of SREBP-2 (2-fold) as early as 6 hours after start of treatment. In contrast to SREBP-1c, the mRNA level of which remained increased at 12 and 24 hours of forskolin treatment, the mRNA level of SREBP-2 was not elevated by forskolin at 12 and 24 hours of forskolin treatment. The levels of pSREBP-1 and pSREBP-2 were increased by forskolin approximately 3.5- and 1.8-fold, respectively (Figure 3, C and D). Levels of nSREBP-1 and nSREBP-2 were elevated by forskolin approximately 95- and 4-fold, respectively (Figure 3, C and D). These findings strongly indicated that forskolin, like TSH, stimulates both gene transcription and maturation of both SREBP isoforms in FRTL-5 cells, and that the stimulatory effect of TSH/forskolin on SREBP expression is mediated via cAMP-response elements (CREs) present in the regulatory region of the SREBP genes. In order to identify possible CRE binding sites we conducted a bioinformatical study of rat SREBP-1 and SREBP-2 promoter regions with MatInspector software (Genomatix, Munich, Germany). The analysis, indeed, revealed three putative CREs each in the rat SREBP-1 promoter (at positions −1695 [matrix similarity, 0.91], −1644 [matrix similarity, 0.91], and −2047 [matrix similarity, 1]) and the rat SREBP-2 promoter (at positions −142 [matrix similarity, 0.95], −384 [matrix similarity, 1], and −474 [matrix similarity, 0.91]). Although their functionality remains to be demonstrated, these findings indicated that SREBP-1 and SREBP-2 are regulated by TSH through the adenylate cyclase/cAMP pathway.
Figure 3.
The cAMP Agonist Forskolin (Forsk) Mimics the Effects of TSH on Expression and Maturation of SREBPs and Expression of SREBP Target Genes in[b] FRTL-5 Cells FRTL-5 cells were grown in 6H medium until 70%–80% confluent, then switched to 5H medium (without TSH) for 24 hours, and subsequently treated without (control) or with forskolin (10 μmol/L) for 6, 12, and/or 24 hours. A and B, Relative mRNA levels of SREBP-1c and SREBP-2, respectively, at different time points. C and D, Relative protein levels of precursor (p) and nuclear (n) forms of SREBP-1 and SREBP-2 determined in cytosolic and nuclear extracts, respectively, at 24 hours. Representative immunoblots are shown at the top, and results from densitometric analysis are given below. E, Relative mRNA levels of FAS, LDLR, GPAT, and HMGCR. A–E, Bars represent means ± SD from 3 independent experiments and are expressed as fold of control (−Forskolin). *, Different from control, P < .05.
To elucidate whether the activation of SREBP-1c and SREBP-2 by forskolin also causes an induction of cholesterogenic and lipogenic genes in FRTL-5 thyrocytes, we treated FRTL-5 cells without (control) or with forskolin (10 μmol/L) for 6, 12, and 24 hours. As shown in Figure 3E, the mRNA levels of FAS, GPAT, LDLR, and HMGCR in FRTL-5 cells were increased by treatment with forskolin. Interestingly, the extent of induction of these genes as well as the temporal pattern of induction was almost identical to that observed with TSH (Figure 1E). Thus, these results obtained from experiments with the cAMP activator forskolin confirmed our observations that the SREBP-1c and SREBP-2 pathways are regulated through the TSH/TSHR/cAMP signaling pathway.
The expression of SREBP-1 and SREBP-2 in the thyroid is reduced in TSHR-deficient mice
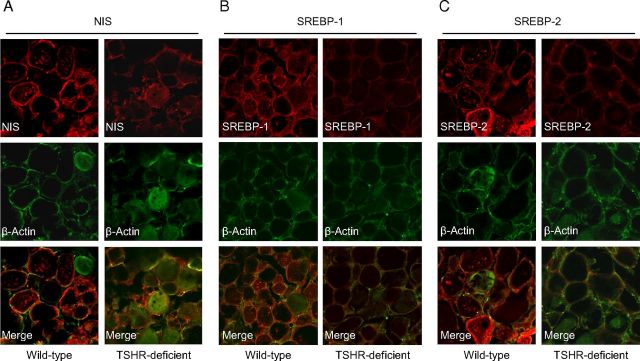
To study whether SREBP isoforms are also regulated by TSH in the thyroid in vivo, we studied expression of SREBPs in the thyroid of TSHR-deficient mice. In these mice, TSH-dependent gene regulation is blocked due to the lack of TSHR expression. TSHR-deficient mice are profoundly hypothyroid, with no detectable thyroid hormone and elevated TSH, and die within 1 week of weaning unless fed a diet supplemented with thyroid powder (100 mg/kg diet) from day 21 postpartum (39), which was also done in this study. At an age of 16–18 weeks, the mice were humanely destroyed and the thyroids were excised for immunohistochemical analysis. As shown in Figure 4, β-actin staining of thyroidal cells revealed a mostly normal follicle structure but slightly fewer follicle-associated cells within the thyroid gland in TSHR-deficient mice compared with wild-type mice, which is in line with observations from Marians et al. (39). Also in agreement with recent observations (39), thyroid sections incubated with a NIS-specific antibody showed a dramatically reduced red fluorescence in the thyroid epithelium of thyroid glands from TSHR-deficient mice compared with wild-type mice (Figure 4A). In addition, thyroid sections incubated with SREBP-1- and SREBP-2-specific antibodies displayed a markedly less pronounced red fluorescence in the thyroid epithelium of thyroid tissues from TSHR-deficient mice compared with wild-type mice, indicating a lower expression of SREBP-1 (Figure 4B) and SREBP-2 (Figure 4C), respectively, in TSHR-deficient mice. This indicated that expression of SREBP-1c and SREBP-2 in the thyroid gland is dependent on TSH also in vivo.
Figure 4.
Expression of SREBP-1 and SREBP-2 in the Thyroid Is Reduced in TSHR-Deficient Mice A–C, Cryosections were prepared from wild-type and TSHR-deficient mice (each n = 6), and incubated with primary antibodies against NIS, SREBP-1, and SREBP-2. Representative cryosections are shown for one animal per genotype. Images for the other animals revealed similar results.
Sterol-mediated inhibition of SREBP maturation and/or SREBP knockdown reduces expression of NIS and NIS-specific iodide uptake
To explore whether SREBPs also influence the expression of genes involved in thyroid hormone synthesis, FRTL-5 cells were treated with 25-HC (5 μmol/L), which is known to decrease nSREBP-1c and nSREBP-2 levels through inhibition of pSREBP-1c and pSREBP-2 maturation, both in the presence and absence of either TSH (10 U/L) or forskolin (10 μmol/L) for 24 hours. As expected, protein levels of NIS were markedly elevated by 24-hour treatment with TSH (Figure 5, A and B) and forskolin (Figure 5, C and D). However, when FRTL-5 cells were treated with 25-HC, protein levels of NIS were strongly reduced, both in the presence and absence of TSH/forskolin (Figure 5, A–D). This finding provided indication that both basal and cAMP-stimulated expression of NIS is regulated by SREBP-1c and SREBP-2. To confirm the importance of SREBP-1c and SREBP-2 in regulating NIS expression, we studied the expression of NIS in FRTL-5 cells with a targeted knockdown of either SREBP-1 or SREBP-2. Transfection of FRTL-5 cells with knockdown siRNAs targeting SREBP-1 caused a reduction in the protein levels of pSREBP-1 and nSREBP-1 by about 73 and 65%, respectively, after 24 hours compared with cells transfected with control siRNAs (Figure 5E). Likewise, transfection of FRTL-5 cells with knockdown siRNAs targeting SREBP-2 resulted in a decrease in the protein levels of pSREBP-2 and nSREBP-2 by about 50 and 20%, respectively, after 24 hours compared with cells transfected with control siRNAs (Figure 5E). Similar as observed in response to inhibition of SREBP maturation by 25-HC, expression of NIS was reduced by about 21 and 28% after 24 hours and 32 and 41% after 48 hours in response to siRNA-mediated knockdown of SREBP-1 and SREBP-2, respectively (Figure 5F), which again indicated that NIS expression is regulated by SREBP-1c and SREBP-2.
Figure 5.
Sterol-Mediated Inhibition of SREBP Maturation and/or SREBP Knockdown Reduces Expression of NIS and NIS-Specific Iodide Uptake A–D, FRTL-5 cells were grown in 6H medium until 70%–80% confluent, and subsequently treated with 25-HC (5 μmol/L) both in the presence and absence of either TSH (10 U/L) or forskolin (10 μmol/L) for 24 hours and analyzed for relative protein levels of NIS. Representative immunoblots are shown, and results from densitometric analysis are given below. Bars represent means ± SD from 3 independent experiments. Bars with different lowercase letters differ, P < .05. E, Relative protein levels of precursor (p) and nuclear (n) forms of SREBP-1 and SREBP-2 determined in cytosolic and nuclear extracts, respectively, in FRTL-5 cells transfected with knockdown siRNAs targeting either SREBP-1 or SREBP-2 and subsequently treated with 6H medium for 24 hours. F, Relative protein levels of NIS in FRTL-5 cells transfected with knockdown siRNAs targeting either SREBP-1 or SREBP-2 and subsequently treated with 6H medium for 24 and 48 hours. E and F, Representative immunoblots are shown, and results from densitometric analysis are given below (E) and at the right (F). Bars represent means ± SD from 3 independent experiments. *, Different from control (Control siRNA). G, FRTL-5 cells were grown in 6H medium until 70%–80% confluent, then switched to 5H medium (without TSH) for 5 days, and subsequently treated with 25-HC (5 μmol/L) in the absence or presence of TSH (10 U/L) for 24 hours. To calculate NIS-specific I uptake, Na125I uptake by FRTL-5 cells was measured both in the presence and absence of K-perchlorate (1 mmol/L). Bars represent means ± SD from 1 of 3 independent experiments, each performed in triplicate. Bars with different lowercase letters differ, P < .05.
To test whether inhibition of pSREBP maturation influences NIS-specific Na125I-uptake into FRTL-5 cells, FRTL-5 cells were treated with 25-HC (5 μmol/L), to reduce nSREBP-1c and nSREBP-2 levels. To calculate NIS-specific I uptake, Na125I uptake by FRTL-5 cells was measured both, in the presence and absence of K-perchlorate (1 mmol/L). We observed that NIS-specific I uptake was reduced by about 20% in response to treatment of FRTL-5 cells with 25-HC (Figure 5G). Because I-uptake into thyroid follicular epithelial cells is a prerequisite step for thyroid hormone synthesis, this finding strongly suggested that SREBP-1c and -2 are important for thyroid hormone synthesis.
Nuclear SREBP-1c and SREBP-2 stimulate transcriptional activity of rat NIS 5′-UTR in HepG2 and FRTL-5 cells
To evaluate whether the putative SREs in the rat NIS promoter are responsive to SREBP-1c and SREBP-2, we generated 5 plasmid constructs containing 5′-deleted fragments of rat NIS promoter upstream of a firefly luciferase reporter gene. These constructs were transiently transfected into HepG2 cells with cotransfection of either SREBP-1c or SREBP-2 expression plasmids or empty vector (pcDNA3.1), and the luciferase activity of the NIS promoter constructs was assayed after 24 hours. As shown in Figure 6, there was a significant increase in luciferase activity in response to SREBP-1c and SREBP-2 in cells transfected with NIS promoter construct rNIS-3089 containing 10 putative SREs (sequences and positions are shown in Figure 6C) and the NIS upstream enhancer (NUE) region, which is located between −2495 and −2264 bp of the 5′-flanking region of the NIS gene and reported to be essential for thyroid-specific TSH-dependent regulation of the NIS gene (51). This indicated that the NIS promoter is transcriptionally regulated by SREBP-1c and SREBP-2. The luciferase reporter activities of the NIS promoter constructs rNIS-1974, rNIS-1109, rNIS-472, and rNIS-199, in which the 6 most distal SREs relative to the transcription start site were serially deleted and the NUE region was absent, also showed a marked response to SREBP-1c and SREBP-2, indicating that the SRE at −2660, −1764, −1644, −670, −481, and −211 in the NIS promoter are not functional (Figure 6). Nevertheless, deletion of the SREs at −670 and −481 (construct rNIS-472) caused a marked reduction in the reporter response to SREBP-1c and SREBP-2. This indicated that the region containing these SREs is not decisive but is at least required to achieve the maximal NIS promoter activity. One explanation for this observation might be that this region contains binding sites for transcriptional coactivators that are necessary for the proper function of SREBPs. In addition, these results suggested that the NUE region is not of importance for SREBP-dependent transcriptional regulation of the NIS gene.
Figure 6.
Nuclear SREBP-1c and SREBP-2 Stimulate the[b] 5′-Flanking Region of the Rat NIS A, HepG2 cells were transiently transfected with rat NIS promoter constructs pGL4.10-rNIS-3089, -1974, -1109, -472, -199 and cotransfected with either pcDNA3.1 (empty vector) or plasmids expressing nuclear forms of rat SREBP-1c and rat SREBP-2 for 12 hours. B, HepG2 cells were transiently transfected with pGL4.23–2× hLDLR-SRE (positive control) and cotransfected with either pcDNA3.1 (empty vector) or plasmids expressing nuclear forms of rat SREBP-1c and rat SREBP-2 for 12 hours. After transfection, medium was changed to RPMI 1640 supplemented with 10% FBS for 24 hours. Afterward, cells were lysed, and luciferase activities were measured. Bars represent means ± SD from at least 3 independent experiments each performed in triplicate. C, Alignment of consensus SRE sequence from human LDLR promoter with sequences of the putative SRE in the rat NIS promoter. Matching nucleotides are underlined.
To clarify whether one or more of the remaining SREs are functional, we generated 2 further NIS reporter gene constructs, construct SRE-7 and construct SRE+19/+37/+59, both of which were lacking the minimal promoter region located within −199 and −110 bp containing binding sites for several thyroid-specific transcription factors, such as thyroid transcription factor (TTF)-2 (40, 41). Reporter construct SRE-7 contained only a small fragment of the NIS promoter including the SRE at −7 and the 5′-UTR (until +16) of the rat NIS gene, whereas construct SRE+19/+37/+59 contained only the 5′-UTR of rat NIS including the three SREs at +19, +37, and +59 (Figure 7A). In cells transfected with reporter construct SRE-7, no response at all to either SREBP-1c or SREBP-2 could be observed, indicating that the SRE at −7 of rat NIS promoter is also not functional (Figure 7A). In contrast, in cells transfected with construct SRE+19/+37/+59, the luciferase reporter activity was activated by both SREBP-1c and SREBP-2. This indicated that one or more of the SREs in the 5′-UTR of the rat NIS gene are functional. To find out the decisive SRE of the NIS 5′-UTR, we generated three mutant versions of construct SRE+19/+37/+59 in which either SRE at +19, +37, or +59 was selectively mutated. When cells were transfected with the mutant versions of construct SRE+19/+37/+59 harboring a mutation in either the SRE at +19 or the SRE at +37, the response of the reporter activity to rat SREBP-1c and SREBP-2 was similar to that observed with the nonmutated construct SRE+19/+37/+59, suggesting that these 2 SREs are also not functional (Figure 7A). However, when cells were transfected with a mutant construct SRE+19/+37/+59, in which only the SRE at +59 was mutated, the reporter response to rat SREBP-1c and SREBP-2 was completely abolished, indicating that the SRE at +59 is functional. To further prove the importance of this SRE for SREBP-1c/-2-dependent regulation of the rat NIS gene, we generated a reporter gene construct containing 2 copies of the SRE at +59 in front of the minipromoter of the luciferase reporter vector. As shown in Figure 7B, cells transfected with this construct showed a pronounced response to SREBP-1c and SREBP-2, whereas cells transfected with a reporter construct containing 2 copies of SRE-481 as negative control showed no response at all. To get further indication for the regulatory importance of the identified SRE we also performed sequence alignment of the SRE at +59 of rat NIS 5′-UTR with the mouse NIS 5′-UTR sequence and the consensus SRE. We found that the identified SRE shows a relatively high degree of conservation of the sequence (70% homology) and its position between mouse and rat, which is indicative of the regulatory importance of this binding site (Figure 7C).
Figure 7.
Nuclear SREBP-1c and SREBP-2 Stimulate Transcriptional Activity of Rat NIS 5′-UTR in HepG2 A, HepG2 cells were transiently transfected with NIS reporter gene constructs pGL4.23-rNIS_SRE-7 or wild-type or mutant pGL4.23-rNIS_SRE+19/+37/+59 and cotransfected with either pcDNA3.1 (empty vector) or plasmids expressing nuclear forms of rat SREBP-1c and rat SREBP-2 for 12 hours. B, HepG2 cells were transiently transfected with NIS reporter gene constructs pGL4.23–2x SRE+59 or pGL4.23–2x SRE-481 (negative control) and cotransfected with either pcDNA3.1 (empty vector) or plasmids expressing nuclear forms of rat SREBP-1c and rat SREBP-2 for 12 hours. After transfection, medium was changed to RPMI 1640 supplemented with 10% FBS for 24 hours. Afterward, cells were lysed, and luciferase activities were measured. Bars represent means ± SD from at least 3 independent experiments each performed in triplicate. C, Sequence alignment of consensus SRE with rat NIS 5′-UTR sequence (nucleotide positions from +59 to +68; NM_052983) and mouse NIS 5′-UTR sequence (nucleotide positions from +71 to +80; NM_053248). Matching nucleotides between rat and mouse are shown by asterisks. The nucleotides corresponding to the SRE at +59 are underlined.
To study whether the results obtained in HepG2 cells are replicated in FRTL-5 cells, we transiently transfected FRTL-5 cells with selected rat NIS promoter constructs (construct rNIS-3089, construct SRE+19/+37/+59, and construct SRE+19/+37/+59mut) and either SREBP-1c or SREBP-2 expression plasmids or empty vector (pcDNA3.1). As shown in Figure 8 A, there was a 2- to 3-fold increase in luciferase activity in response to SREBP-1c and SREBP-2 in cells transfected with construct rNIS-3089 and construct SRE+19/+37/+59, but not with mutant construct SRE+19/+37/+59mut. Although the reporter response to SREBP-1c and SREBP-2 in FRTL-5 cells was clearly less than in HepG2 cells, these findings indicated that the rat NIS promoter is transcriptionally regulated by SREBPs also in thyroid cells. The generally weaker response of the reporter to SREBP expression plasmids in FRTL-5 cells compared with HepG2 cells was also observed with the positive control vector containing 2 copies of the SRE from the human LDL receptor in front of the luciferase reporter. We found that the luciferase activity of the positive control increased only about 6- to 10-fold (HepG2 cells: 20–150 fold) in response to SREBP expression plasmids in FRTL-5 cells (Figure 8B).
Figure 8.
Nuclear SREBP-1c and SREBP-2 Stimulate Transcriptional Activity of Rat NIS 5′-UTR in FRTL-5 Cells A, FRTL-5 cells were transiently transfected with rat NIS promoter construct pGL4.10-rNIS-3089 or NIS reporter gene constructs of wild-type or mutant pGL4.23-rNIS_SRE+19/+37/+59 and cotransfected with either pcDNA3.1 (empty vector) or plasmids expressing nuclear forms of rat SREBP-1c and rat SREBP-2 for 24 hours. B, FRTL-5 cells were transiently transfected with pGL4.23–2× hLDLR-SRE (positive control) and cotransfected with either pcDNA3.1 (empty vector) or plasmids expressing nuclear forms of rat SREBP-1c and rat SREBP-2 for 24 hours. After an additional incubation period of 24 hours in 6 H medium, cells were lysed, and luciferase activities were measured. Bars represent means ± SD from at least 2 independent experiments, each performed in duplicate.
Nuclear SREBP-1c and SREBP-2 bind in vitro and in vivo to a functional SRE located at +59 in the 5′-UTR of the rat NIS gene
To confirm the functionality of SRE+59 in the rat NIS 5′-UTR, we studied in vitro binding of rat SREBP-1c or SREBP-2 to this sequence using in vitro-translated rat SREBP-1c and SREBP-2 and an oligonucleotide corresponding to SRE+59 in gel shift assays (EMSA). As shown in Figure 9, a major DNA-protein complex was formed between the specific probe of human LDLR-SRE and the in vitro-translated rat SREBP-1c (lane 2, Figure 9A) and SREBP-2 (lane 2, Figure 9B) as positive control, whereas no complex was formed between the nonspecific probe of mutated human LDLR-SRE and in vitro-translated rat SREBP-1c and SREBP-2 as negative control (lane 3, Figure 9, A and B). A strong DNA-protein complex formation was also observed between the specific probe of rat NIS-SRE+59 and in vitro-translated rat SREBP-1c and SREBP-2 (lane 4, Figure 9, A and B), whereas no DNA-protein complex formation was observed between the mutant oligonucleotide rat NIS-SRE+59mut and in vitro-translated rat SREBP-1c and SREBP-2 (lane 5, Figure 9, A and B). In addition, competition experiments using the oligonucleotide corresponding to rat NIS-SRE+59 and increasing molar excess of unlabeled specific probes (human LDLR-SRE) were performed to test the binding specificity of SREBP-1c and SREBP-2 (lanes 6–8, Figure 9, A and B). This revealed that the complex intensity was successively reduced with increasing molar excess of unlabeled specific probe. At the highest molar excess of unlabeled probe, complex formation was almost completely absent being indicative of specific binding. These results demonstrated that both SREBP-1c and SREBP-2 bind specifically to SRE+59 of rat NIS 5′-UTR.
Figure 9.
In Vivo and in VitroBinding of Nuclear SREBP-1c and SREBP-2 to Rat NIS 5′-UTR A and B, In vitro binding of rat nuclear SREBP-1c (A) and rat nuclear SREBP-2 (B) to the SRE+59 of rat NIS 5′-UTR. EMSA was performed using in vitro-translated rat nuclear SREBP-1c or rat nuclear SREBP-2 and DIG-labeled oligonucleotide corresponding to either wild-type or mutated SRE+59. Fold molar excess of unlabeled specific probe for competition (human LDLR-SRE) is indicated. The use of DIG-labeled specific probe (human LDLR-SRE) and nonspecific probe (mutated human LDLR-SRE) is also indicated. EMSA is representative for 1 of 2 independent experimentations, each providing similar results. C and D, Chromatin immunoprecipitation of SRE+59 of rat NIS 5′-UTR using antibodies against rat SREBP-1 and SREBP-2. The gene sequence spanning the SRE+59 of rat NIS 5′-UTR and a random control sequence were analyzed by conventional PCR (C) and qPCR (D) in the immunoprecipitated chromatin of FRTL-5 cells. FRTL-5 cells were grown in 6H medium in 150-mm dishes until 70%–80% confluent, then switched to 5H medium (without TSH) for 5 days, and subsequently treated with 25-HC (5 μmol/L) in the absence or presence of TSH (10 U/L) for 24 hours. Rabbit IgG was used as control. The image from agarose gel electrophoresis is representative for 1 of 3 independent ChIP experiments, each providing similar results. Data from qPCR analysis represent means ± SD for the 3 independent experiments. M, DNA fragment size marker.
To clarify whether SREBP-1c and SREBP-2 are bound to the SRE+59 of rat NIS 5′-UTR in vivo, ChIP was performed using antibodies against rat SREBP-1 and SREBP-2. As shown in Figure 9, C and D, we observed binding of SREBP-1 and SREBP-2 to a 144-bp sequence spanning the SRE+59 in FRTL-5 cells treated with TSH (10 U/L) for 24 hours but only negligible binding in cells treated without TSH. In addition, no binding of SREBP-1 and SREBP-2 to the 144-bp sequence spanning the SRE+59 was found in FRTL-5 cells treated with TSH and 25-HC (5 μmol/L) in parallel for 24 hours. There was no immunoprecipitation of the SRE+59 with rabbit IgG, and no binding of SREBP-1 and SREBP-2 to a random control sequence (168 bp) was observed (Figure 9, C and D). These data suggested that SREBP-1c and SREBP-2 bind in vivo to the sequence containing the SRE+59.
Discussion
The sterol-sensitive SREBPs are well documented to act as master regulators of lipid metabolism, in particular Chol and fatty acid synthesis (20, 21). Due to their prominent role in controlling lipid metabolism, the regulation and function of SREBPs have been largely investigated in cells and tissues in which lipid biosynthesis pathways play a major role, such as liver, intestine, adipose tissue, and mammary gland (27–30). In the present study it is shown for the first time that SREBPs are also functional in thyroid epithelial cells and that TSH, the master regulator of thyroid growth, differentiation, and hormone synthesis (8), acts as a regulator of SREBP expression and maturation in thyroid epithelial cells. A key finding of the present study, however, is that SREBP-1c and SREBP-2 are novel transcriptional regulators of NIS. NIS is essential for thyroid hormone synthesis due to its function in facilitating active iodide uptake into thyroid epithelial cells by coupling the inward movement of Na+ to the translocation of iodide against its electrochemical gradient, with the Na+ gradient serving as the driving force (42, 43). Using reporter gene assays we could clearly demonstrate that a 3.1-kb DNA fragment of the NIS promoter, which included the NUE region (−2495 to −2264 bp) containing two binding sites each for TTF-1 and Pax8 and a CRE-like binding site and the proximal promoter region (−199 to −110 bp) containing binding sites for several thyroid-specific transcription factors (eg, TTF-1 and NTF-1; Ref. 44), is activated by the active forms of SREBP-1c and SREBP-2. To identify the exact region involved in SREBP-dependent regulation of the NIS gene we tested the activation of several deletion reporter constructs of the NIS promoter. We observed that neither the NUE region nor the proximal promoter region was required for SREBP-dependent activation of the NIS promoter. Rather, by analyzing the reporter response of a further construct containing only the 5′-UTR of the NIS gene with 3 putative SREs, and site-directed mutagenesis of each of these SREs, we identified a critical SRE located downstream from the transcription start site at +59. The critical role of this SRE could be evidenced by demonstrating that mutating this SRE caused a complete loss of the reporter response to active SREBP-1c and SREBP-2. Moreover, the importance of this SRE in regulating the NIS gene could be further confirmed by showing that the reporter activity of an artificial construct containing 2 copies of the SRE +59 was dramatically increased by both active SREBP-1c and active SREBP-2. Finally, we confirmed the functionality of this SRE in showing by EMSA and ChIP experiments that both active SREBP-1c and SREBP-2 bind specifically to this site in the NIS 5′-UTR.
Because nucleotide sequences that are important for gene regulation typically show a high degree of conservation, such sites can be successfully predicted from genome comparison of closely related species like rats, mice, or humans (45, 46). Therefore, to provide further indication of the regulatory importance of the identified SRE, we also performed sequence alignment of the SRE at +59 of rat NIS 5′-UTR with the mouse NIS 5′-UTR sequence, and, indeed, found that the identified SRE shows a relatively high degree of conservation of the sequence (70% homology) and its position between mouse and rat being indicative of the importance of this binding site. Hence, these additional findings from sequence comparisons, together with our experimental findings, underscore the importance of the identified SRE site for the SREBP-mediated activation of the NIS gene.
Our finding that the NUE region and the minimal promoter region are not involved in the SREBP-dependent regulation of the NIS gene might be explained by the fact that NIS is also an important iodide transporter in nonthyroidal tissues, such as small intestine, mammary gland, gastric mucosa, and salivary glands (47–49), in which TSH is not the primary regulator. Because the NUE region and the minimal promoter region stimulate NIS transcription in a thyroid-specific, TSH/cAMP-dependent manner due to the presence of CRE-L sites and binding sites for thyroid-specific transcription factors (40, 50, 51), it is conceivable that the SREBP-dependent regulation of the NIS gene in mainly TSH-independent tissues (intestine, mammary gland, gastric mucosa, salivary glands) does not involve the thyroid-specific binding sites of the NIS promoter. However, from this nonthyroid-specific regulation pattern of the NIS gene by SREBPs it cannot be deduced that the SREBP-dependent regulation is not important for NIS function in thyroid epithelial cells. Rather, the importance of the SREBP-dependent regulatory pathway for NIS function in thyroid epithelial cells, in which iodide uptake by NIS is a prerequisite step for thyroid hormone synthesis, could be evidenced by the observation that inhibition of SREBP maturation by 25-HC caused a decrease of NIS-specific iodide uptake by approximately 20%.
A further important result of the present study supporting a role for SREBPs in transcriptional regulation of the NIS gene is that treatment of FRTL-5 cells with 25-HC caused a decrease in the expression of NIS. Studies with nonthyroidal cells, such as hepatocytes, showed that oxysterols, like 25-HC, inhibit SREBP maturation through blocking the SCAP-mediated movement of SREBPs from the endoplasmic reticulum to the Golgi (52). Blockade of SCAP function by 25-HC is mediated by binding of 25-HC to insulin-induced genes (Insigs), which causes binding of Insigs to SCAP (53). Consequently, binding of coat protein II proteins to SCAP is blocked, and movement of the SCAP-SREBP complex to the Golgi is prevented (54, 55), leading to a decrease in the transcriptionally active nSREBPs in the nucleus, which was also shown in the present study in FRTL-5 thyroid epithelial cells. The 25-HC-induced increase of the inactive pSREBP-1c and pSREBP-2 in the cytoplasm of FRTL-5 cells is also in line with observations in nonthyroidal cells (56–58) and is largely explained by the 25-HC-mediated inhibition of pSREBP processing, which leads to an accumulation of the precursors in the endoplasmic reticulum. Thus, in addition to the description of SREBPs as novel regulators of NIS gene expression, our observations in FRTL-5 cells suggest that SREBP processing is regulated in thyroidal cells similarly as in nonthyroidal cells.
The novel role for TSH in regulating SREBP expression in thyroid epithelial cells, as shown in this study, was evidenced by the observation that FRTL-5 cells treated with TSH had increased mRNA levels and protein levels of inactive and active SREBP-1c and SREBP-2, indicating that TSH stimulates expression of SREBPs in thyroid epithelial cells. Whether the increased levels of nSREBPs are also indicative of a stimulation of SREBP maturation by TSH cannot be definitely answered because TSH may have caused an inhibition of nSREBP degradation that has not been investigated in the present study. In any case, the elevated levels of transcriptionally active nSREBPs suggest that TSH activates the SREBP-1c and SREBP-2 pathway in FRTL-5 cells. Almost identical results were obtained when FRTL-5 cells were treated with the adenylate cyclase activator forskolin, instead of TSH. Forskolin and other cAMP agonists perfectly mimic the effects of TSH on thyrocytes, because most of the cellular effects of TSH after activation of the TSHR are mediated through elevation of cellular cAMP leading to activation of protein kinase A-dependent and -independent signaling pathways (59, 60). One important downstream effect of the TSH-/forskolin-mediated raise in cellular cAMP includes the phosphorylation of cAMP response element-binding protein (CREB), which itself interacts with the transcriptional coactivator CREB-binding protein, and, together with CREB-binding protein, binds to a degenerate CRE site (CRE-L), thereby, increasing NIS gene transcription (61, 62). Therefore, our finding that expression of SREBP-1c and SREBP-2 in FRTL-5 cells was increased in response to TSH and forskolin strongly favors the assumption that expression of SREBPs in FRTL-5 cells is regulated through the cAMP pathway. To confirm that the expression of SREBPs in thyrocytes is indeed regulated via the TSH/TSHR/cAMP pathway, we also determined the expression of SREBP-1 and SREBP-2 in the thyroid gland of TSHR-deficient mice, in which TSH/TSHR/cAMP-dependent gene regulation is blocked due to the lack of TSHR expression (39). In agreement with a recent report (39), the expression of NIS in thyroid epithelial cells of TSHR-deficient mice was only barely detectable by immunohistochemistry compared with wild-type mice. It is noteworthy that we found that the expression of SREBP-1 and SREBP-2 in thyroid epithelial cells from TSHR-deficient mice was markedly lower than in wild-type mice, indicating that expression of SREBP-1c and SREBP-2 in thyrocytes is indeed dependent on TSH also in vivo. Although we did not study the exact TSH/TSHR/cAMP-dependent regulation of the SREBP-1 and SREBP-2 genes, it may involve one or more functional CRE or CRE-L sites in the regulatory region, eg, promoter, of the SREBP-1 and SREBP-2 genes. Using bioinformatic tools, we indeed found 3 putative CREs each in the rat SREBP-1 the rat SREBP-2 promoters. Although the functionality of the predicted binding sites remains to be demonstrated, these findings strengthen our assumption that SREBP-1c and SREBP-2 are regulated by TSH through the adenylate cyclase/cAMP pathway.
Our observation that TSH acts as a molecular regulator of genes involved in Chol homeostasis is in agreement with earlier findings that HMGCR, the rate-limiting enzyme in the Chol biosynthetic pathway, and malic enzyme are up-regulated by TSH in FRTL-5 cells (13–15). These authors demonstrated that the HMGCR gene is directly regulated by TSH through cAMP-dependent activation of a CRE-L sequence in the HMGCR promoter that is closely homologous to the consensus CRE sequence (63). Similar findings of HMGCR regulation by TSH/TSHR have been recently reported from studies in hepatocytes (19), in which the TSHR is also present and TSH stimulates production of cAMP (64). The authors of this study clearly demonstrated that TSH increases HMGCR transcription through activating the cAMP/protein kinase A/CREB signaling pathway leading to the binding of phosphorylated CREB to a CRE in the proximal HMGCR promoter region (19). Our observation that TSH causes activation of SREBP-2 in FRTL-5 cells might also provide an explanation for the TSH-mediated up-regulation of the HMGCR gene in hepatocytes, because regulation of the HMGCR promoter by SREBP-2 has been well established (65, 66). It is also conceivable that SREBP-2 activates HMGCR transcription in concert with CREB, because CREB was demonstrated to be an important coregulatory protein for efficient activation of genes of the Chol biosynthetic pathway by SREBP-2 (67). Irrespective of the exact involvement of SREBP-2 in the TSH-dependent regulation of genes involved in Chol homeostasis in FRTL-5 cells, our findings indicate that the SREBP-2-dependent Chol biosynthesis pathway is of great importance in thyrocytes for regulating NIS-dependent iodide uptake and thereby for production of thyroid hormones.
From a medical perspective it is worth mentioning that NIS activity is required for radioiodide uptake, which has long played a role in the diagnosis and treatment of thyroid cancers and their metastases after thyroidectomy (33), with a major therapeutic limitation being that several thyroid carcinomas have lost their radioiodide transport activity due to defective expression of NIS. Restoration of NIS expression in thyroid cancer cells, therefore, has been postulated to be essential for the efficacy of radioiodide therapy (11). The identification of novel regulatory pathways of NIS, as described in this study, may therefore help at developing new strategies to induce NIS for effective radioiodine therapy.
In conclusion, the present study shows, for the first time, that the sterol-sensitive SREBPs are not only functional in tissues with pronounced lipid biosynthesis activity, such as liver or adipose tissue, but also in thyroid epithelial cells. The observation from this study that SREBP-1c and SREBP-2 are novel transcriptional regulators of NIS, which is essential for thyroid hormone synthesis, indicates that SREBPs are possible novel targets for pharmacologic modulation of thyroid hormone synthesis and for induction of NIS for effective radioiodine therapy.
Acknowledgments
We thank Dr Patrick Schäfer and Professor Dr Kogel (both, Institute of Phytopathology and Applied Zoology, University of Giessen, Germany) for kindly providing access to their confocal microscope for immunohistochemical analysis.
This work was supported by the Deutsche Forschungsgemeinschaft (Grant RI 1537/2-1).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALLN
- N-acetyl-Leu-Leu-Norleucinal
- ChIP
- chromatin immunoprecipitation
- CRE
- cAMP-response element
- CREB
- CRE-binding protein
- DIG
- digoxigenin
- FAS
- fatty acid synthase
- FBS
- fetal bovine serum
- GPAT
- glycerolphosphate-acyltransferase
- 25-HC
- 25-hydroxycholesterol
- HMGCR
- methylglutaryl coenzyme A reductase
- LDL
- low-density lipoprotein
- NIS
- sodium-iodide symporter
- nSREBP
- nuclear SREBP
- NUE
- NIS upstream enhancer
- pSREBP
- precursor SREBP
- qPCR
- quantitative PCR
- RT
- room temperature
- SCAP
- SREBP cleavage activating protein
- siRNA
- small interfering RNA
- SRE
- sterol-regulatory element
- SREBP
- sterol regulatory element-binding protein
- TSHR
- TSH receptor
- TTF
- thyroid transcription factor
- UTR
- untranslated region.
References
- 1. Oppenheimer JH , Schwartz HL , Mariash CN , Kinlaw WB , Wong NC , Freake HC. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987;8:288–308. [DOI] [PubMed] [Google Scholar]
- 2. Dai G , Levy O , Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. [DOI] [PubMed] [Google Scholar]
- 3. Fujiwara H , Tatsumi K , Miki K , et al. Recurrent T354P mutation of the Na+/I− symporter in patients with iodide transport defect. J Clin Endocrinol Metab. 1998;83:2940–2943. [DOI] [PubMed] [Google Scholar]
- 4. Matsuda A , Kosugi S. A homozygous missense mutation of the sodium/iodide symporter gene causing iodide transport defect. J Clin Endocrinol Metab. 1997;82:3966–3971. [DOI] [PubMed] [Google Scholar]
- 5. Spitzweg C , Morris JC. Genetics and phenomics of hypothyroidism and goiter due to NIS mutations. Mol Cell Endocrinol. 2010;322:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolff J. Transport of iodide and other anions in the thyroid gland. Physiol Rev. 1964;44:45–90. [DOI] [PubMed] [Google Scholar]
- 7. Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. [DOI] [PubMed] [Google Scholar]
- 8. Vassart G , Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. [DOI] [PubMed] [Google Scholar]
- 9. Laglia G , Zeiger MA , Leipricht A , et al. Increased cyclic adenosine 3′,5′-monophosphate inhibits G protein-coupled activation of phospholipase C in rat FRTL-5 thyroid cells. Endocrinology. 1996;137:3170–3176. [DOI] [PubMed] [Google Scholar]
- 10. Levy O , Dai G , Riedel C , et al. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci USA. 1997;94:5568–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicola JP , Nazar M , Mascanfroni ID , Pellizas CG , Masini-Repiso AM. NF-κB p65 subunit mediates lipopolysaccharide-induced Na(+)/I(−) symporter gene expression by involving functional interaction with the paired domain transcription factor Pax8. Mol Endocrinol. 2010;24:1846–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kogai T , Endo T , Saito T , Miyazaki A , Kawaguchi A , Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138:2227–2232. [DOI] [PubMed] [Google Scholar]
- 13. Grieco D , Beg ZH , Romano A , Bifulco M , Aloj SM. Cell cycle progression and 3-hydroxy-3-methylglutaryl coenzyme A reductase are regulated by thyrotropin in FRTL-5 rat thyroid cells. J Biol Chem. 1990;265:19343–19350. [PubMed] [Google Scholar]
- 14. Aloj SM , Grieco D , Kohn AD , Nikodem VM , Kohn LD. Thyrotropin regulation of malic enzyme in FRTL-5 rat thyroid cells. Mol Endocrinol. 1990;4:611–622. [DOI] [PubMed] [Google Scholar]
- 15. Bifulco M , Perillo B , Saji M , et al. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in FRTL-5 cells. I. Identification and characterization of a cyclic AMP-responsive element in the rat reductase promoter. J Biol Chem. 1995;270:15231–15236. [DOI] [PubMed] [Google Scholar]
- 16. Giuliani C , Noguchi Y , Harii N , et al. The flavonoid quercetin regulates growth and gene expression in rat FRTL-5 thyroid cells. Endocrinology. 2008;149:84–92. [DOI] [PubMed] [Google Scholar]
- 17. Tran N , Valentín-Blasini L , Blount BC , et al. Thyroid-stimulating hormone increases active transport of perchlorate into thyroid cells. Am J Physiol Endocrinol Metab. 2008;294:E802–E806. [DOI] [PubMed] [Google Scholar]
- 18. Ambesi-Impiombato FS , Parks LA , Coon HG. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci USA. 1980;77:3455–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tian L , Song Y , Xing M , et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52:1401–1409. [DOI] [PubMed] [Google Scholar]
- 20. Yokoyama C , Wang X , Briggs MR , et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 21. Hua X , Yokoyama C , Wu J , Briggs MR , et al. SREBP-2, a second basic-helix-loop-helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc Natl Acad Sci USA. 1993;90:11603–11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horton JD , Goldstein JL , Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang T , Espenshade PJ , Wright ME , et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. [DOI] [PubMed] [Google Scholar]
- 24. Yabe D , Brown MS , Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci USA. 2002;99:12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Espenshade PJ , Li WP , Yabe D. Sterols block binding of COPII proteins to SCAP, thereby controlling SCAP sorting in ER. Proc Natl Acad Sci USA. 2002;99:11694–11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldstein JL , Rawson RB , Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys. 2002;397:139–148. [DOI] [PubMed] [Google Scholar]
- 27. Shimomura I , Shimano H , Horton JD , Goldstein JL , Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horton JD , Bashmakov Y , Shimomura I , Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA. 1998;95:5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Field FJ , Born E , Murthy S , Mathur SN. Gene expression of sterol regulatory element-binding proteins in hamster small intestine. J Lipid Res. 2001;42:1–8. [PubMed] [Google Scholar]
- 30. Barber MC , Vallance AJ , Kennedy HT , Travers MT. Induction of transcripts derived from promoter III of the acetyl-CoA carboxylase-alpha gene in mammary gland is associated with recruitment of SREBP-1 to a region of the proximal promoter defined by a DNase I hypersensitive site. Biochem J. 2003;375:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horton JD , Shah NA , Warrington JA , et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reed BD , Charos AE , Szekely AM , Weissman SM , Snyder M. Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genet. 2008;4:e1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reiners C , Hänscheid H , Luster M , Lassmann M , Verburg FA. Radioiodine for remnant ablation and therapy of metastatic disease. [published correction appears in Nat Rev Endocrinol. 2012;8:130]. Nat Rev Endocrinol. 2011;7:589–595. [DOI] [PubMed] [Google Scholar]
- 34. Livak KJ , Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 35. Hua X , Nohturfft A , Goldstein JL , Brown MS. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell. 1996;87:415–426. [DOI] [PubMed] [Google Scholar]
- 36. Murphy C , Ledmyr H , Ehrenborg E , Gåfvels M. Promoter analysis of the murine squalene epoxidase gene. Identification of a 205 bp homing region regulated by both SREBP'S and NF-Y. Biochim Biophys Acta. 2006;1761:1213–1227. [DOI] [PubMed] [Google Scholar]
- 37. Inoue S , Yoshinari K , Sugawara M , Yamazoe Y. Activated sterol regulatory element-binding protein-2 suppresses hepatocyte nuclear factor-4-mediated Cyp3a11 expression in mouse liver. Mol Pharmacol. 2011;79:148–156. [DOI] [PubMed] [Google Scholar]
- 38. Wen G , Ringseis R , Eder K. Mouse OCTN2 is directly regulated by peroxisome proliferator-activated receptor α (PPARα) via a PPRE located in the first intron. Biochem Pharmacol. 2010;79:768–776. [DOI] [PubMed] [Google Scholar]
- 39. Marians RC , Ng L , Blair HC , Unger P , Graves PN , Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA. 2002;99:15776–15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chun JT , Di Dato V , D'Andrea B , Zannini M , Di Lauro R. The CRE-like element inside the 5′-upstream region of the rat sodium/iodide symporter gene interacts with diverse classes of b-Zip molecules that regulate transcriptional activities through strong synergy with Pax-8. Mol Endocrinol. 2004;18:2817–2829. [DOI] [PubMed] [Google Scholar]
- 41. Riesco-Eizaguirre G , Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol. 2006;155:495–512. [DOI] [PubMed] [Google Scholar]
- 42. Eskandari S , Loo DD , Dai G , Levy O , Wright EM , Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272:27230–27238. [DOI] [PubMed] [Google Scholar]
- 43. Dohán O , De la Vieja A , Paroder V , et al. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. [DOI] [PubMed] [Google Scholar]
- 44. Endo T , Kaneshige M , Nakazato M , Ohmori M , Harii N , Onaya T. Thyroid transcription factor-1 activates the promoter activity of rat thyroid Na+/I− symporter gene. Mol Endocrinol. 1997;11:1747–1755. [DOI] [PubMed] [Google Scholar]
- 45. Dubchak I , Brudno M , Loots GG , et al. Active conservation of noncoding sequences revealed by three-way species comparisons. Genome Res. 2000;10:1304–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alotaibi H , Yaman E , Salvatore D , et al. Intronic elements in the Na+/I− symporter gene (NIS) interact with retinoic acid receptors and mediate initiation of transcription. Nucleic Acids Res. 2010;38:3172–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De La Vieja A , Dohan O , Levy O , Carrasco N. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 2000;80:1083–1105. [DOI] [PubMed] [Google Scholar]
- 48. Tazebay UH , Wapnir IL , Levy O , et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6:871–878. [DOI] [PubMed] [Google Scholar]
- 49. Cho JY , Léveillé R , Kao R , et al. Hormonal regulation of radioiodide uptake activity and Na+/I− symporter expression in mammary glands. J Clin Endocrinol Metab. 2000;85:2936–2943. [DOI] [PubMed] [Google Scholar]
- 50. Kogai T , Taki K , Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006;13:797–826. [DOI] [PubMed] [Google Scholar]
- 51. Ohno M , Zannini M , Levy O , Carrasco N , di Lauro R. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol. 1999;19:2051–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nohturfft A , Yabe D , Goldstein JL , Brown MS , Espenshade PJ. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102:315–323. [DOI] [PubMed] [Google Scholar]
- 53. Radhakrishnan A , Ikeda Y , Kwon HJ , Brown MS , Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA. 2007;104:6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goldstein JL , DeBose-Boyd RA , Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. [DOI] [PubMed] [Google Scholar]
- 55. Sun LP , Li L , Goldstein JL , Brown MS. Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J Biol Chem. 2005;280:26483–26490. [DOI] [PubMed] [Google Scholar]
- 56. Wang X , Sato R , Brown MS , Hua X , Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. [DOI] [PubMed] [Google Scholar]
- 57. DeBose-Boyd RA , Ou J , Goldstein JL , Brown MS. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc Natl Acad Sci USA. 2001;98:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yokoyama A , Makishima M , Choi M , et al. Induction of SREBP-1c mRNA by differentiation and LXR ligand in human keratinocytes. J Invest Dermatol. 2009;129:1395–1401. [DOI] [PubMed] [Google Scholar]
- 59. Taki K , Kogai T , Kanamoto Y , Hershman JM , Brent GA. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3′,5′-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells. Mol Endocrinol. 2002;16:2266–2282. [DOI] [PubMed] [Google Scholar]
- 60. Venkateswaran A , Marsee DK , Green SH , Jhiang SM. Forskolin, 8-Br-3′,5′-cyclic adenosine 5′-monophosphate, and catalytic protein kinase A expression in the nucleus increase radioiodide uptake and sodium/iodide symporter protein levels in RET/PTC1-expressing cells. J Clin Endocrinol Metab. 2004;89:6168–6172. [DOI] [PubMed] [Google Scholar]
- 61. Dremier S , Milenkovic M , Blancquaert S , et al. Cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases, but not exchange proteins directly activated by cAMP (Epac), mediate thyrotropin/cAMP-dependent regulation of thyroid cells. Endocrinology. 2007;148:4612–4622. [DOI] [PubMed] [Google Scholar]
- 62. Fenton MS , Marion KM , Hershman JM. Identification of cyclic adenosine 3′,5′-monophosphate response element modulator as an activator of the human sodium/iodide symporter upstream enhancer. Endocrinology. 2008;149:2592–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montminy MR , Sevarino KA , Wagner JA , Mandel G , Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA. 1986;83:6682–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang W , Tian LM , Han Y , et al. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med. 2009;13:4636–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vallett SM , Sanchez HB , Rosenfeld JM , Osborne TF. A direct role for sterol regulatory element binding protein in activation of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. J Biol Chem. 1996;271:12247–12253. [DOI] [PubMed] [Google Scholar]
- 66. Kawabe Y , Suzuki T , Hayashi M , Hamakubo T , Sato R , Kodama T. The physiological role of sterol regulatory element-binding protein-2 in cultured human cells. Biochim Biophys Acta. 1999;1436:307–318. [DOI] [PubMed] [Google Scholar]
- 67. Ngo TT , Bennett MK , Bourgeois AL , Toth JI , Osborne TF. A role for cyclic AMP response element-binding protein (CREB) but not the highly similar ATF-2 protein in sterol regulation of the promoter for 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 2002;277:33901–33905. [DOI] [PubMed] [Google Scholar]