Abstract
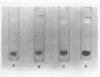
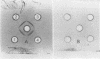
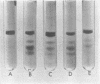
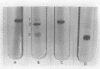
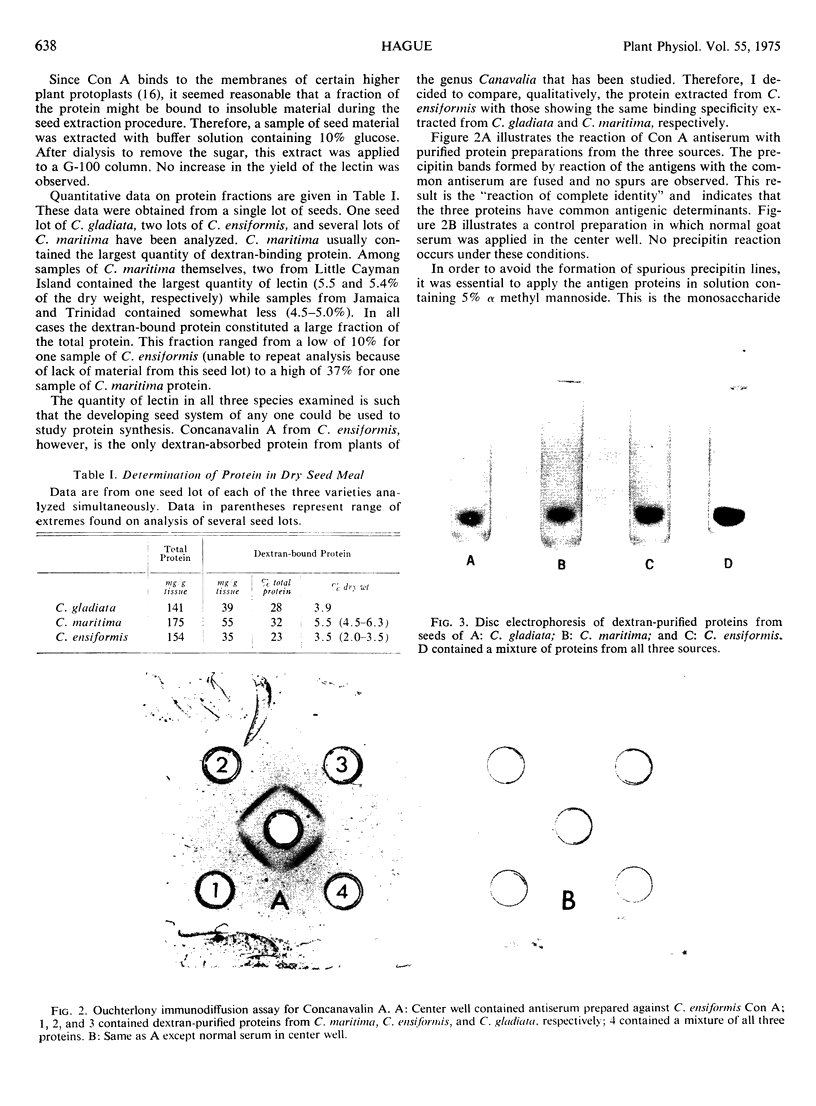
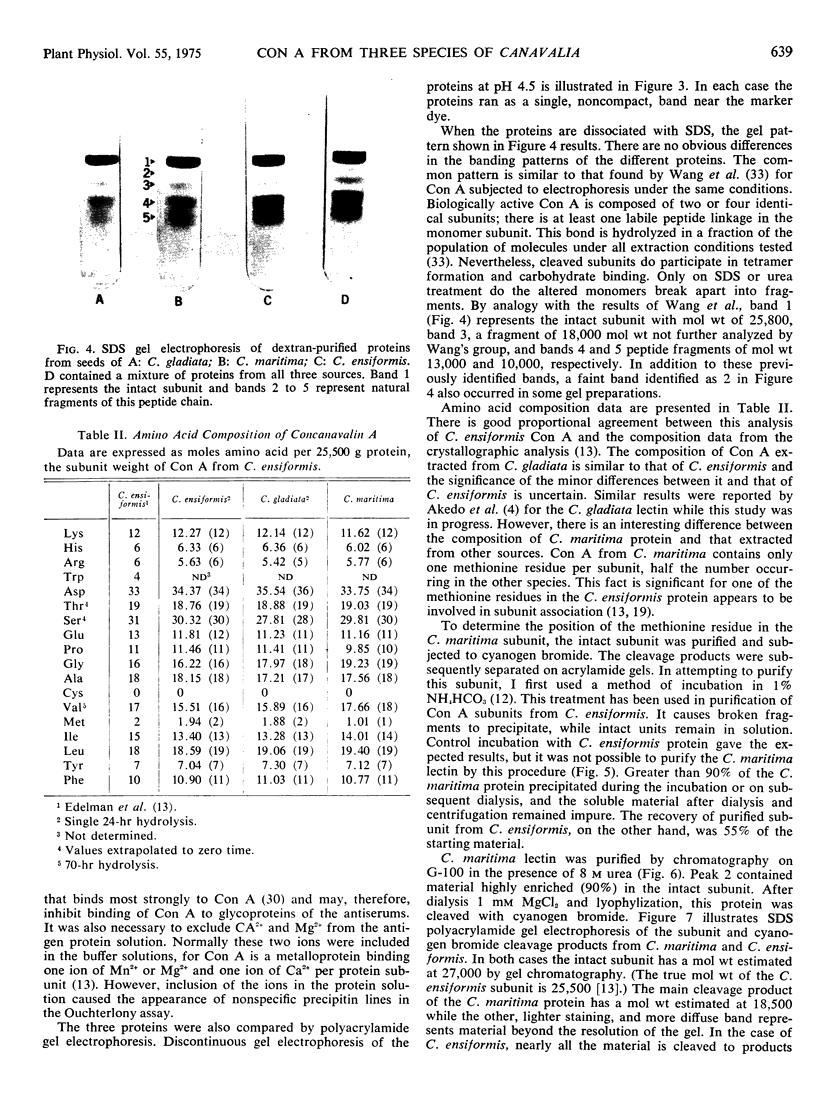
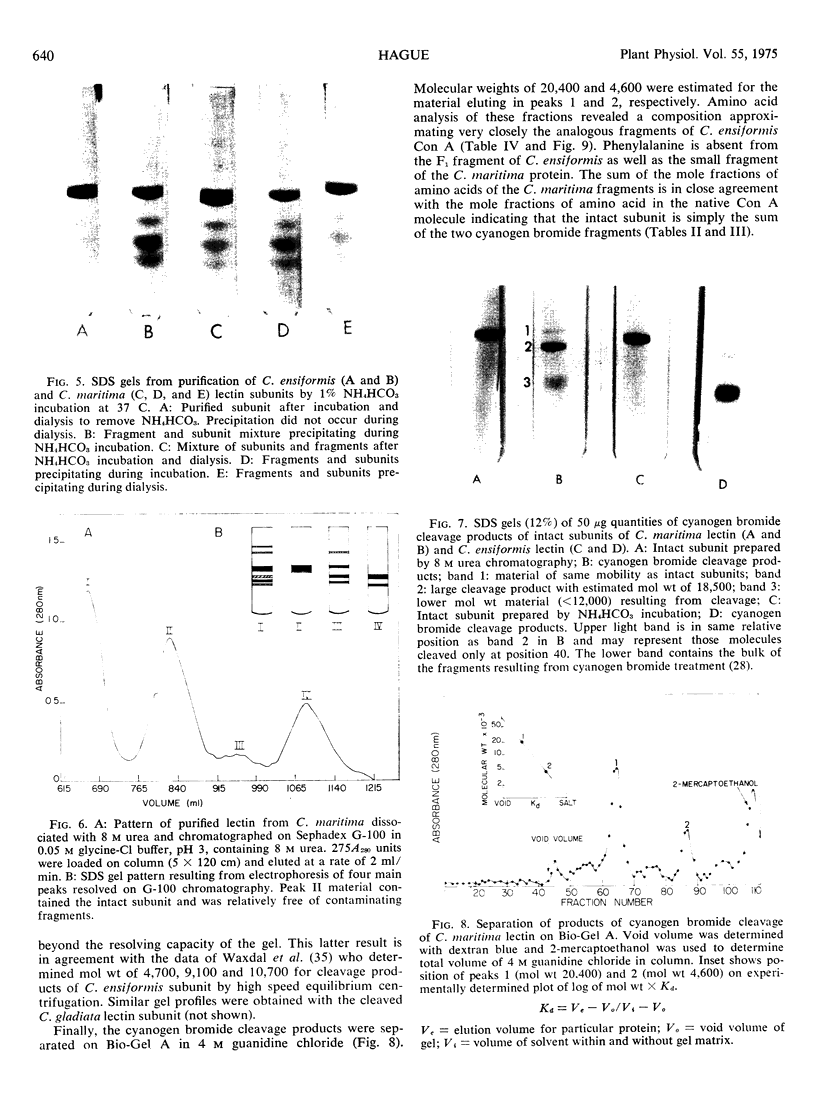
Concanavalin A, the lectin of the Jack bean, Canavalia ensiformis, was extracted and compared with homologous proteins from Canavalia gladiata and Canavalia maritima. All proteins were bound to Sephadex G-100 and eluted from the gel with buffered glucose solution. Quantitative recoveries indicated that large quantities (23 to 28% of dry seed protein) of these lectins are synthesized by all three species. Antibody preparations made against C. ensiformis lectin failed to discriminate among the three proteins; the pattern of the precipitin bands indicated identical antigenic determinants in the Ouchterlony double-diffusion assay. Native and sodium dodecyl sulfate polyacryl-amide gel electrophoresis also failed to distinguish differences in the proteins. The storage protein active in carbohydrate binding is composed, in each case, of identical subunits. However, the amino acid composition of the subunit chains from the three sources is not identical. In particular, the lectins from C. ensiformis and C. gladiata contain two methionine residues per protein subunit, while only one methionine residue is found in the C. martima lectin. Cyanogen bromide cleavage of the purified subunit from C. maritima yieded two fragments with molecular weights estimated at 20,400 and 4,600, respectively. Amino acid analysis of the separated fragments indicated that the methionine residue at position 130 in C. ensiformis is absent in the lectin from C. maritima.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Iwabuchi M., Ishii S. I. Multiple forms in the subunit structure of concanavalin A. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1271–1278. doi: 10.1016/0006-291x(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VII. Physical and chemical studies on concanavalin A, the hemagglutinin of the jack bean. Arch Biochem Biophys. 1968 Mar 20;124(1):218–229. doi: 10.1016/0003-9861(68)90322-6. [DOI] [PubMed] [Google Scholar]
- Akedo H., Mori Y., Tanigaki Y., Shinkai K., Morita K. Isolation of concanavalin A binding protein(s) from rat erythrocyte stroma. Biochim Biophys Acta. 1972 Jul 21;271(2):378–387. doi: 10.1016/0005-2795(72)90213-9. [DOI] [PubMed] [Google Scholar]
- Albersheim P., Anderson A. J. Proteins from plant cell walls inhibit polygalacturonases secreted by plant pathogens. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1815–1819. doi: 10.1073/pnas.68.8.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. J., Boulter D. The structure of legumin, a storage protein of broad bean (Vicia faba) seed. Eur J Biochem. 1970 Dec;17(3):460–466. doi: 10.1111/j.1432-1033.1970.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Pflumm M. N., Edelman G. M. Isolation and proteolytic cleavage of the intact subunit of concanavalin A. Biochemistry. 1972 Aug 15;11(17):3233–3239. doi: 10.1021/bi00767a016. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Hamblin J., Kent S. P. Possible role of phytohaemagglutinin in Phaseolus vulgaris L. Nat New Biol. 1973 Sep 5;245(140):28–30. doi: 10.1038/newbio245028a0. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J. Isolation and characterization of a phytohemagglutinin from the lentil. Biochemistry. 1969 Jun;8(6):2436–2441. doi: 10.1021/bi00834a028. [DOI] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Millerd A., Simon M., Stern H. Legumin Synthesis in Developing Cotyledons of Vicia faba L. Plant Physiol. 1971 Oct;48(4):419–425. doi: 10.1104/pp.48.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. O., Liener I. E. Some physical and chemical properties of concanavalin A, the phytohemagglutinin of the jack bean. Biochemistry. 1967 Jan;6(1):105–111. doi: 10.1021/bi00853a018. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxdal M. J., Konigsberg W. H., Henley W. L., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. II. Isolation and characterization of the cyanogen bromide fragments. Biochemistry. 1968 May;7(5):1959–1966. doi: 10.1021/bi00845a046. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]