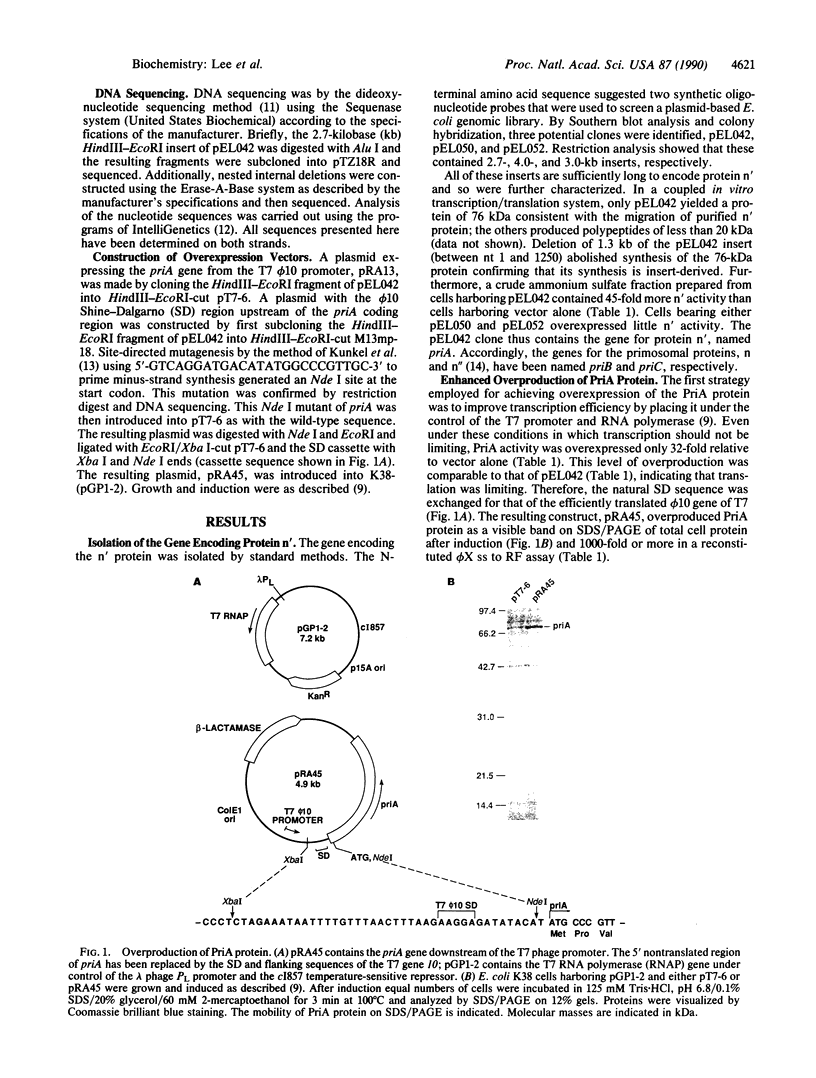
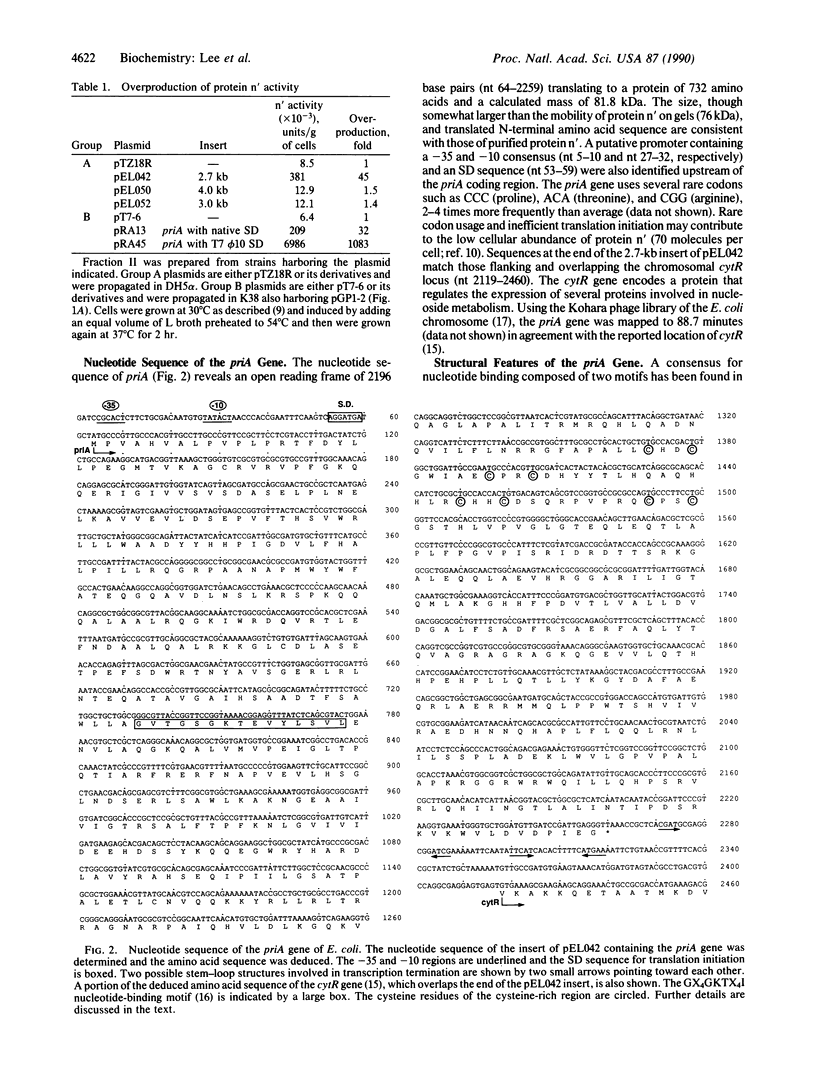
Abstract
The Escherichia coli gene encoding protein n' has been isolated and named priA for primosomal protein A. Protein n' is absolutely required for the conversion of single-stranded phi X174 DNA to the duplex replicative form in an in vitro-reconstituted system. The gene maps to 88.7 minutes on the chromosome adjacent to the cytR locus. Soluble protein extracts from cells harboring the priA gene on a multicopy plasmid contained 45-fold more n' replication activity than wild-type extracts. Enhanced overproduction of greater than 1000-fold was achieved by replacing the natural Shine-Dalgarno sequence with that of the phage T7 phi 10 gene and placing this priA under the control of the T7 phage promoter and RNA polymerase. The priA sequence reveals a 732-amino acid open reading frame and a nucleotide-binding consensus site consistent with the size and ATPase activity of the purified protein. The gene for protein n has been named priB and the putative gene for protein n", priC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arikan E., Kulkarni M. S., Thomas D. C., Sancar A. Sequences of the E. coli uvrB gene and protein. Nucleic Acids Res. 1986 Mar 25;14(6):2637–2650. doi: 10.1093/nar/14.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L., Clayton J., Friedland P., Kedes L. H. SEQ: a nucleotide sequence analysis and recombination system. Nucleic Acids Res. 1982 Jan 11;10(1):279–294. doi: 10.1093/nar/10.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Hampe A., Walker J. E. Nucleotide sequence of the Rhodospirillum rubrum atp operon. Biochem J. 1985 Jun 1;228(2):391–407. doi: 10.1042/bj2280391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Emmerson P. T. The nucleotide sequence of the uvrD gene of E. coli. Nucleic Acids Res. 1984 Jul 25;12(14):5789–5799. doi: 10.1093/nar/12.14.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist C. A., Denhardt D. T. Escherichia coli rep gene: sequence of the gene, the encoded helicase, and its homology with uvrD. Nucleic Acids Res. 1987 Jan 26;15(2):465–475. doi: 10.1093/nar/15.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B., Hansen F. G., von Meyenburg K. The nucleotide sequence of the dnaA gene and the first part of the dnaN gene of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 25;10(22):7373–7385. doi: 10.1093/nar/10.22.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Sancar A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J Biol Chem. 1986 Apr 15;261(11):4895–4901. [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lark C. A., Riazi J., Lark K. G. dnaT, dominant conditional-lethal mutation affecting DNA replication in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1008–1017. doi: 10.1128/jb.136.3.1008-1017.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Marians K. J. The Escherichia coli primosome can translocate actively in either direction along a DNA strand. J Biol Chem. 1989 Aug 25;264(24):14531–14542. [PubMed] [Google Scholar]
- Low R. L., Shlomai J., Kornberg A. Protein n, a primosomal DNA replication protein of Escherichia coli. Purification and characterization. J Biol Chem. 1982 Jun 10;257(11):6242–6250. [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Arai N., Bond M. W., Kaziro Y., Arai K. Nucleotide sequence of dnaB and the primary structure of the dnaB protein from Escherichia coli. J Biol Chem. 1984 Jan 10;259(1):97–101. [PubMed] [Google Scholar]
- Nurse P., DiGate R. J., Zavitz K. H., Marians K. J. Molecular cloning and DNA sequence analysis of Escherichia coli priA, the gene encoding the primosomal protein replication factor Y. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4615–4619. doi: 10.1073/pnas.87.12.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. Sequential early stages in the in vitro initiation of replication at the origin of the Escherichia coli chromosome. J Biol Chem. 1988 May 25;263(15):7124–7130. [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. A prepriming DNA replication enzyme of Escherichia coli. I. Purification of protein n': a sequence-specific, DNA-dependent ATPase. J Biol Chem. 1980 Jul 25;255(14):6789–6793. [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. A prepriming DNA replication enzyme of Escherichia coli. II. Actions of protein n': a sequence-specific, DNA-dependent ATPase. J Biol Chem. 1980 Jul 25;255(14):6794–6798. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Larsen J. E., Højrup P., Short S. A., Barbier C. S. Nucleotide sequence of the CytR regulatory gene of E. coli K-12. Nucleic Acids Res. 1986 Mar 11;14(5):2215–2228. doi: 10.1093/nar/14.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. R., Matson S. W. The molecular cloning of the gene encoding the Escherichia coli 75-kDa helicase and the determination of its nucleotide sequence and gentic map position. J Biol Chem. 1989 May 15;264(14):8297–8303. [PubMed] [Google Scholar]
- Yin K. C., Blinkowa A., Walker J. R. Nucleotide sequence of the Escherichia coli replication gene dnaZX. Nucleic Acids Res. 1986 Aug 26;14(16):6541–6549. doi: 10.1093/nar/14.16.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]