Abstract
Sex steroids are important regulators of neuronal cell morphology, and this is critical for gender differences in brain function and dysfunction. Neuronal morphology is controlled by multiprotein complexes including moesin (a member of the ezrin/radixin/moesin family), focal adhesion kinase (FAK), or the Wiskott-Aldrich syndrome protein-family verprolin homologous (WAVE1) protein, controlling dynamic remodeling of the cytoskeleton and cell membrane. We investigated the actions of natural progesterone (P) and of the synthetic progestin medroxyprogesterone acetate (MPA) on actin remodeling, focal adhesion complex formation, and actin branching in rat cortical neurons. Treatment with P and, to a lesser extent, MPA, increases the number and density of dendritic spines. P increases the phosphorylation of moesin, FAK, and WAVE1, and their redistribution toward cell membrane sites where spines are formed. Signaling to moesin is achieved by PR via a Gα/Gβ-dependent signaling to the small GTPase Ras homolog gene family, member A and its related kinase, Rho-associated kinase-2. In parallel, WAVE1 recruitment is triggered by a Gαi/Gβ-dependent signaling of PR to c-Src, FAK, and Rac1 GTPase. Rac1 recruits cyclin-dependent kinase-5, which phosphorylates WAVE1. Silencing of moesin, FAK, or WAVE1 abrogates the increase in dendritic spines induced by progesterone. In all applications, MPA is found to act similar to P, albeit with a lower efficacy. In conclusion, our findings indicate that the control of actin polymerization and branching and focal adhesion complex formation via moesin, FAK, and WAVE1 is a key function of progesterone receptor in neurons, which may be relevant for the regulation of dendritic spine turnover and neuronal plasticity.
Excitatory synaptic transmission in the central nervous system requires spines, highly motile protrusions on the dendritic surface (1). Dendrites, the main neuronal sensory structures, are dynamic structures, and their arbors and spines change in response to environmental changes (2), injuries (3, 4), diseases (5, 6), and throughout aging (7, 8). Dendritic spines undergo rapid changes in their distribution and morphology. This plasticity involves a number of proteins controlling the organization of the actin cytoskeleton (1). Filamentous actin represents the major cytoskeletal component of dendrite spines (9, 10). Changes in spine shape, size, and number are determined by local actin dynamics (11). Actin-dependent reorganization of spine morphology is believed to play a role in memory and learning in the mammalian brain (12).
Steroid hormones influence dendritic morphology (13–16). This may explain, in part, the actions of these hormones on brain plasticity (17–19). Clinical studies suggest that estrogens and progestin may protect against degenerative insults implicated in neurodegenerative diseases (18, 20–26).
Sex steroid hormones are fundamental regulators of cell morphology and movement in diverse cellular types, including neurons (27–33). We have recently shown that estrogen controls actin remodeling and the development of specialized membrane structures, including dendritic spines (30). These events depend on the rapid regulation of actin-binding proteins belonging to the ezrin/radixin/moesin (ERM) family (30). Activated moesin triggers the depolymerization of actin fibers and the reassembly toward the cell membrane edge, leading to the formation of cortical actin complexes and specialized cell membrane structures implicated in the generation of the cellular locomotive force (28, 30, 31, 34). The Wiskott-Aldrich syndrome protein (WASP) family verprolin homologous (WAVE) family protein, WAVE1 is a scaffold that relays signals from small GTPases to the Arp2/3 complex, leading to a burst of actin polymerization. Actin nucleation, responsible for the branching of actin filaments, is critical for the rapid formation of an actin network at the leading edge of the cell (35–38) that provides the protrusive force required for dendritic spine formation (39). The formation and turnover of focal adhesions are also required for cells to develop membrane protrusions and get in contact with the extracellular matrix via anchorage proteins (40). Focal adhesion kinase (FAK) (41) is a nonreceptor tyrosine kinase that controls cell movement (42, 43) and is involved in the formation and turnover of focal adhesion sites (42–44).
The aim of the present study was to identify the molecular basis of the actions of progesterone (P) and of the synthetic progestin medroxyprogesterone acetate (MPA) on neuronal dendritic spine formation. In particular, we wished to identify whether these actions may require the regulation of the actin cytoskeleton via moesin, FAK, and WAVE1, and to characterize the intracellular cascades recruited during this signaling.
Materials and Methods
Neuronal cell cultures and treatments
Primary cultures of cortical neurons were obtained from embryonic day 18 rat fetuses as described elsewhere (45). Cortical cells were dissected with 0.02% trypsin (Invitrogen, Carlsbad, California) for 5 minutes at 37°C and then dissociated. Cells were plated on poly-d-lysine-coated 60-mm Petri dishes at a density of 0.5–1 × 105 cells/cm2. Neurons were grown in Neurobasal medium (Invitrogen) supplemented with 10 U/mL penicillin, 10 μg/mL streptomycin, 0.5 mM glutamine, 25 μM glutamate, and 2% B27 (Invitrogen). These culture conditions generate cultures that are more than 95% neuronal and less than or equal to 5% glial (1). P, MPA, E2, and Y-27632 were from Sigma-Aldrich (St. Louis, Missouri); roscovitine and PP2 were from Calbiochem (La Jolla, California); FAK inhibitor (FAKi) was from (Santa Cruz Biotechnology, Santa Cruz, California). ORG 31710 was obtained from Organon Akzo Nobel (Oss, The Netherlands). Whenever an inhibitor was used, the compound was added 30–45 minutes before starting the treatments.
Immunoblottings
Cell lysates were separated by SDS-PAGE. Antibodies used were: moesin (clone 38), p-FAK (Y397), FAK (BD Transduction Laboratories, Lexington, Kentucky); Actin (C-11), progesterone receptor (PR) (C-20), Gα13 (A-20), Gαi1 (R4), Gβ1 (C-16), c-Src (H-12), Rac1 (C-14), p-Rac1 (sc-135641), p-cyclin-dependent kinase 5 (Cdk5) (B-4), p-FAK(Tyr397) (sc-11765-R), Rho-associated kinase-2 (ROCK-2) (C-20), Thr558-p-moesin (sc-12895), (Santa Cruz Biotechnology); Thr98-p-myelin basic protein (MBP; 05-429) (Upstate Biotechnology, Inc., Lake Placid, New York); phospho-Src (Tyr416) (Cell Signaling Technology, Danvers, Massachusetts); WAVE1 (SAB4503508), p-WAVE1 (pSer397) (W2768) (Sigma-Aldrich); Cdk5 (268–283) (Calbiochem). Primary and secondary antibodies were incubated with the membranes with standard technique. Immunodetection was accomplished using enhanced chemiluminescence.
Cell immunofluorescence
Neuronal cells were grown on coverslips. Cells were fixed with 4% paraformaldehyde for 30 minutes and permeabilized with 0.1% Triton for 5 minutes. Blocking was performed with PBS containing 3% bovine serum albumin for 30 minutes. When indicated, cells were incubated with antiphospho WAVE1 (1:750; Sigma-Aldrich Laboratories) (1:300; Santa Cruz Biotechnology) overnight at 4°C followed by incubation with a fluorescein-conjugated goat antirabbit secondary antibody (1:200; Vector Laboratories, Burlingame, California). Then cells were incubated with Texas Red-phalloidin (Sigma) for 30 minutes. After washing the nuclei were counterstained with or 4′-6-diamidino-2-phenylindole (Sigma) and mounted with Vectashield mounting medium (Vector Laboratories). Immunofluorescence was visualized using an Olympus BX41 microscope (Olympus, Lake Success, New York) and recorded with a DP70 Olympus digital camera.
Kinase assays
Cortical neuronal cells were harvested in 20 mM Tris-HCl, 10 mM EDTA, 100 mM NaCl, 0.5% IGEPAL, and 0.1 mg/mL phenylmethylsulfonyl fluoride. Equal amounts of cell lysates were immunoprecipitated with vs ROCK-2 (C-20, Santa Cruz) antibody, 40 μL 1:1 protein A-agarose was added, and the immunoprecipitates (IPs) were washed three times with buffer containing 20 mM Tris-HCl, 10 mM EDTA, 150 mM NaCl, 0.1% IGEPAL, and 0.1 mg/mL phenylmethylsulfonyl fluoride. For ROCK-2 activity assay, two additional washes were performed in kinase assay buffer (20 mM 3[N-morpholino]propane sulfonic acid, 25 mM β-glycerophosphate, 5 mM EGTA, 1 mM dithiothreitol), and the samples were therefore resuspended in this buffer. Dephosphorylated MBP (5 μg; Upstate Biotechnology) together with 500 μM ATP and 75 mM MgCl2 were added to each sample, and the reaction was started at 30°C for 20 minutes. The reaction was stopped on ice and by resuspending the samples in Laemmli buffer. The samples were separated with SDS-PAGE and Western analysis was performed using antibodies recognizing Ras homolog gene family, member A (sc-418, Santa Cruz Biotechnology) or Thr98-P-MBP (05-429, Upstate Biotechnology).
Gene silencing with RNA interference
Synthetic small interfering RNAs targeting ROCK-2 (siRNA SMARTpool ROCK2), FAK (siRNA SMARTpool FAK), WAVE-1 (siRNA SMARTpool WAVE-1), PR-A/-B (ON-TARGETplus SMARTpool Pgr siRNA), and control siRNAs (D-001810–01-05) were purchased from Dharmacon (Thermo Fisher Scientific, Inc, Pittsburgh, Pennsylvania). Gβ1 siRNAs was from Santa Cruz Biotechnology. The siRNAs were used at the final concentration of 50–75 nM. Validated antisense phosphorothioate oligonucleotides (PONs) (S-modified) complementary to the 1–15 position of the human moesin gene-coding region were obtained. The sequence was 5-TACGGGTTTTGCTAG-3 for moesin antisense PON. The complementary sense PON was used as control (5-CTAGCAAAACCCGTA-3). Cortical neurons were treated 48 hours after siRNA or PON transfection. The efficacy of gene silencing was checked with Western analysis and found to be optimal at 48 hours.
Transfection experiments
The dominant-negative constructs for Gαi1 (Gαi1 G202T), Rac1 (Rac1 T17N), and Gα13 (Gα13 Q226L/D294N) were from the Guthrie cDNA Resource Center (www.cdna.org). The inserts were cloned in pcDNA3.1+. The plasmids (10 μg) were transfected into cortical neurons using Lipofectamine (Invitrogen). Parallel cells were transfected with empty pcDNA3.1+ plasmid. Cells (60%–70% confluent) were treated 24 hours after transfection.
Statistical analysis
All values are expressed as mean ± SD. Statistical analyses and graphics were done using InStat from GraphPad Prism Software (San Diego, California). Statistical differences between mean values were determined by ANOVA, followed by the Fisher's protected least significance difference.
Results
P rapidly induces moesin, FAK, and WAVE1 phosphorylation and increases dendritic spine formation in cortical neurons
As a first step in identifying the effects of P and MPA on neuronal cell morphology, we studied the actions of a rapid P and MPA exposure on three regulators of the actin cytoskeleton, moesin, FAK, and WAVE1 in rat cortical neurons. A 20-minute treatment with increasing amounts of P (0.1–100 nM) resulted in a rapid increase of Thr558-moesin, Tyr397-FAK, and Ser397-WAVE1 phosphorylation. Phosphorylation of such residues is established to turn into functional activation of these proteins, leading to modifications in actin organization (30, 31, 46), (Figure 1A). This experiment allowed selection of 1 nM (physiological concentration of P outside pregnancy) as the concentration for the later experiments.
Figure 1.
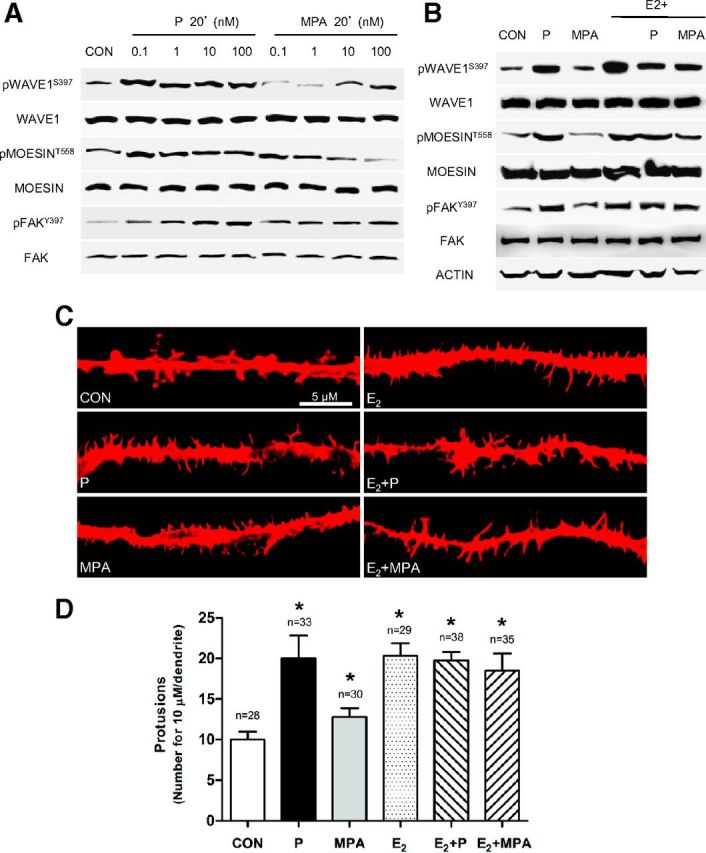
P Activates Moesin, FAK, and WAVE1 and Induces Dendritic Spine Formation in Cortical Neurons A, Dose-dependent moesin, FAK, and WAVE1 phosphorylation in neurons after treatment with P and MPA. Total cell amount of wild-type (moesin, FAK, and WAVE1) or Thr588-phosphorylated moesin, Tyr397-phosphorylated FAK, and Ser397-phosphorylated WAVE1 are shown with Western blot. Phospho-moesin588, phospho-FAK397, and phospho-WAVE1397 densitometry values were adjusted to moesin, FAK, and WAVE1 intensity, and then normalized to the control sample. *P < .05 vs corresponding control (Supplemental Figure, panels A–C published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). B, Moesin, FAK, and WAVE1 phosphorylation induced by progestogens alone or in combination with E2 (1 nM). C, Dendritic spine morphology and number were measured with immunofluorescence after staining actin fibers with phalloidin/Texas Red. The scale bar corresponds to 5 μm. D, Graph shows the quantitative analysis of spine density expressed as the number of spines per 10 μm dendrite length. Results are expressed as the mean ± SD. *P < .05 vs control (CON). The experiments were performed in triplicate and representative images are shown. Densitometric quantifications of all the blots (including those not shown) were performed, and the relative mean ± SD of each condition is presented in a graph as supplemental data.
In parallel, a similar treatment with MPA (0.1–100 nM), a synthetic progestin that is approximately 100-fold more effective in binding PR in comparison to P, resulted in minor increases in phosphorylation of the three proteins (Figure 1A).
As previously described (30, 42), moesin, FAK, and WAVE1 phosphorylation were markedly increased by the addition of 17β-estradiol (E2, 1 nM) (Figure 1B). The addition of either progestogen resulted in visible decreases in the estrogen-related Ser397-phosphorylation of WAVE1, and slight reductions on the phosphorylation of moesin and FAK (Figure 1B).
The reported actions on moesin, FAK, or WAVE1 of P and MPA, either alone or with E2, were related to modifications of dendritic spine density in neuronal cell protrusions at high-resolution fluorescence imaging (Figure 1, C and D).
P and MPA activate moesin, FAK. and WAVE1 via PR
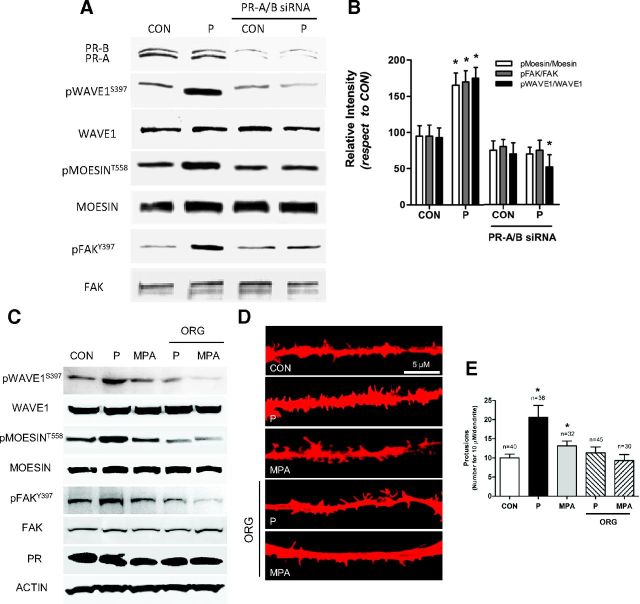
Rat cortical neurons express both isoforms of PR, PR-A and PR-B, as shown in Figure 2A. Silencing of PR-A and PR-B expression with specific siRNAs abolished phosphorylation of WAVE1, moesin, and FAK induced by P (Figure 2, A and B). In agreement, blockade of PR with the pure PR antagonist ORG 31710 completely abolished P- and MPA-dependent moesin, FAK, and WAVE1 phosphorylation and the related spine formation, confirming that P activates these signals via PR (Figure 2, C and E).
Figure 2.
P Signals to Moesin, FAK, and WAVE1 via[b] PR A and B, Cortical neurons were exposed for 20 minutes to 1 nM P in the presence or absence of siRNAs vs PR-A and PR-B. Expression of PR-A/-B, along with phosphorylation of moesin, FAK, and WAVE1 were assayed with Western analysis. C, Cortical neurons were exposed for 20 minutes to 1 nM P in the presence or absence of the PR antagonist ORG 31710 (ORG; 1 μM). Phosphorylation of moesin, FAK, and WAVE1 were assayed with Western analysis. D, Dendritic spine density was measured with immunofluorescence after staining actin fibers with phalloidin/Texas Red. The scale bar corresponds to 5 μm. E, Graph shows the quantitative analysis of spine density expressed as the number of spines per 10 μm dendrite length. Results are expressed as the mean ± SD. *P < .05 vs control (CON). The experiments were performed in triplicates and representative images are shown. Densitometric quantifications of all the blots (including those not shown) were performed, and the relative mean ± SD of each condition is presented in a graph as supplemental data.
P and MPA phosphorylate moesin, FAK, and WAVE1 through G protein-dependent signaling pathways
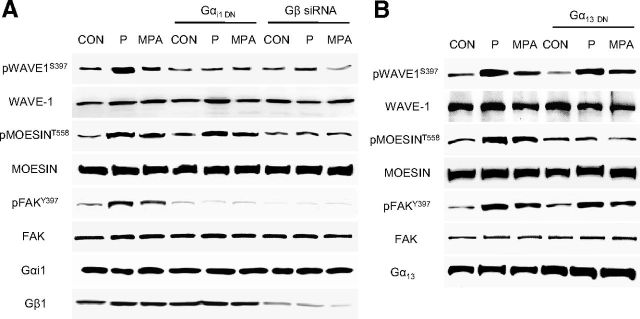
Signaling through Gαi/Gβ is used by estrogen receptors to regulate FAK (42) and WAVE1 (30), whereas these receptors use Gα13/Gβ to control moesin (31). We blocked Gαi or Gα13 with dominant-negative constructs and Gβ with small interfering RNAs (30, 42). Gαi silencing impaired FAK and WAVE1 phosphorylation in the presence of P and MPA, but not of moesin (Figure 3A). Silencing of Gβ decreased the phosphorylation of moesin, FAK, and WAVE1 (Figure 3A). Blockade of Gα13 turned into impaired moesin phosphorylation by P or MPA, but not of FAK or WAVE1 (Figure 3, A and B).
Figure 3.
PR Signals to Moesin via Gα13 and to FAK/WAVE1 via Gαi/β A and B, Neurons were treated with P and MPA (1 nM) before or after transfection with dominant-negative Gα13 or Gαi constructs or siRNAs vs Gβ1. Gα13, Gαi, Gβ1, moesin or phospho-moesin, FAK or phospho-FAK and WAVE1 or phospho-WAVE1 were assayed in cell extracts. All experiments were performed three times and representative blots are presented. Densitometric quantifications of all the blots (including those not shown) were performed, and the relative mean ± SD of each condition are presented in a graph as supplemental data. CON, control.
ROCK-2, c-Src, and Cdk5 are involved in P signaling to moesin, FAK, and WAVE1
In the search for the signaling pathways through which PR leads to moesin, FAK, and WAVE1 phosphorylation, we interfered with a number of signaling cascades that are known to be involved in these processes. The c-Src kinase inhibitor (PP2 10 μM), blockade of Rac1 with a dominant-negative construct and a selective inhibitor of Cdk5 (roscovitine 50 μM), all impaired WAVE1 phosphorylation by P or MPA, but did not affect moesin phosphorylation (Figure 4A). In parallel, FAK activation was also prevented by the c-Src kinase inhibitor (PP2) (Figure 4A).
Figure 4.
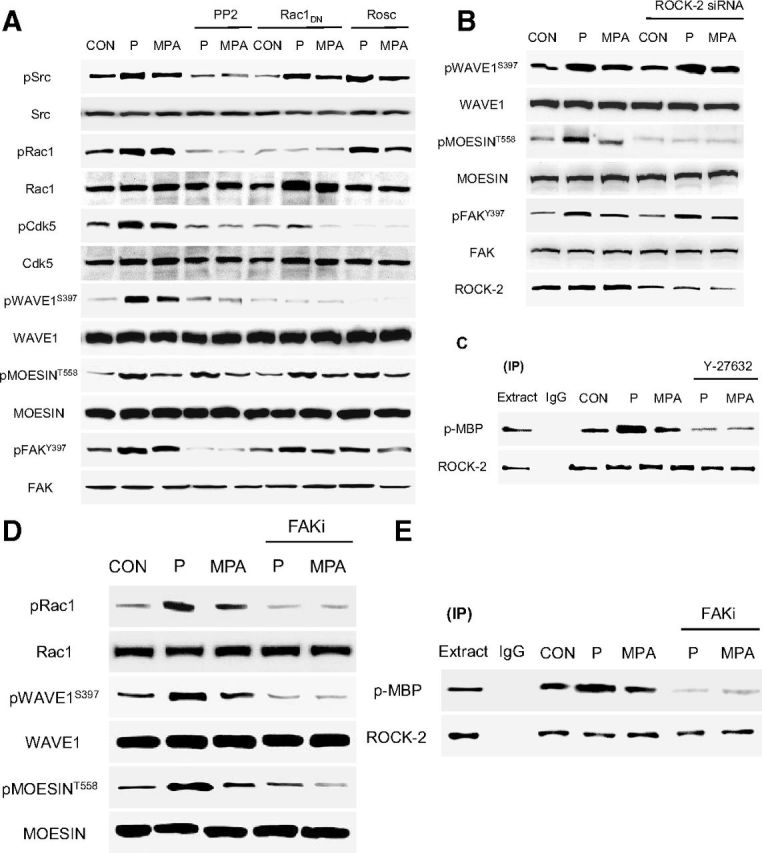
PR Signaling Cascades to Moesin, FAK, and WAVE1 A, Neurons were exposed to 1 nM P or MPA for 20 minutes, in the presence or absence of the Src kinase inhibitor, PP2 (10 μM), after transfection with a dominant-negative Rac1 (RAC1TN17DN) construct, or of the specific Cdk5 inhibitor, roscovitine (Rosc; 50 μM) or B) silencing of ROCK2 with siRNAs (panel B) or with a specific inhibitor of FAK (panel D) (FAKi, 1 μM). A, B, and D, Neuronal cell content of c-Src, Rac1, Cdk5, ROCK2, moesin, FAK, and WAVE1 or of the relative phosphorylated forms were assayed with Western analysis. C and E, Cells were treated with P or MPA (both 1 nM) for 20 minutes in the presence or absence of the ROCK inhibitor, Y-27632 (Y - 10 μM) (panel C) or with a specific inhibitor of FAK (FAKi, 1 μM) (panel D). C and E, ROCK-2 was immunoprecipitated and the IPs were used to phosphorylate the bait protein, MBP. ROCK-2 kinase activity is shown as the amount of phosphorylated MBP (P-MBP). All experiments were performed in triplicates and representative images are shown. Densitometric quantifications of all the blots (including those not shown) were performed, and the relative mean ± SD of each condition is presented in a graph as supplemental data. CON, control.
Silencing of ROCK-2 with small interfering RNAs resulted in decreased moesin phosphorylation during exposure to P or MPA, but not of FAK or WAVE1 (Figure 4B). These findings suggest that PR signals to moesin via a ROCK-2-dependent pathway. In parallel, PR uses a c-Src/Rac1/Cdk5-dependent pathway to recruit WAVE1 and requires c-Src to control FAK.
ROCK-2 was functionally activated in the presence of P (1 nM) as shown by enhanced Thr-phosphorylation of the bait protein MBP by ROCK-2 IPs (Figure 4C).
In addition, moesin and WAVE1 phosphorylation induced by P or MPA was inhibited by blockade of FAK with a specific inhibitor (FAKi) (Figure 4D), which also blocked Rac1 phosphorylation (Figure 4D). This suggests that PR recruitment of c-Src and FAK leads to WAVE1 phosphorylation via Rac1 activation, consistent with previous work showing that FAK controls Wiskott-Aldrich Syndrome family proteins during cellular movement (47, 48). To further test the requirement of ROCK-2 for P signaling in neurons as a possible target of FAK in the regulation to moesin, we immunoprecipitated ROCK-2 and used the IPs to perform kinase assays using dephosphorylated MBP as the target for ROCK-2. P administration resulted in a rapid increase in ROCK-2 activity, which was sensitive to FAKi (μM) (Figure 4E).
Based on these experiments, FAK is suggested to represent a central controller modulating different pathways recruited by PR, favoring its control of actin remodeling via moesin and of spine formation via WAVE1.
FAK mediates P signaling to moesin and WAVE1 and the formation of dendritic spines in cortical neurons
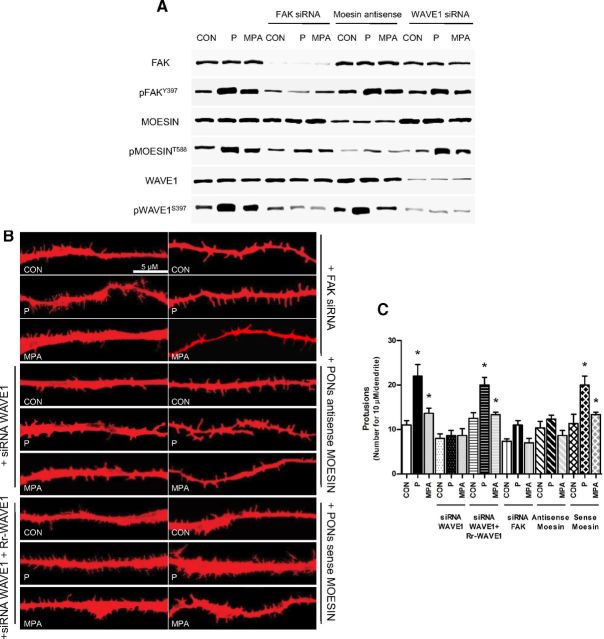
To test this concept, we silenced FAK, moesin, or WAVE1 during treatment with P (1 nM) or MPA (1 nM). Blockade of FAK resulted in impaired Thr588-moesin and Ser397-WAVE1 phosphorylation (Figure 5A). Blockade of WAVE1 did not affect FAK and moesin phosphorylation by P or MPA (Figure 5A), and silencing of moesin did not alter FAK Tyr397 and WAVE1 Ser397 phosphorylation (Figure 5A), supporting the idea that FAK is central in modulating PR-related phosphorylation of moesin and WAVE1.
Figure 5.
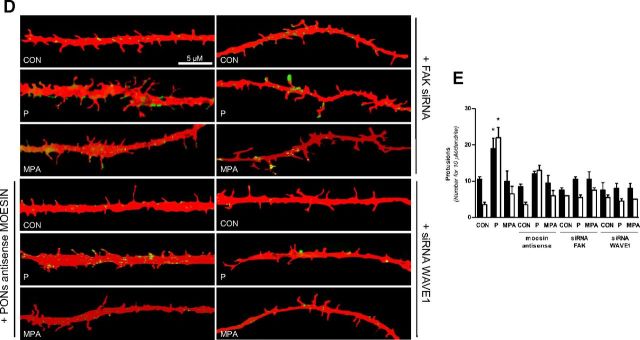
P Signaling to Moesin and FAK/WAVE1 Turns into a Dendrite Spine Formation and Membrane Redistribution of WAVE1 A, Neuronal cells were treated for 20 minutes with 1 nM P and MPA with or without transfection with moesin antisense phosphorothioate oligonucleotides and siRNAs vs FAK and WAVE1. Moesin, Thr588 phospho-moesin, FAK, Tyr397 phospho-FAK, WAVE1, and Ser397 phospho-WAVE1 were assayed in cell extracts. B, Dendritic spine density was measured with immunofluorescence after staining actin fibers with phalloidin/Texas Red. The scale bar corresponds to 5 μm. C, Graph shows the quantitative analysis of spine density expressed as the number of spines per 10 μm dendrite length. D and E, Quantification of the cell membrane dendrite-localized phospho-WAVE1 in the different conditions. Results are expressed as percent vs control cells (mean ± SD). *P < .05 vs control (CON). Dendrite-localized phospho-WAVE1 was counted in 40 different cells. The experiments were repeated three times with consistent results. Densitometric quantifications of all the blots (including those not shown) were performed, and the relative mean ± SD of each condition is presented in a graph as supplemental data.
Actin staining in cortical neurons demonstrated a net enrichment in dendritic spine density after treatment with P, and much less so with MPA (Figure 5, B and C). Silencing of WAVE1 significantly decreased the number of spines induced by P or MPA (Figure 5, B and C). As control, the transfection of a siRNA-resistant WAVE1 construct reversed the effect of the siRNA (Figure 5, B and C). FAK and moesin silencing also prevented the increase in dendritic spines by P or MPA (Figure 5, B and C).
We therefore studied the distribution of Ser397-phosphorylated (active) WAVE1 in dendritic protrusions of cortical neurons during exposure to the progestogens. In control cells, Ser397-phospho-WAVE1 was diffusely distributed throughout the dendrites (Figure 5D). Short-term exposure to P or MPA (1 nM, 20 min) led to an increase in WAVE1 Ser397 phosphorylation and translocation of the signal at the edge of the dendrites, particularly where spines were formed (Figure 5, D and E). This paralleled a visible increase in spine formation (Figure 5, D and E). The subcellular redistribution of active WAVE1 and the increase in the spine density were counteracted by silencing of FAK and moesin (Figure 5, D and E).
Discussion
Sex steroids, and particularly estrogen and P, control brain plasticity through neuronal/glial remodeling, which is critical for memory, learning, and cognition (49). At the basis of brain plasticity is the ability of neurons and glial cells to remodel their mutual connections, which requires major changes in cell morphology (50). These morphologic modifications depend on the generation of dynamic structural changes of the actin cytoskeleton and on the development of protrusive membrane structures, such as dendritic spines (51). These processes require a number of modulators orchestrating the interactions of cytoskeletal proteins with the cell membrane and anchorage proteins (51, 52). The cytoskeleton forms the principal machinery involved in such phenomena in neurons. During changes in cell morphology, globular actin readily polymerizes to form fibrillar actin and de novo actin polymerization occurs, resulting in the formation of membrane protrusions providing a structural basis for interactions with extracellular matrix proteins or nearby cells through anchorage proteins and focal adhesions (53, 54). These processes are important for the morphologic changes required for dendritic spine formation (30, 55). This manuscript highlights that such processes are under the control of P and of synthetic progestins, and identifies the molecular events involved.
P influences embryonic neuronal development and continues to act on mature neurons regulating their structural plasticity at synapses and modulating dendritic spine density through the dynamic control of actin filaments (18). Understanding the basis through which P drives neurons to enact plasticity and transmission heralds profound biological and medical implications.
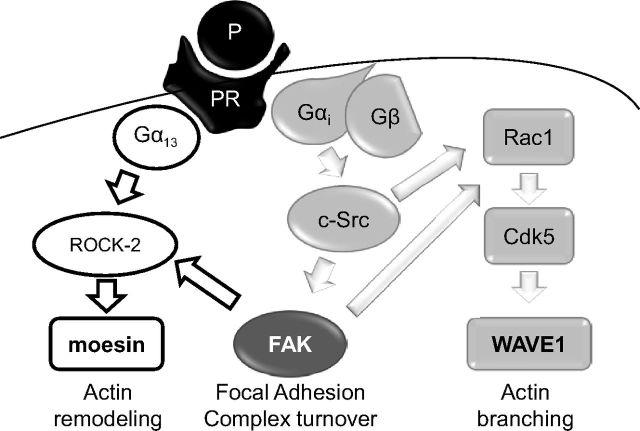
Our work shows that natural P uses the actin controllers moesin, FAK, and WAVE1 in neurons to induce rapid changes of cell membrane morphology, enhancing the ability of neurons to form dendritic spines, which may contribute to the effects of this hormone on neuronal plasticity and transmission. This is achieved through at least two principal cascades of events, both linked to PR-dependent signaling. Through the rapid signaling of PR to the G protein Gα13, P is able to recruit the small GTPase Ras homolog gene family, member A and the Rho-associated kinase, ROCK-2, leading to moesin activation. In parallel, PR recruits a Gαi/Gβ/c-Src/FAK/Rac1/Cdk5/WAVE1 cascade, which turns into WAVE1 Ser397-phosphorylation and translocation to sites where dendritic spines are formed. These parallel sets of events are both required for the increase in dendritic spine formation associated with P exposure (Figure 6). It is also interesting to note that because silencing of conventional PRs abolishes these signaling chains, it seems likely that the recently identified membrane-bound PRs (65) may not be important in this system.
Figure 6.
Proposed intracellular signaling of P to moesin/FAK/WAVE1 implicated in dendritic spine formation in rat cortical neurons.
We also identify FAK as a potential central controller of both pathways, which may suggest this nonreceptor tyrosine kinase could be particularly relevant in steroid-related events in the central nervous system. In addition, these findings also highlight the relevance of FAK for neuronal physiology. The identification of FAK regulation as a key action of P may offer in the future mechanistic insights to better understand the role of this hormone on neuronal plasticity and transmission.
WAVE1 is an established player for actin branching and spine formation in neurons. Loss of WAVE1 function in vivo or in cultured neurons results in a decrease in mature dendritic spines (56), and WAVE1 knockout mice exhibit deficits in learning and memory (56, 57), suggesting the intriguing hypothesis that the increased prevalence of cognitive impairment and of some degenerative disorders observed in conditions of P and estrogen withdrawal might be due, to some extent, to lack of WAVE1 activation/control by these steroids in brain cells.
It is interesting to note that the comparison between the administration of natural P and the synthetic progestin MPA turns in all experiments into similar effects, albeit MPA is significantly less potent. Due to its inherent higher affinity with PR, one derives that such observation is related to differences in the ability to drive PR to achieve extranuclear signaling, which has been shown before in other cell types (58, 59). The growing indications that progestogens are not all alike adds perspective to the clinical observations that the use of natural P or specific synthetic progestins may turn into different effects on the cardiovascular system, on breast tissue, and on the brain, likewise. It also fits with the past observations that P and MPA differ significantly in many biological actions in neurons. This applies to protection from glutamate excitotoxicity (60, 61), neuronal progenitor cell proliferation (62), neuronal mitochondrial function (63), and with the vast amount of data on the modulation of estrogen-linked spine formation in vivo (64). It is also interesting to see that in the presence of estradiol, the action of P or MPA, although slightly inhibitory on WAVE1 phosphorylation, does not turn into significant modifications of the enhancement of spine formation associated with estrogen in this setting; this observation points to the fact that molecular events are not necessarily linked to functional differences. This also indicates that complexity grows when steroid hormones sharing signaling pathways are jointly present, suggesting that the modulation of dendritic spine turnover may vary throughout life based on the reciprocal levels of estrogens and P available to brain cells. However, these finding may also indicate that pharmacologic use of progestins that may turn into concentrations that are very high (such as during hormonal adjuvant therapies for endocrine-sensitive cancers) or stably elevated (such as during certain depot or oral administration of progestins), may have some functional impact on neuronal spine turnover.
In conclusion, the present manuscript suggests that, within the broader range of actions of PRs, rapid extranuclear signaling to the actin cytoskeleton through the Gαi/Gβ/c-Src/FAK/Rac1/Cdk5/WAVE1 and Gα13/Gβ/ROCK-2/moesin cascade is relevant for the generation of P-dependent dendritic spine formation in rat cortical neurons. These extranuclear signaling avenues may represent a useful way selected by nature to use P as a flexible modulator of neuronal function, allowing dynamic changes in response to surrounding stimuli. Further investigation in this area will lead to better understanding of the mechanistic basis through which sex steroids dynamically control brain physiology and could help to engineer newer and more selective pharmacologic tools for endocrine therapies against important neurological diseases.
Acknowledgments
We thank Dr. Yong Kim and Dr. Paul Greengard (The Rockefeller University, New York, New York) for providing the siRNA-resistant WAVE1 construct.
This work has been supported by Programma di Ricerca di Interesse Nazionale grant 2004057090_007 (to T.S.) by the Italian University and Scientific Research Ministry.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Cdk5
- cyclin-dependent kinase 5
- E2
- 17β-estradiol
- FAK
- focal adhesion kinase
- FAKi
- FAK inhibitor
- IPs
- immunoprecipitates
- MBP
- myelin basic protein
- MPA
- medroxyprogesterone acetate
- P
- progesterone
- PON
- phosphorothioate oligonucleotide
- PR
- progesterone receptor
- ROCK-2
- Rho-associated kinase-2
- siRNA
- small interfering RNA
- WASP
- Wiskott-Aldrich syndrome protein
- WAVE
- Wiskott-Aldrich syndrome protein-family verprolin homologous.
References
- 1. Schubert V , Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–212. [DOI] [PubMed] [Google Scholar]
- 2. Horner CH. Plasticity of the dendritic spine. Prog Neurobiol. 1993;41:281–321. [DOI] [PubMed] [Google Scholar]
- 3. Tseng GF , Hu ME. Axotomy induces retraction of the dendritic arbor of adult rat rubrospinal neurons. Acta Anat (Basel). 1996;155:184–193. [DOI] [PubMed] [Google Scholar]
- 4. Chen JR , Wang YJ , Tseng GF. The effect of epidural compression on cerebral cortex: a rat model. J Neurotrauma. 2003;20:767–780. [DOI] [PubMed] [Google Scholar]
- 5. Ferrer I , Guionnet N , Cruz-Sanchez F , Tunon T. Neuronal alterations in patients with dementia: a Golgi study on biopsy samples. Neurosci Lett. 1990;114:11–16. [DOI] [PubMed] [Google Scholar]
- 6. Chen JR , Yan YT , Wang TJ , Chen LJ , Wang YJ , Tseng GF. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex. 2009;19:2719–2727. [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ , Chen JR , Wang YJ , Tseng GF. The cytoarchitecture and soma-dendritic arbors of the pyramidal neurons of aged rat sensorimotor cortex: an intracellular dye injection study. Neuroscience. 2009;158:776–785. [DOI] [PubMed] [Google Scholar]
- 8. Scheibel ME , Lindsay RD , Tomiyasu U , Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. [DOI] [PubMed] [Google Scholar]
- 9. Cohen RS , Chung SK , Pfaff DW. Immunocytochemical localization of actin in dendritic spines of the cerebral cortex using colloidal gold as a probe. Cell Mol Neurobiol. 1985;5:271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fifkova E , Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer M , Kaech S , Wagner U , Brinkhaus H , Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000;3:887–894. [DOI] [PubMed] [Google Scholar]
- 12. Kasai H , Matsuzaki M , Noguchi J , Yasumatsu N , Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. [DOI] [PubMed] [Google Scholar]
- 13. Cooke BM , Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. [DOI] [PubMed] [Google Scholar]
- 14. McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. [DOI] [PubMed] [Google Scholar]
- 15. McEwen BS , Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–436. [DOI] [PubMed] [Google Scholar]
- 16. Genazzani AR , Stomati M , Morittu A , et al. Progesterone, progestagens and the central nervous system. Hum Reprod 2000;15(suppl 1):14–27. [DOI] [PubMed] [Google Scholar]
- 17. Foy MR. Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiol Learn Mem. 2011;95:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foy MR , Baudry M , Akopian GK , Thompson RF. Regulation of hippocampal synaptic plasticity by estrogen and progesterone. Vitam Horm. 2010;82:219–239. [DOI] [PubMed] [Google Scholar]
- 19. Hojo Y , Murakami G , Mukai H , et al. Estrogen synthesis in the brain-Role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. [DOI] [PubMed] [Google Scholar]
- 20. Baum LW. Sex, hormones, and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–743. [DOI] [PubMed] [Google Scholar]
- 21. Bourque M , Dluzen DE , Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30:142–157. [DOI] [PubMed] [Google Scholar]
- 22. Brinton RD , Nilsen J. Effects of estrogen plus progestin on risk of dementia. JAMA 2003;290:1706; author reply 1707–1708. [DOI] [PubMed] [Google Scholar]
- 23. Brinton RD , Thompson RF , Foy MR , et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carroll JC , Rosario ER , Chang L , et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh M , Sumien N , Kyser C , Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vallee M , Mayo W , Koob GF , Le Moal M. Neurosteroids in learning and memory processes. Int Rev Neurobiol. 2001;46:273–320. [DOI] [PubMed] [Google Scholar]
- 27. Flamini MI , Sanchez AM , Goglia L , Tosi V , Genazzani AR , Simoncini T. Differential actions of estrogen and SERMs in regulation of the actin cytoskeleton of endometrial cells. Mol Hum Reprod. 2009;15:675–685. [DOI] [PubMed] [Google Scholar]
- 28. Fu XD , Giretti MS , Baldacci C , et al. Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS One. 2008;3:e2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giretti MS , Fu XD , De Rosa G , et al. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS One. 2008;3:e2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanchez AM , Flamini MI , Fu XD , et al. Rapid signaling of estrogen to WAVE1 and moesin controls neuronal spine formation via the actin cytoskeleton. Mol Endocrinol. 2009;23:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simoncini T , Scorticati C , Mannella P , et al. Estrogen receptor alpha interacts with Gα13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756–1771. [DOI] [PubMed] [Google Scholar]
- 32. Li C , Brake WG , Romeo RD , et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sakamoto H , Mezaki Y , Shikimi H , Ukena K , Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144:4466–4477. [DOI] [PubMed] [Google Scholar]
- 34. Flamini MI , Fu XD , Sanchez AM , et al. Effects of raloxifene on breast cancer cell migration and invasion through the actin cytoskeleton. J Cell Mol Med. 2009;13:2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bailly M , Macaluso F , Cammer M , Chan A , Segall JE , Condeelis JS. Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J Cell Biol. 1999;145:331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higgs HN , Blanchoin L , Pollard TD. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry. 1999;38:15212–15222. [DOI] [PubMed] [Google Scholar]
- 37. Higgs HN , Pollard TD. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem. 1999;274:32531–32534. [DOI] [PubMed] [Google Scholar]
- 38. Mullins RD , Heuser JA , Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95:6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takenawa T , Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. [DOI] [PubMed] [Google Scholar]
- 40. Yamazaki D , Kurisu S , Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fu XD , Goglia L , Sanchez AM , et al. Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase. Endocr Relat Cancer. 2010;17:431–443. [DOI] [PubMed] [Google Scholar]
- 42. Sanchez AM , Flamini MI , Baldacci C , Goglia L , Genazzani AR , Simoncini T. Estrogen receptor-α promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol Endocrinol. 2010;24:2114–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitra SK , Hanson DA , Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. [DOI] [PubMed] [Google Scholar]
- 44. McLean GW , Carragher NO , Avizienyte E , Evans J , Brunton VG , Frame MC. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. [DOI] [PubMed] [Google Scholar]
- 45. Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71:143–155. [DOI] [PubMed] [Google Scholar]
- 46. Zheng S , Huang J , Zhou K , et al. Progesterone enhances vascular endothelial cell migration via activation of focal adhesion kinase. J Cell Mol Med. 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim AS , Kakalis LT , Abdul-Manan N , Liu GA , Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. [DOI] [PubMed] [Google Scholar]
- 48. Schlaepfer DD , Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. [DOI] [PubMed] [Google Scholar]
- 49. Duffau H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J Clin Neurosci. 2006;13:885–897. [DOI] [PubMed] [Google Scholar]
- 50. Kulkarni VA , Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50:10–20. [DOI] [PubMed] [Google Scholar]
- 51. Sanchez AM , Flamini MI , Polak K , et al. Actin cytoskeleton remodelling by sex steroids in neurones. J Neuroendocrinol. 2012;24:195–201. [DOI] [PubMed] [Google Scholar]
- 52. Sanchez AM , Simoncini T. Extra-nuclear signaling of ERα to the actin cytoskeleton in the central nervous system. Steroids. 2010;75:528–532. [DOI] [PubMed] [Google Scholar]
- 53. Pollard TD , Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. [DOI] [PubMed] [Google Scholar]
- 54. Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. [DOI] [PubMed] [Google Scholar]
- 55. Kim Y , Sung JY , Ceglia I , et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. [DOI] [PubMed] [Google Scholar]
- 56. Soderling SH , Langeberg LK , Soderling JA , et al. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA. 2003;100:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Soderling SH , Guire ES , Kaech S , et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simoncini T , Mannella P , Fornari L , et al. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004;145:5745–5756. [DOI] [PubMed] [Google Scholar]
- 59. Fu XD , Giretti MS , Goglia L , et al. Comparative actions of progesterone, medroxyprogesterone acetate, drospirenone and nestorone on breast cancer cell migration and invasion. BMC Cancer. 2008;8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mannella P , Sanchez AM , Giretti MS , Genazzani AR , Simoncini T. Oestrogen and progestins differently prevent glutamate toxicity in cortical neurons depending on prior hormonal exposure via the induction of neural nitric oxide synthase. Steroids. 2009;74:650–656. [DOI] [PubMed] [Google Scholar]
- 61. Nilsen J , Morales A , Brinton RD. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol. 2006;22:355–361. [DOI] [PubMed] [Google Scholar]
- 62. Liu L , Zhao L , She H , et al. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology. 2010;151:5782–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Irwin RW , Yao J , Ahmed SS , Hamilton RT , Cadenas E , Brinton RD. Medroxyprogesterone acetate antagonizes estrogen up-regulation of brain mitochondrial function. Endocrinology. 2011;152:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Srivastava DP , Woolfrey KM , Evans PD. Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity [published online December 13, 2012]. Neuroscience. doi:10.1016/j.neuroscience.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 65. Pang Y , Dong J , Thomas P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ϵ (mPRδ and mPRϵ) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology. 2013;154(1):283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]