Abstract
Immediately following exposure to light, a postillumination burst of CO2 has been detected in Crassulacean acid metabolism plants. A detailed study with pineapple (Ananas comosus) leaves indicates that the postillumination burst changes its amplitude and kinetics during the course of a day. In air, the postillumination burst in pineapple leaves generally is exhibited as two peaks. The postillumination burst is sensitive to atmospheric CO2 and O2 concentrations as well as to the light intensity under which plants are grown. We propose that the CO2 released in the first postillumination burst peak is indicative of photorespiration since it is sensitive to either O2 or CO2 concentration while the second CO2 evolution peak is likely due to decarboxylation of organic acids involved in Crassulacean acid metabolism.
In marked contrast to other higher plants, the postillumination burst in Crassulacean acid metabolism plants can be equal to or greater than the rate of photosynthesis. Photosynthesis in pineapple leaves also varies throughout a day. Both photosynthesis and the postillumination burst have a daily variation which apparently is a complex function of degree of leaf acidity, growth light intensity, ambient gas phase, and the time a plant has been exposed to a given gas.
Full text
PDF
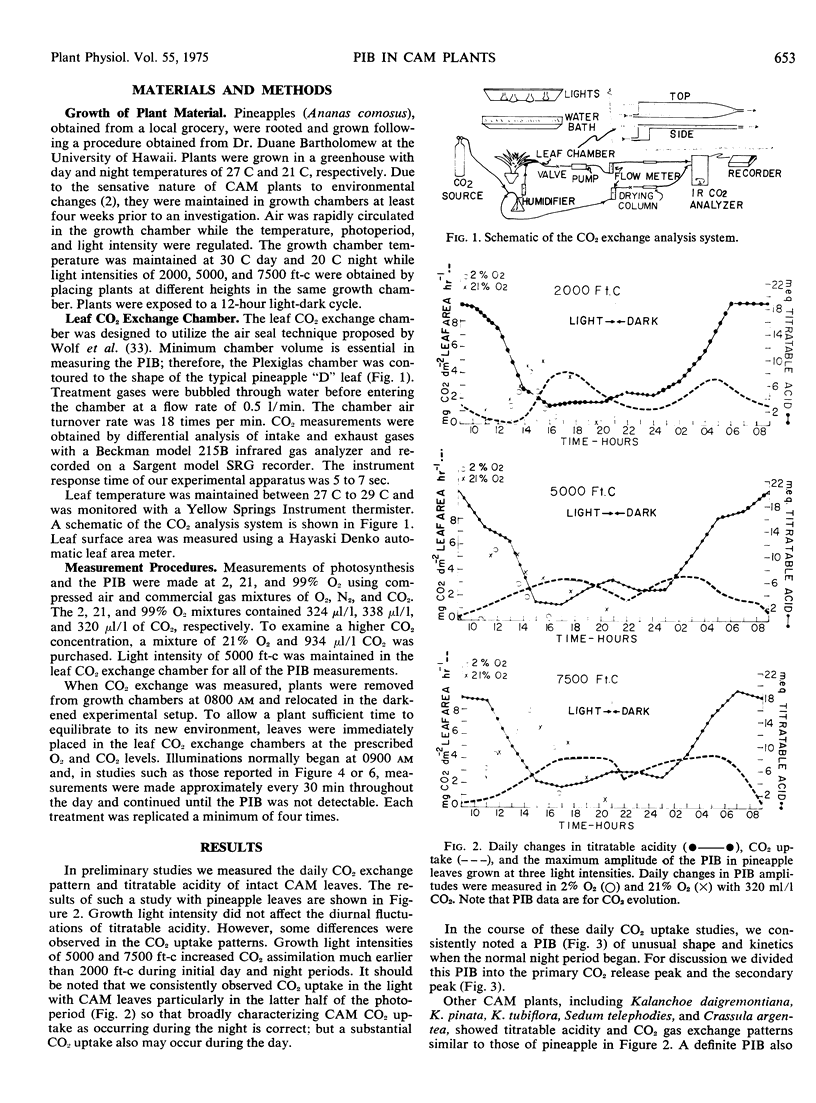
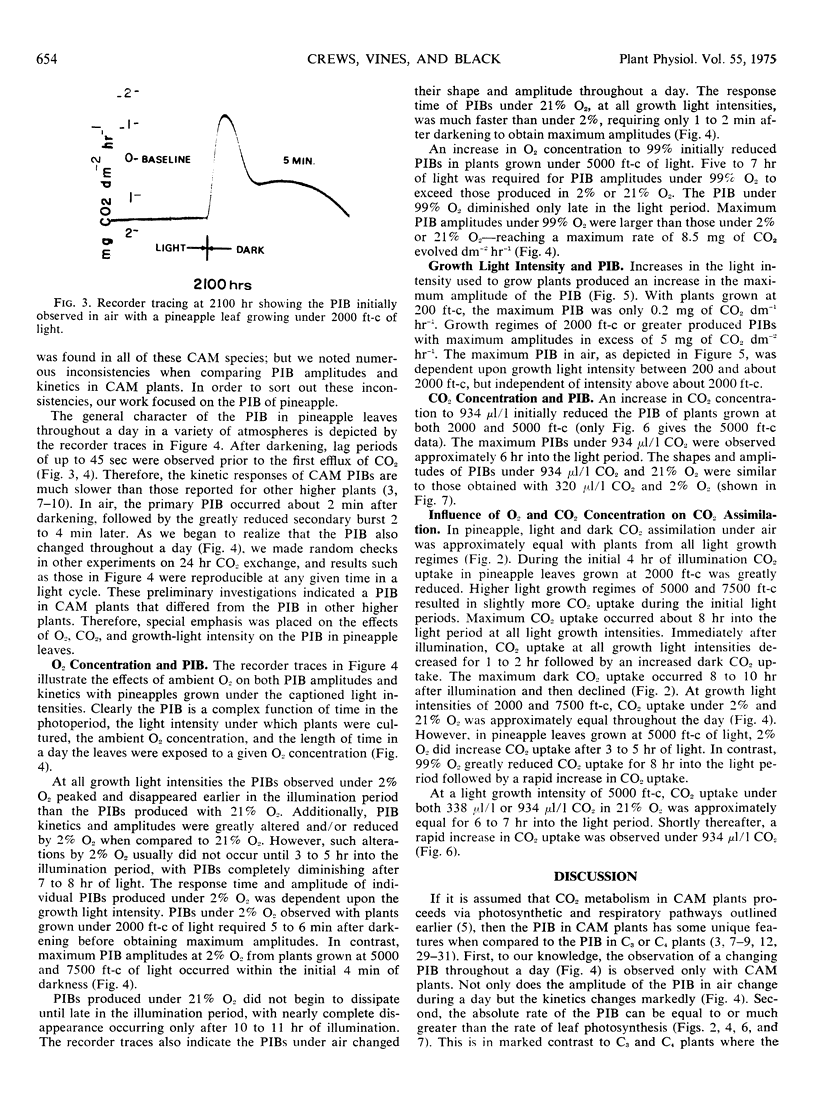
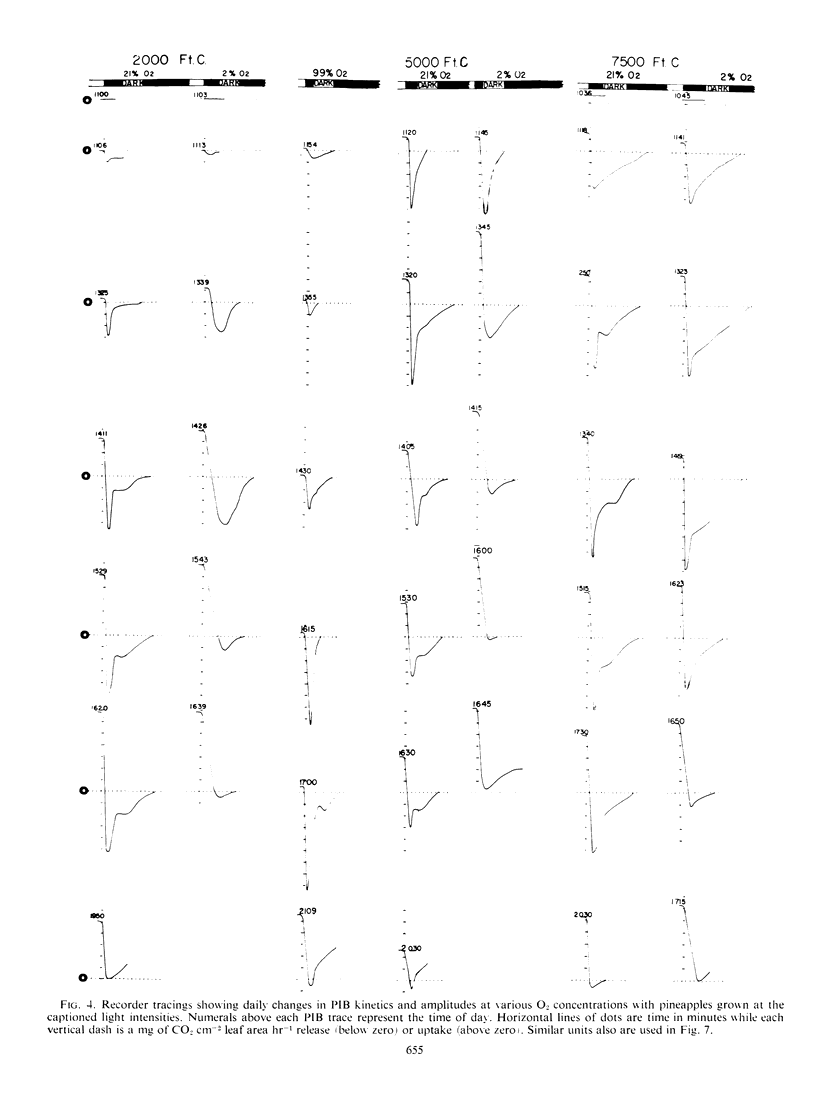
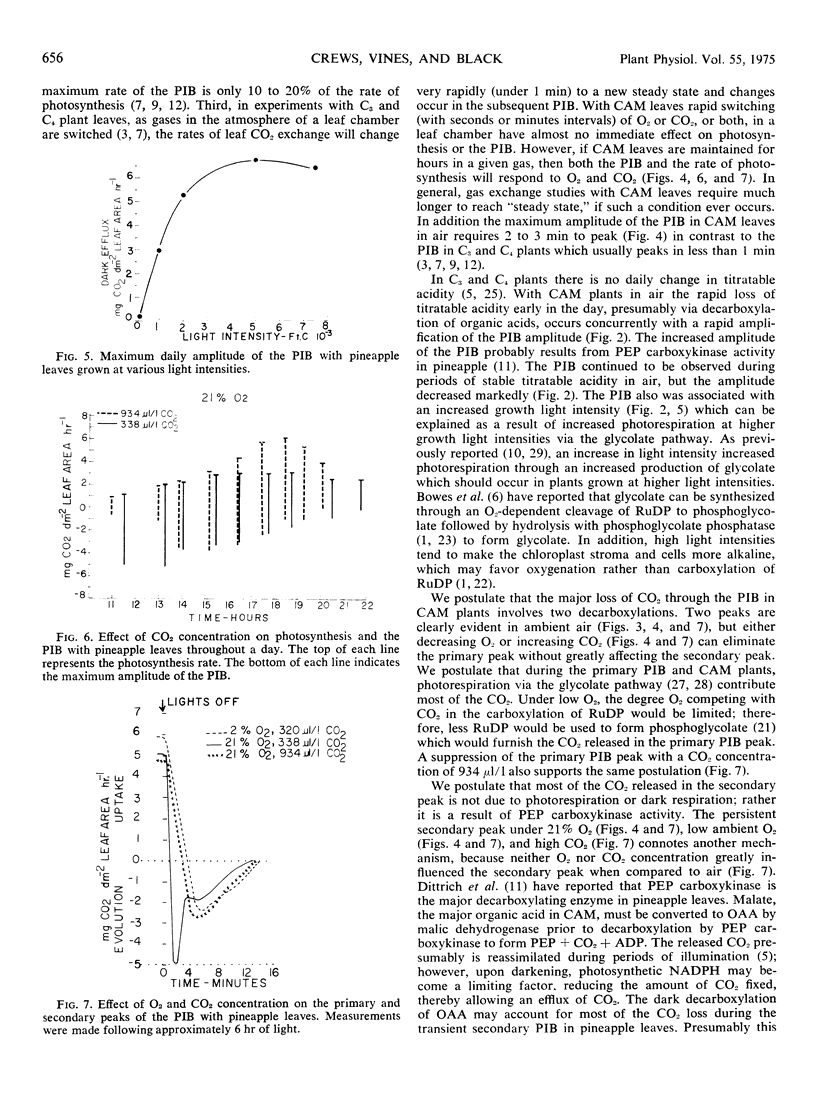

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Decker J. P. A Rapid, Postillumination Deceleration of Respiration in Green Leaves. Plant Physiol. 1955 Jan;30(1):82–84. doi: 10.1104/pp.30.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker J. P. Comparative Responses of Carbon Dioxide Outburst and Uptake in Tobacco. Plant Physiol. 1959 Mar;34(2):100–102. doi: 10.1104/pp.34.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich P., Campbell W. H., Black C. C. Phosphoenolpyruvate carboxykinase in plants exhibiting crassulacean Acid metabolism. Plant Physiol. 1973 Oct;52(4):357–361. doi: 10.1104/pp.52.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Kagawa T. NAD malic enzyme in leaves with C-pathway photosynthesis and its role in C4 acid decarboxylation. Arch Biochem Biophys. 1974 Jan;160(1):346–349. doi: 10.1016/s0003-9861(74)80043-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heichel G. H. Response of Respiration of Tobacco Leaves in Light and Darkness and the CO(2) Compensation Concentration to Prior Illumination and Oxygen. Plant Physiol. 1971 Aug;48(2):178–182. doi: 10.1104/pp.48.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitake G., Saltman P. Dark Fixation of CO(2) by Succulent Leaves: Conservation of the Dark Fixed CO(2) Under Diurnal Conditions. Plant Physiol. 1958 Nov;33(6):400–403. doi: 10.1104/pp.33.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren W. L., Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971 Mar 31;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- STRIEBEL A., VON PLANTA P., VIOLLIER G. Calcium- und Magnesiumaus-scheidung im Urin bei Mamma-Karzinomen zur Beurteilung der Testosterontherapie. Schweiz Med Wochenschr. 1955 Feb 19;85(8):167–170. [PubMed] [Google Scholar]
- Takabe T., Akazawa T. Oxidative formation of phosphoglycolate from ribulose-1,5-diphosphate catalysed by Chromatium ribulose-1,5-diphosphate carboxylase. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1173–1179. doi: 10.1016/0006-291x(73)90588-3. [DOI] [PubMed] [Google Scholar]
- Thompson C. M., Whittingham C. P. Intracellular localisation of phosphoglycollate phosphatase and glyoxalate reductase. Biochim Biophys Acta. 1967;143(3):642–644. doi: 10.1016/0005-2728(67)90074-6. [DOI] [PubMed] [Google Scholar]
- VARNER J. E., BURRELL R. C. Use of C14 in the study of the acid metabolism of Bryophyllum calycinum. Arch Biochem. 1950 Feb;25(2):280–287. [PubMed] [Google Scholar]


